All of our recommendations, unless specified, relate to acute COVID-19 in adults.
Some of our recommendations vary according to the severity of COVID-19 illness. Definitions of the categories are based on World Health Organization (WHO) criteria and can be viewed by clicking the plus (+) signs below.
RECOMMENDATION:
We recommend against use of Favipiravir in the treatment of COVID-19 except in the context of a randomized controlled trial.
DATE OF RECOMMENDATION: 14 February 2023
Definition of mild:
- Symptomatic (any acute COVID-19 related symptoms)
- AND respiratory rate <24/min
- WITHOUT pneumonia or hypoxia
Definition of moderate illness:
- Pneumonia (clinical or radiological) OR hypoxia (SpO2 <94% in adults with no underlying lung disease)
- AND respiratory rate ≤30/min
- AND SpO2 ≥90% on room air
Definition of Severe illness
Pneumonia with ANY ONE of the following:
- severe respiratory distress or respiratory rate >30/min
- SpO2 <90% on room air
- NO invasive or non-invasive respiratory support needed
Definition of critical:
- Requirement for high-level respiratory support: noninvasive ventilation, high-flow oxygen (≥20 litres per minute) or invasive mechanical ventilation
- OR acute respiratory distress syndrome (PaO2/FiO2 ratio of <300)
- OR sepsis
- OR shock
Favipiravir is an antiviral drug that has been evaluated in the past for the treatment of Influenza and Ebola. Favipiravir was widely used in the initial part of the pandemic in Wuhan, Russia, and many parts of Asia. It was initially used in India as well, after approval from the Drugs Controller General of India in June 2020, and made its way into many state and institutional guidelines.
After detailed review of available evidence, we do not recommend the use of Favipiravir for any category of patients with COVID-19 infection for the following reasons:
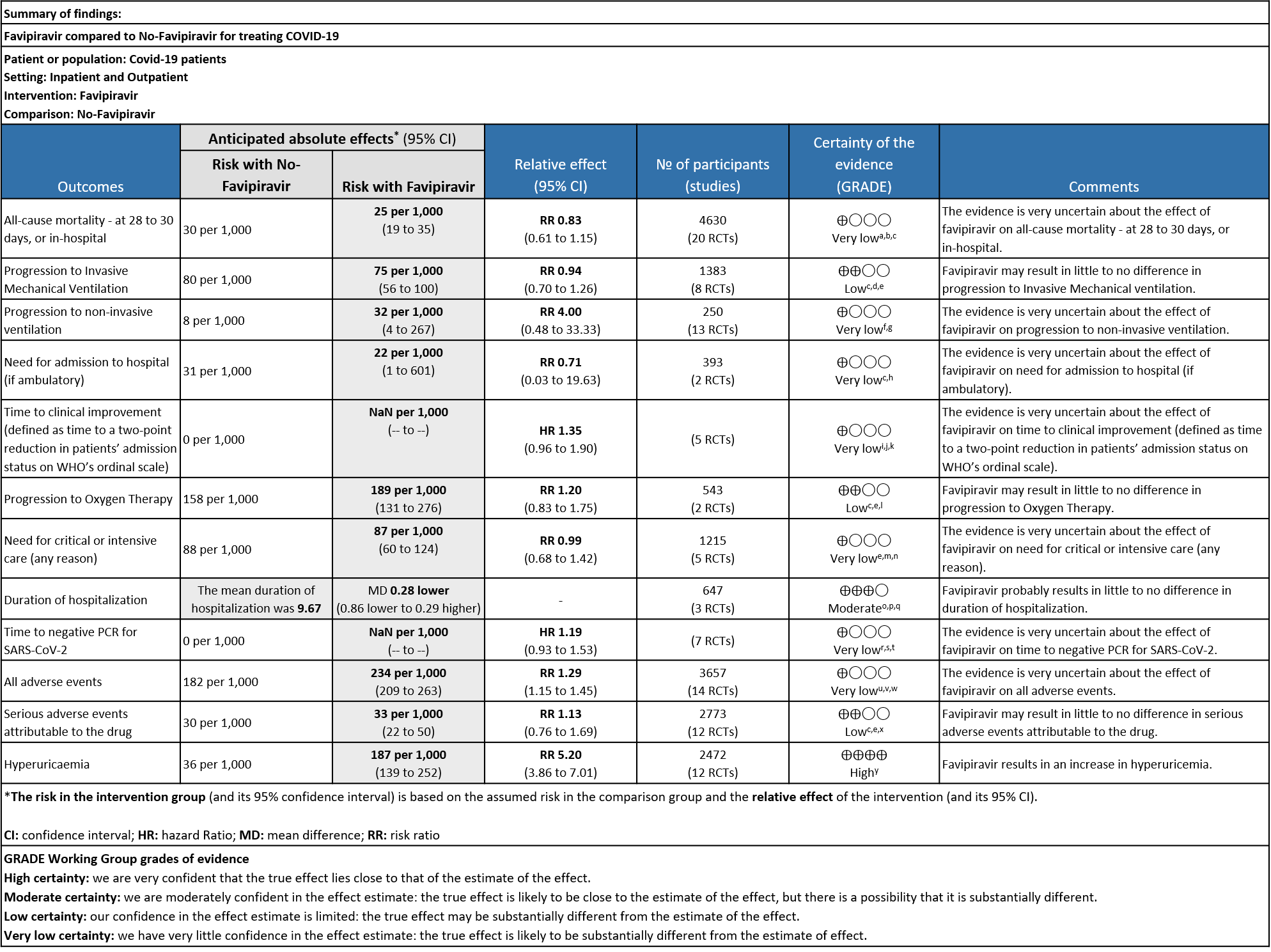
There was very low certainty evidence for clinically relevant outcomes like all-cause mortality, need for hospitalization, time to clinical improvement, need for critical or intensive care, progression to non-invasive ventilation, time to negative PCR and all adverse events indicating that the evidence is very uncertain regarding the effect of Favipiravir.
Further, for outcomes like progression to invasive mechanical ventilation, progression to oxygen therapy and serious adverse events attributable to the drug, the certainty is low indicating that Favipiravir may result in little to no difference in these outcomes.
There was high certainty of evidence to suggest that Favipiravir results in an increase in hyperuricemaia and moderate certainty to suggest that Favipiravir probably results in little to no difference in duration of hospitalization.
Overall, the benefit of Favipiravir in the treatment of COVID-19 is low to uncertain as there was significant heterogeneity among the trials, compared to what would be standard of care today and wide confidence intervals in effect estimates of critical outcomes. Hence at this point, there is insufficient evidence to recommend this drug routinely in the context of Covid-19.
Date of latest search: September 26th 2022
Date of completion & presentation to the Expert Working Group:
Date of planned review:
Evidence Synthesis Team: Hanna Alexander, Pritish Korula, Jisha Sara John,Richard Kirubakaran, Bhagteshwar Singh, Prathap Tharyan, Priscilla Rupali
Explanations
a. Downgraded by two levels for risk of bias: Dmitri Puskar, Holubar M, Solayamani Dodaran M, Shenoy S, Udwadia Z and Zhao H have some concerns and Balykova, Behnam Mahmudi, Chen, Finberg, Lou, Ruzhenstova TA, Shinkai M and Tabarsi have high RoB
b. Downgraded by 1 level for inconsistency: As we identified moderate heterogeneity, (I² = 59%)
c. Downgraded by one level for serious imprecision: lower confidence interval bound represents a mild benefit from Favipiravir whereas the upper bound includes harm.
d. Downgraded by one level for Risk of Bias as Ivaschenko and Solayamani Dodaran M had some concerns in the risk of bias whereas Behnam Maahmudie, Finberg, Lou Y and Ruzhenstova (40%) have high risk of bias.
e. Not downgraded for inconsistency as we did not identify any heterogeneity( I²=0%)
f. Downgraded by 2 levels for Risk of Bias, as Dmitry Pushkar has some concerns and Finberg has high Risk of Bias.
g. Downgraded by 2 levels for very serious Imprecision as the lower bound represents appreciable benefit and upper bound suggests harm (0.48, 33.33)
h. Downgraded two levels due to inconsistency. We identified considerable heterogeneity (I² = 76%).
i. Downgraded by two levels for very serious risk of bias: Balykova reported high risk of bias with regard to missing outcomes and measurement of outcomes; Finberg reported high risk of bias arising from the randomization process and some concerns due to deviations from intended interventions; Ruzhenstova reported high risk of bias due to measurement of outcome; Shenoy reported some concerns due to bias arising from randomization process; Udwadia reported some concerns due to missing outcome data.
j. Downgraded two levels due to inconsistency. We identified considerable heterogeneity (I² = 78%).
k. Downgraded by one level for serious imprecision: lower confidence interval bound represents mild harm from Favipiravir, whereas the upper bound includes appreciable benefit.
l. Downgraded by one level for serious risk of bias: Lou reported a high risk of bias in randomization and some concerns due to deviations from intended interventions.
m. Downgraded by two levels for very serious risk of bias: Ruzhenstova reported a high risk of bias due to measurement of outcome; Solaymani reported some concerns due to bias arising from the selection of reported results whereas Tabarsi reported a high risk of bias arising from deviation from intended interventions, missing outcome data and some concerns due to selection of reported results.
n. Downgraded by one level for serious imprecision: lower confidence interval bound represents an appreciable benefit from Favipiravir whereas the upper bound includes harm.
o. Not downgraded for risk of bias as Chuah H has no risk of bias (92.2% weightage) whereas as studies with minimal weightage like Behnam Mahmudie and Finberg has high risk of bias
p. Not downgraded for inconsistency as we did not identify any important heterogeneity(I²=14%)
q. Downgraded by one level for imprecision: the optimal information size is not met
r. Downgraded by 2 levels for Risk of Bias as Shinkai, Ruzhentsova and Finberg (>40%) have high risk of bias.
s. Downgraded by 1 level for inconsistency: As we identified moderate heterogeneity, (I² = 52%)
t. Downgraded by one level for serious imprecision: lower confidence interval bound represents mild harm from Favipiravir, whereas the upper bound suggests appreciable benefit.
u. Downgraded by 2 levels for Risk of Bias as Balykova, Ruzhnetsova, Shinkai, Chen & Finberg contribute to >50% of weightage and has high risk of bias
v. Downgraded by 1 level for inconsistency: As we identified considerable heterogeneity, (I² = 72%)
w. Not downgraded for Imprecision, even though the confidence of interval varies from 1.25 to 1.61 because the upper and lower bound point towards harm from the intervention.
x. Downgraded by one level for risk of bias: Holubar M, Shenoy S, Udwadia Z and Zhao H has some concerns whereas Balykov, Finberg, Lou Y, Ruzhenstova TA and Shinkai S have high risk of bias
y. Not downgraded for inconsistency as we did not identify any important heterogeneity(I²=46%)
The World Health Organization (WHO) on March 11, 2020, declared COVID-19 a worldwide pandemic because of its widespread prevalence and life-altering consequences (1). The disease is caused by SARS-CoV-2 Virus that largely depends on angiotensin-converting enzyme 2 (ACE-2) receptor binding, RNA-dependent RNA polymerase (RdRp), as well as other host and viral proteins important for successful transmission and replication (2). Several interventions have emerged in the past two years for treating COVID-19, along with numerous existing antivirals currently being investigated in terms of their suitability as potential treatment options, a simple oral antiviral drug for COVID-19 has not yet been developed. (3–5)
Favipiravir (FVP) is one of the many antivirals that are currently being investigated for their efficacy and safety in Covid-19. It is a broad-spectrum antiviral discovered in Japan and was initially used in the management of influenza, Ebola, and other viral diseases. FVP is a nucleoside analog that can be triphosphorylated in cells to become active and serves as a substrate of virus RNA-dependent RNA polymerase (RdRp), leading to an increased mutation frequency, and possibly lethal mutagenesis (6). An excellent bioavailability of ~94%, 54% protein binding, and short half-life with rapid elimination makes favipiravir a promising candidate in the treatment of covid 19. (7)
There is conflicting evidence on the efficacy and safety of favipiravir in Covid 19. FVP was shown to inhibit SARS CoV-2 in vitro (5,8), however, the clinical benefits in Covid-19 patients are relatively low and not established (9,10).With the availability of more studies and the inconsistencies in the data and it is imperative to conduct an updated systematic review to evaluate the clinical benefits of FVP as a treatment for Covid-19
This guideline recommendation was made according to the evidence synthesized by the authors for the systematic review conducted according to the Cochrane protocol available in https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD015219/full
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language published up to September 2022. We also reviewed reference lists of systematic reviews and included studies. We contacted researchers to identify unpublished and ongoing studies.
We included randomized controlled trials (RCTs) testing Favipiravir treatment of any dose or duration in people with acute COVID‐19, whether suspected or confirmed. Trials were included if the intervention arm had Favipiravir with or without another experimental drug, and if the comparator arm did not include Favipiravir (this could involve use of placebo, standard care, or other potentially active drugs). We excluded trials that did not report any outcomes that could provide usable data for the review, those which were quasi-randomized and those lacking a comparator arm.
We extracted data for the following outcomes, pre-defined by the Expert Working Group:
- Critical (primary for this review):
- Progression to:
- Oxygen therapy
- Ventilation: non-invasive or invasive
- Critical or Intensive care (any reason)
- Duration of hospitalization
- Progression to:
- Important (secondary):
- Mortality (all-cause) – at 28-30 days, or in-hospital
- Time for clinical improvement
- Time to negative PCR for SARS-CoV-2
- Complications of COVID-19:
- Thrombotic events
- Pulmonary function/fibrosis
- Long covid/post-acute sequelae
- Secondary infections
- Adverse events:
- All and serious, hyperuricemia
Two reviewers independently assessed the eligibility of search results using the online Rayyan tool. Data extraction was performed by one reviewer, and checked by another, using a piloted data extraction tool on Microsoft Word. Risk of bias (RoB) was assessed using the Cochrane RoB v2.0 tool, with review by a second reviewer and compared with covid-nma database. If there was a difference in more than one domain, it was assessed by a third independent author. We planned to use risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). We planned to meta-analyze if appropriate, i.e., if outcomes were measured and reported in a similar way for similar populations in each trial, using random-effects models with the assumption of substantial clinical heterogeneity (the appropriateness of which would be checked by the Methodology Committee), and the I2 test to calculate residual statistical heterogeneity, using Review Manager (RevMan) v5.4. The sensitivity analysis was done by comparing after splitting into 2 categories -SOC + active comparator (another antiviral) vs together. We performed sensitivity analysis for outcomes with high I2 values by incorporating only trials with Low RoB. We used GRADE methodology to make a summary of findings tables on GradeProGDT.
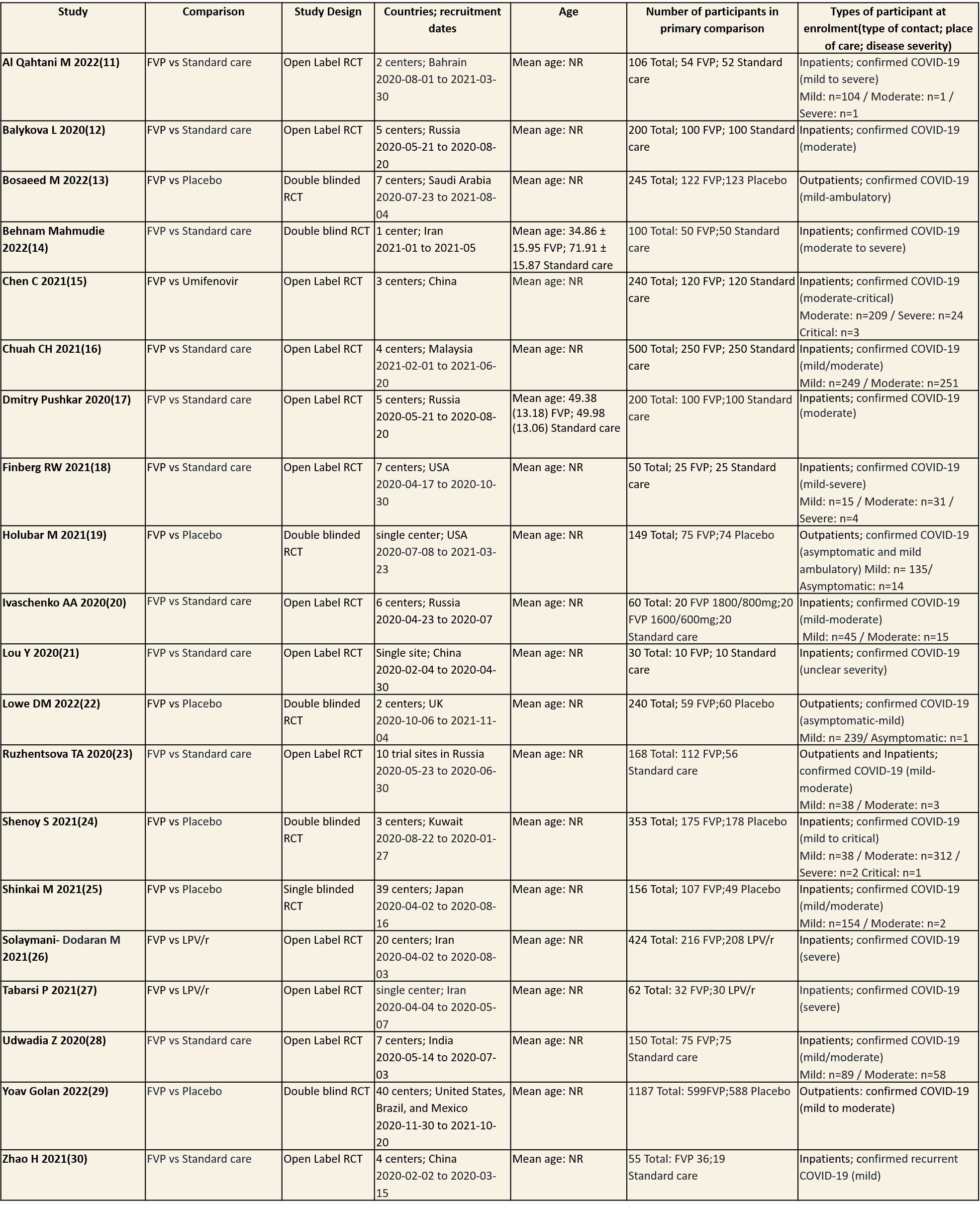
We found 20 RCTs that matched the PICO question as pre-defined by the expert working group. The trials included a total of 4675 participants, all of whom were adults. Three trials were conducted in China, early in the pandemic; all completed recruitment in April 2020(15,21,30). The other trials recruited from April 2020 to November 2021: in Iran (13,26,27); Russia (12,17,20,23); India(28); Bahrain (11); Saudi Arabia(14); Malaysia (16); the UK (22); the USA (18,19); Kuwait (24); Japan(25);one trial recruited from the United states of America, Brazil and Mexico(29) . all trials varied in disease severity ranging from mild to critical. 15 trials recruited hospitalized patients (11-13,15-18,20,21,24-30), 1 trial recruited both outpatients and inpatients(23) whilst the other four trials were focused on ambulatory care and only included outpatients (14,19,22,29).
Severity of COVID‐19 disease at enrolment was reported as asymptomatic, mild, moderate, severe or critical; this was inferred using classification as described by the authors in accordance with the WHO guidance. Of the 4675 participants (20 trials), 15 (0.3%) were asymptomatic, 1412 (30.2%) had mild disease, 1410 (30.1%) moderate disease, 517 (11.1%) severe disease and 4(0.08) had critical disease. Severity was either unclear or not reported in 1317(28.1%) of participants.
Each trial and its results are described below; characteristics of the trials are summarised in the Summary of characteristics of included trials table. All the trials have varying risk of bias across all domains. Risk of bias for each domain per trial is displayed alongside the forest plots below.
The following comparisons were investigated in the trials (we compared outcomes for arms randomized to Favipiravir vs. standard of care or active comparators).
1. Favipiravir (FVP) vs Standard of care without Favipiravir/Placebo
Seventeen trials were included in this comparison (11-13,16-25,28-30). Twelve trials compared FVP to standard of care (11-13,16-21,23,25,28,30) whereas five trials, (14,19,22,24,29), compared FVP to placebo.
2. Favipiravir vs Umifenovir
One Trial was included in this comparison (15).
3. FVP vs Lopinavir/ritonavir
Two trials were included in this comparison, in which participants were randomized 1:1 ratio to receive FVP or LPV/r (26,27)
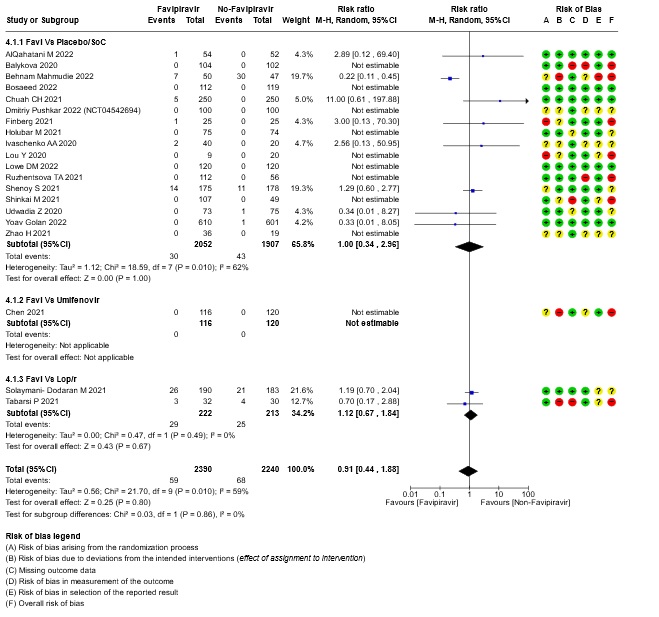
Since Favipiravir was compared with Standard of care or placebo or an active comparator we did a sensitivity analysis to assess the robustness of the pooled estimate by stratifying the comparators separately from standard of care or placebo. We did this for critical outcomes of mortality, time to clinical improvement, time to negative PCR and duration of hospitalization. We concluded that there was no difference between the pooled estimate and the analysis done separately for Favipiravir vs SOC/placebo and Favipiravir vs Active comparator and hence decided to use the pooled estimate for representing the meta-analysis and summary of findings tables.
Our expert working group classified progression to oxygen, non-invasive ventilation (NIV) and invasive mechanical ventilation (IMV) as critical outcomes and mortality, time to clinical improvement and thrombotic or secondary infections as important outcomes. However, as the situation in the country evolved, the guidelines group upgraded mortality, progression to respiratory failure, oxygen requirement and critical and intensive care admission as critical outcomes and others as important outcomes.
Critical (primary) Outcomes
As presented in the ‘Summary of findings’ table, the evidence is of very low certainty for the effect of Favipiravir on mortality, progression to non-invasive ventilation, progression to invasive mechanical ventilation, time to clinical improvement, need for hospital admission, time to negative PCR and adverse events-all and serious. The outcomes of duration of hospitalization were regarded as moderate certainty evidence and adverse event of hyperuricemia as high certainty evidence.
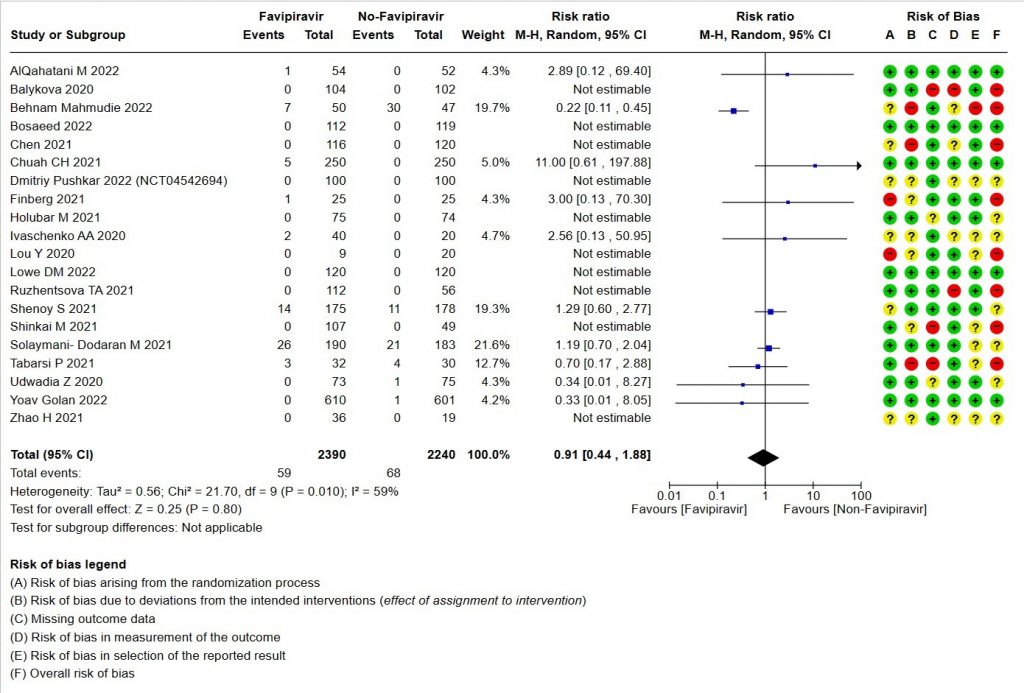
- All-cause mortality: Very low certainty of evidence in 4630 patients in twenty RCTs(11-30) found the evidence is very uncertain about the effect of Favipiravir on all-cause mortality vs. standard of care or active comparator (RR 0.91; 95% CI 0.44 to 1.88). Sensitivity analysis performed with the sole inclusion of low risk of bias studies did not demonstrate an appreciable change in the statistical significance of the pooled estimate results (RR 2.38, 95% CI [0.31, 18.19]).
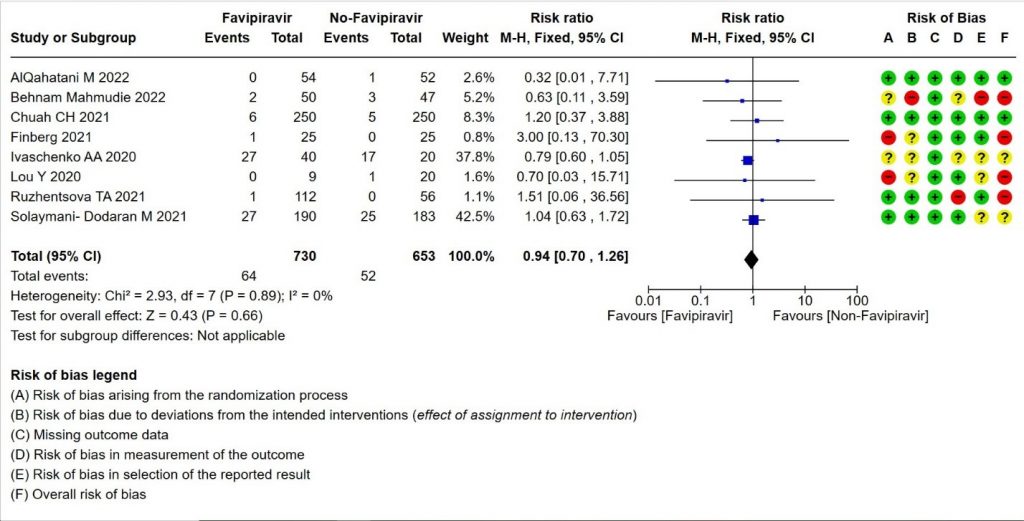
- Progression to Invasive mechanical ventilation:Low certainty evidence in 1383 patients from eight RCTs (11,13,16,18,20,21,23,26) found that the Favipiravir may result in little to no difference in progression to Invasive Mechanical ventilation (RR 0.94; 95% CI 0.70 to 1.26).
- Progression to non-Invasive ventilation:Very low certainty evidence in 250 patients from thirteen RCTs (11-22,29) found that the evidence is very uncertain about the effect of Favipiravir vs. standard of care or active comparator on progression to non-invasive ventilation (RR 4.0; 95% CI 0.48 to 33.33).
- Need for hospital admission: Very low certainty in 393 patients from two RCTs(14,19)found that the evidence is very uncertain for Favipiravir standard of care or active comparator on the need for hospitalization (RR 0.71; 95% CI 0.03 to 19.63).
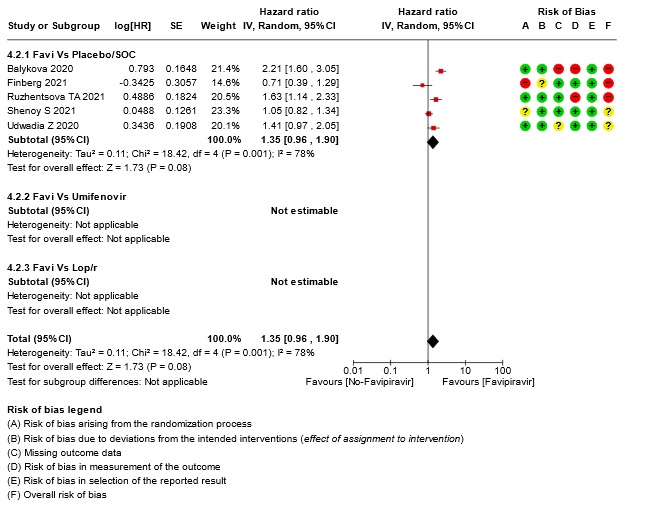
- Time to clinical improvement (>2 point reduction in the WHO ordinal score): Very low certainty in 921 patients from five RCTs (12,18,23,24,28) found that the evidence is very uncertain about the effect of Favipiravir standard of care or active comparator on time to clinical improvement (HR 1.35; 95% CI 0.96 to 1.90). Sensitivity analysis performed with removal of studies with high risk of bias did not change the pooled estimate effect (RR 1.18 [0.89 , 1.56])
- Progression to oxygen therapy: Low certainty evidence in 543 patients from two studies(16,21), found that Favipiravir may result in little to no difference in progression to Oxygen Therapy when compared to standard of care or active comparator (RR 1.20; 95% CI 0.83 to 1.75).
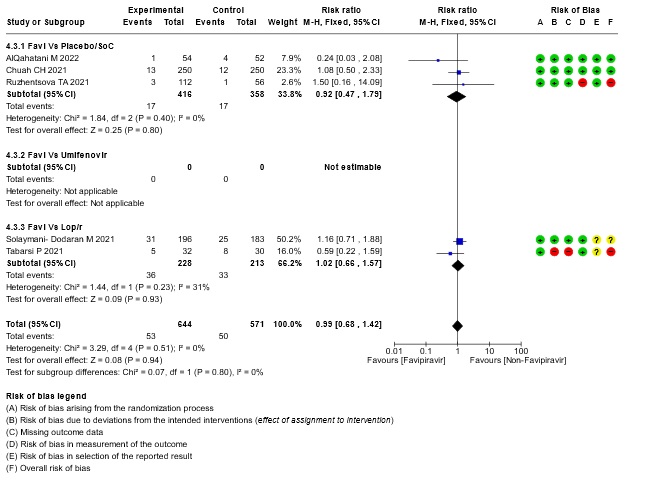
- Need for critical or intensive care for any reason:Very low certainty evidence in 1215 patients from five RCTs (11,16,23,26,27), found that Favipiravir found that the evidence is very uncertain about the effect of Favipiravir on progression to intensive care when compared to standard of care or active comparator (RR 0.99; 95% CI 0.68 to 1.42).
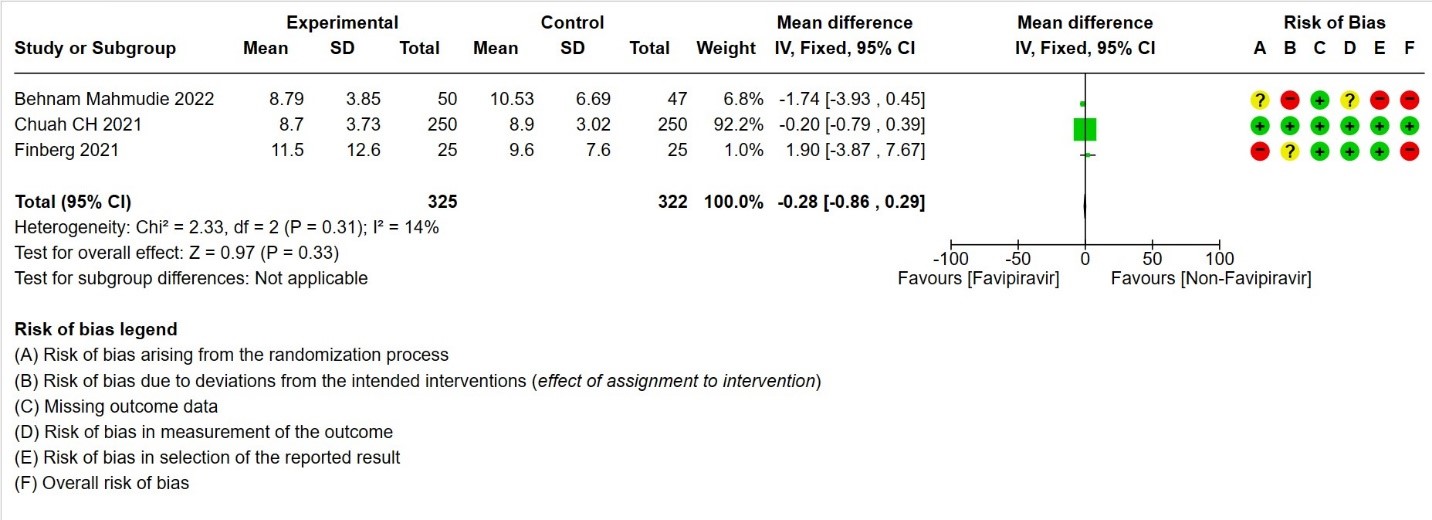
- Duration of hospitalization: Moderate certainty of evidence in 647 patients from three RCTs (13,16,18) reported that Favipiravir probably results in little to no difference in duration of hospitalization. Pooled duration of hospitalization was similar in both groups i.e. it did not differ between participants who received FVP and those who did not (mean difference (MD) 0.28 days shorter with FVP, 95% CI 0.86 lower to 0.29 higher.
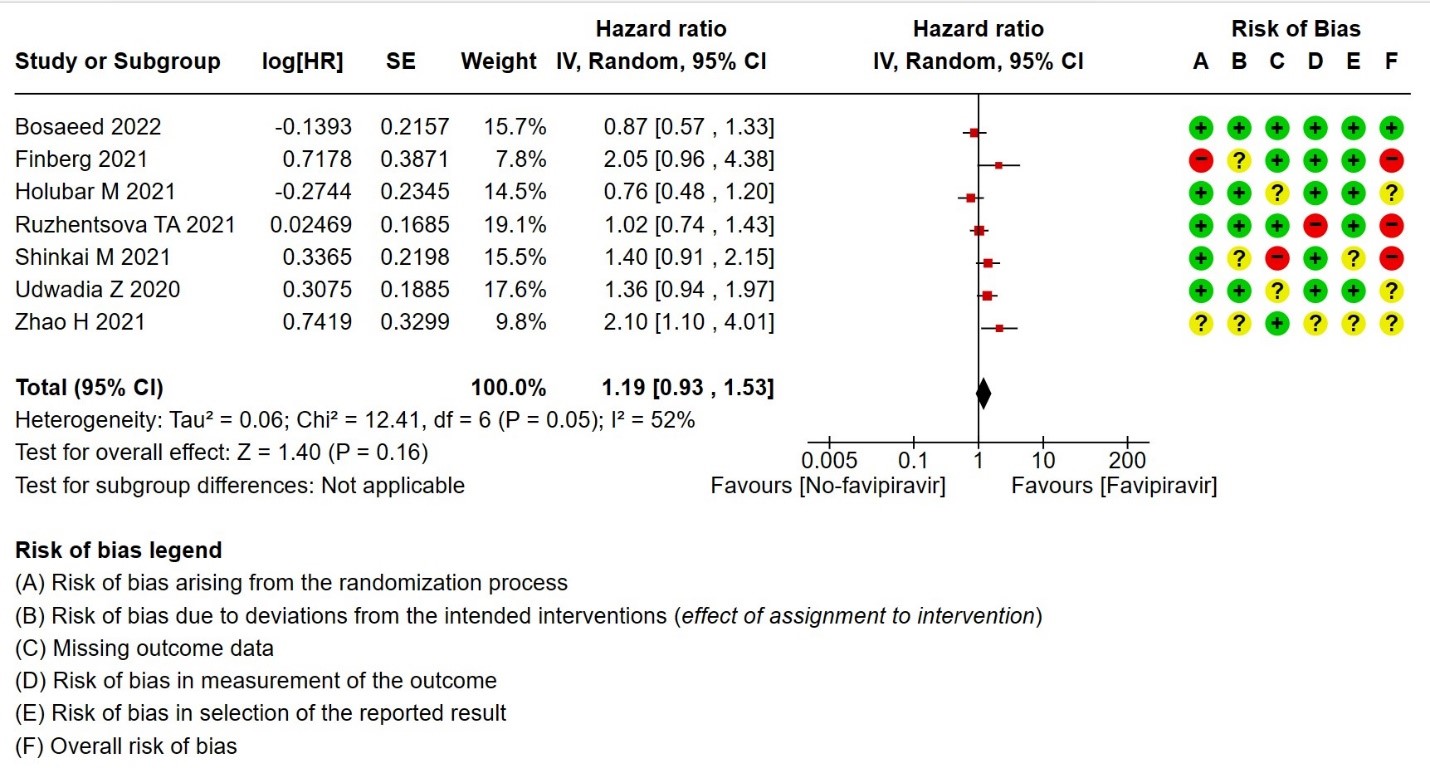
- Time to negative PCR: Very low certainty of evidence from seven RCTs(14,18,19,23,25,28,30) found that the evidence is very uncertain for effect of Favipiravir on time to negative PCR when compared to standard of care or active comparator (HR 1.19 95% CI 0.93 to 1.53). Sensitivity analysis performed after removing studies with high risk of bias (18,23,25) had minimal effect on the pooled hazard ratio (HR: 1.13 [0.76 , 1.68] 4 RCTs).
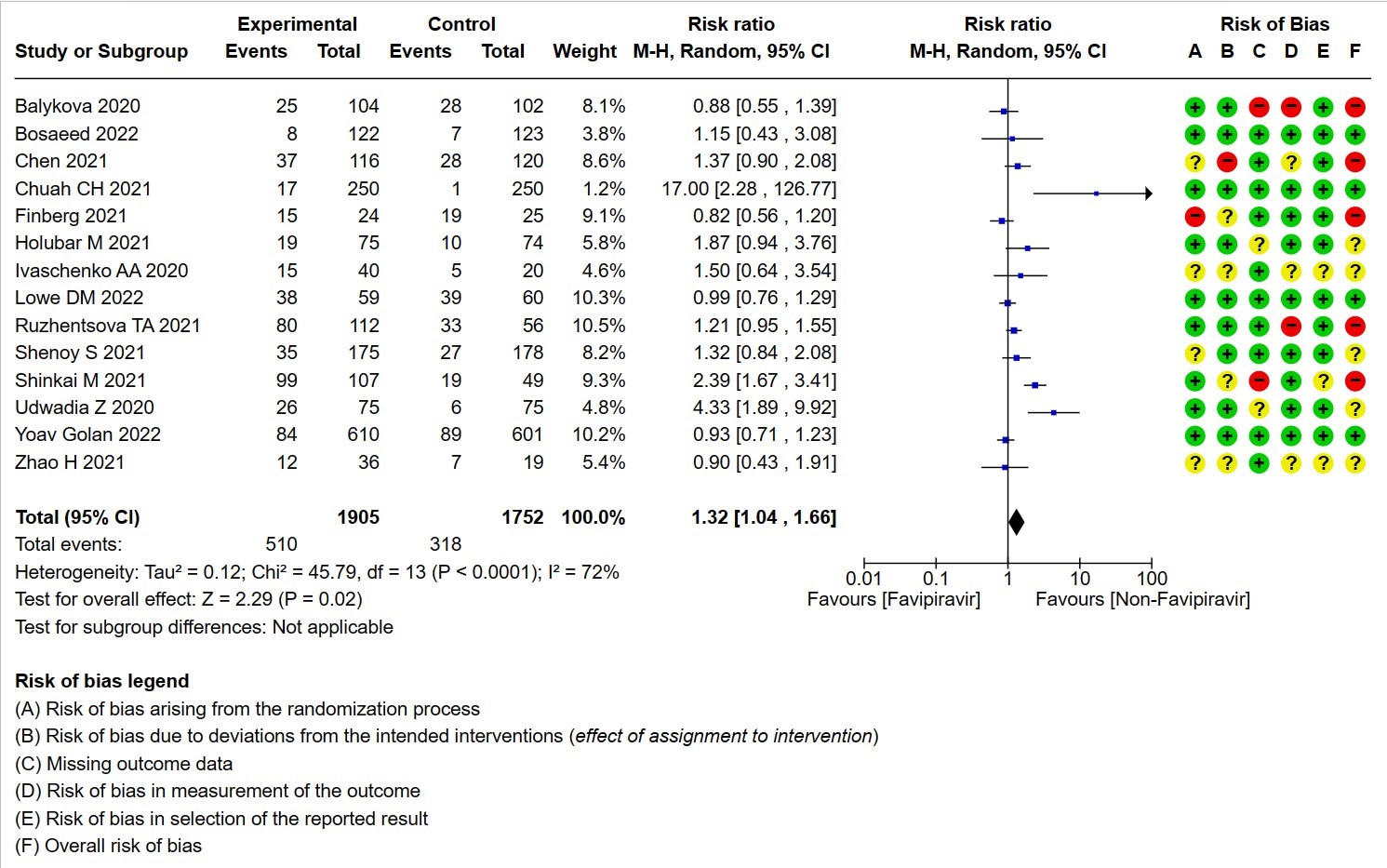
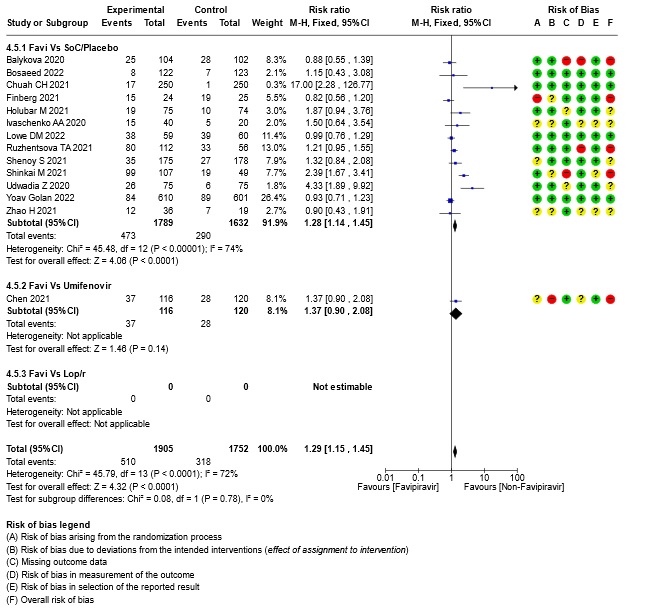
- All Adverse events : Very low certainty of evidence in 3657 patients from fourteen RCTs(12,14-16,18-20,22-25,28,20) found that the evidence is very uncertain on effect of favipiravir on adverse events when compared to standard of care or active comparator (RR 1.29; 95%CI 1.15 to 1.45). sensitivity analysis which incorporated removal of studies with high risk of bias (12,15,18,23,25) demonstrated pooled effect estimate that was not statistically significant (RR 1.40 [1.00 , 1.97]). Sensitivity analysis incorporating a random effects model also did not change the pooled estimate effect significantly (RR 1.32 [1.04 to 1.66] 14RCTs).
- Serious Adverse events (attributable to the drug): Very low certainty of evidence in 2773 patients from twelve RCTs(12,14,15,19,21,22-25,28,30) found that Favipiravir may result in little to no difference in serious adverse events attributable to the drug when compared to standard of care or active comparator (RR 1.13; 95%CI 0.76 to 1.69).
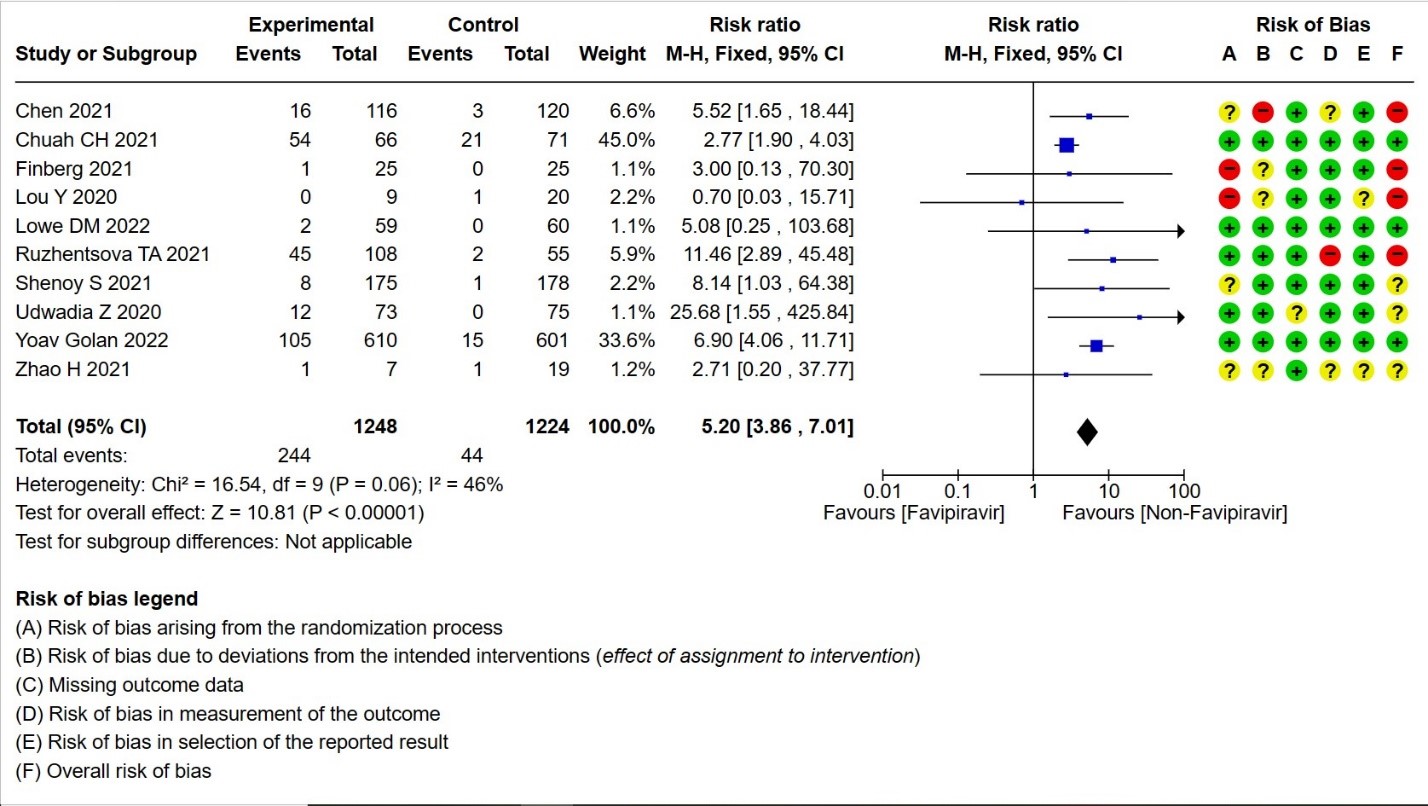
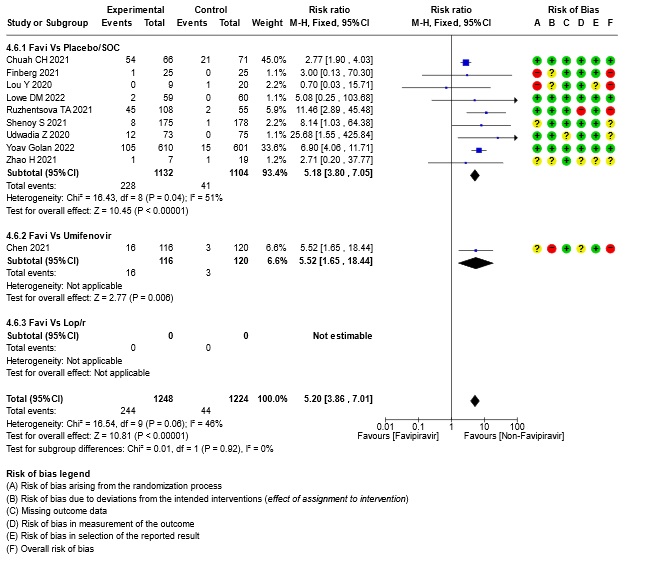
- Adverse Events – Hyperuricemia: High certainty of evidence in 2472 patients from twelve RCTs (15,16,18,21-24,28-30) found that Favipiravir results in an increase in hyperuricemia (RR 5.20; 95%CI 3.86 to 7.01).
1. All‐cause mortality ‐ at 28 to 30 days, or in hospital.
2. Progression to Invasive Mechanical Ventilation
3. Progression to non‐invasive ventilation
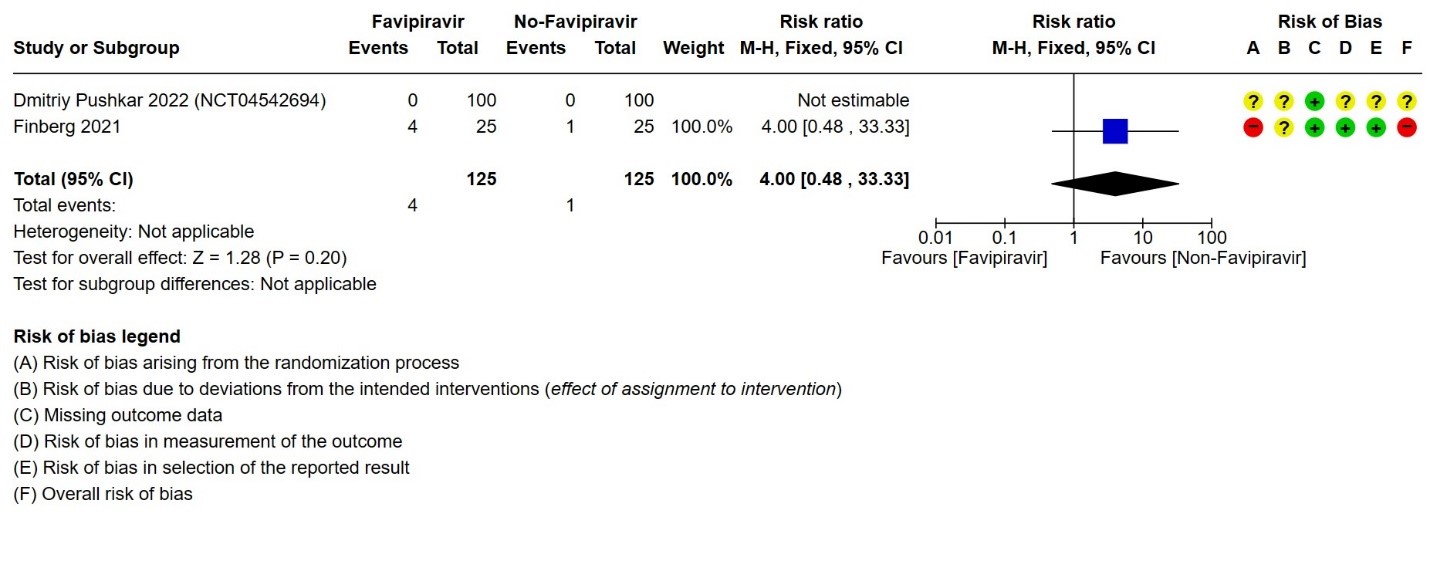
4. Need for admission to hospital (if ambulatory)
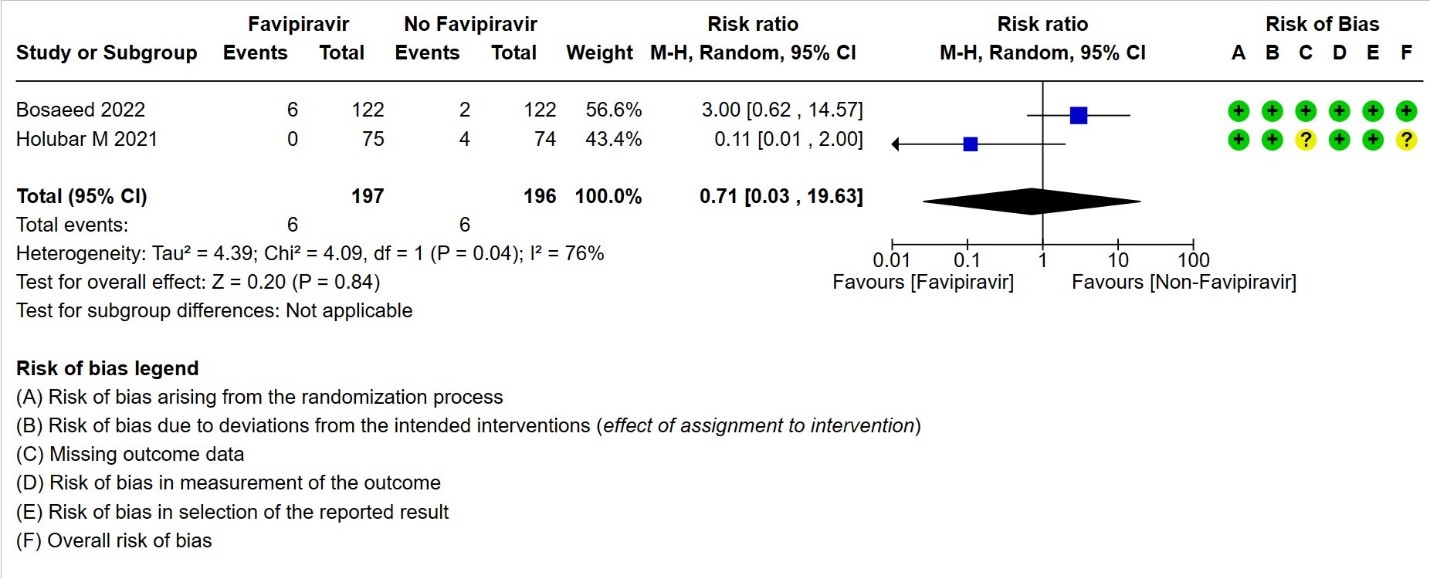
5. Time to clinical improvement (defined as time to a two‐point reduction in patients’ admission status on WHO’s ordinal scale).
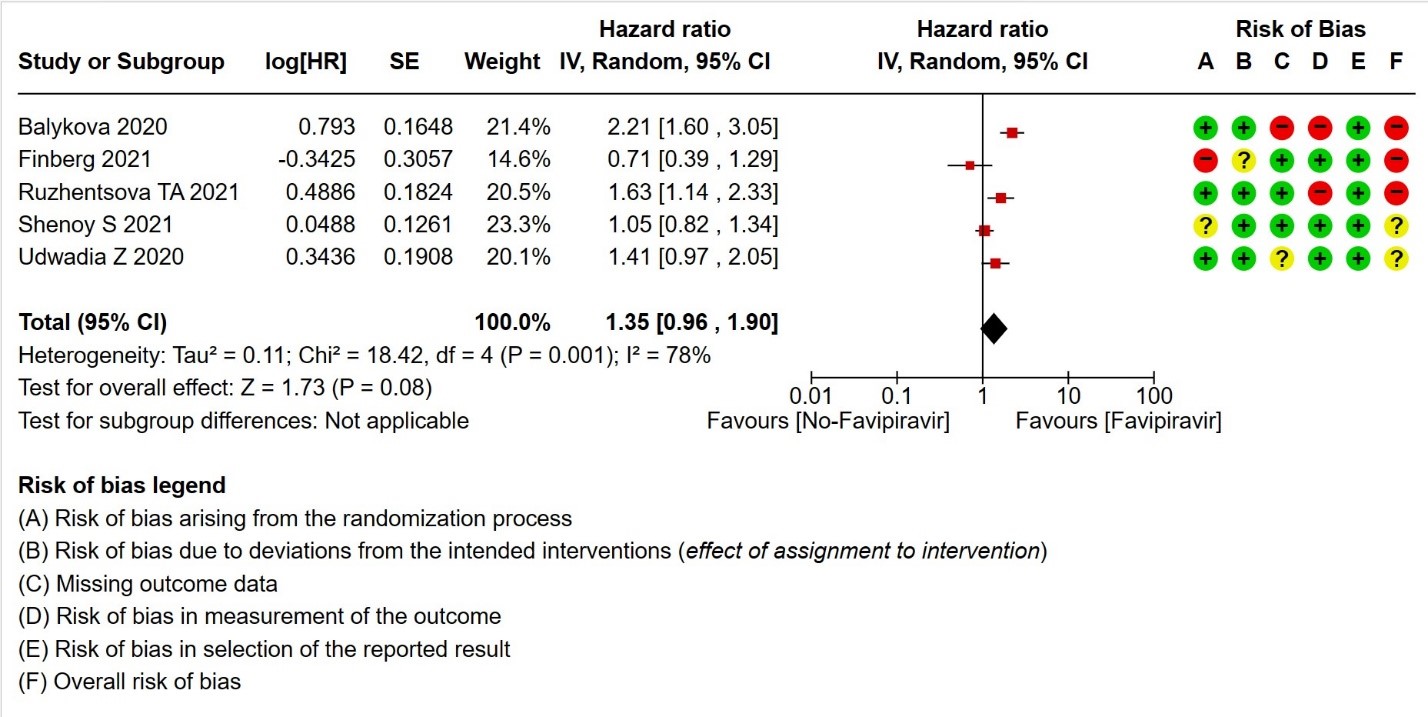
6. Progression to Oxygen Therapy
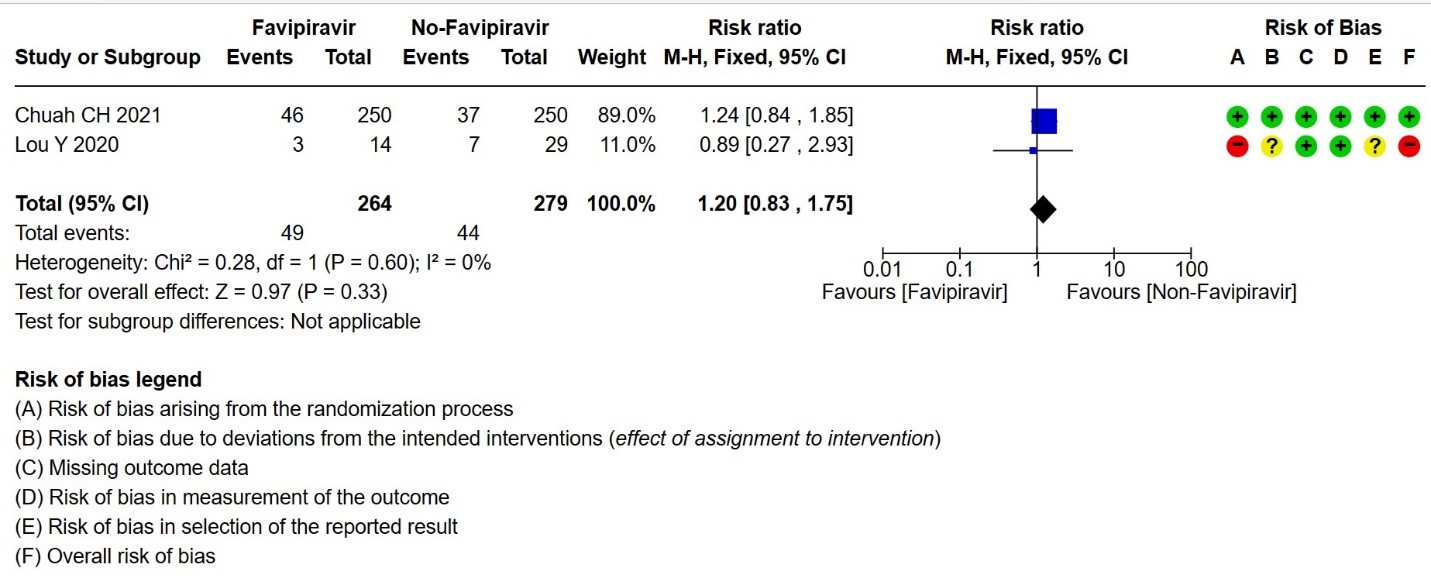
7. Need for critical or intensive care (any reason)
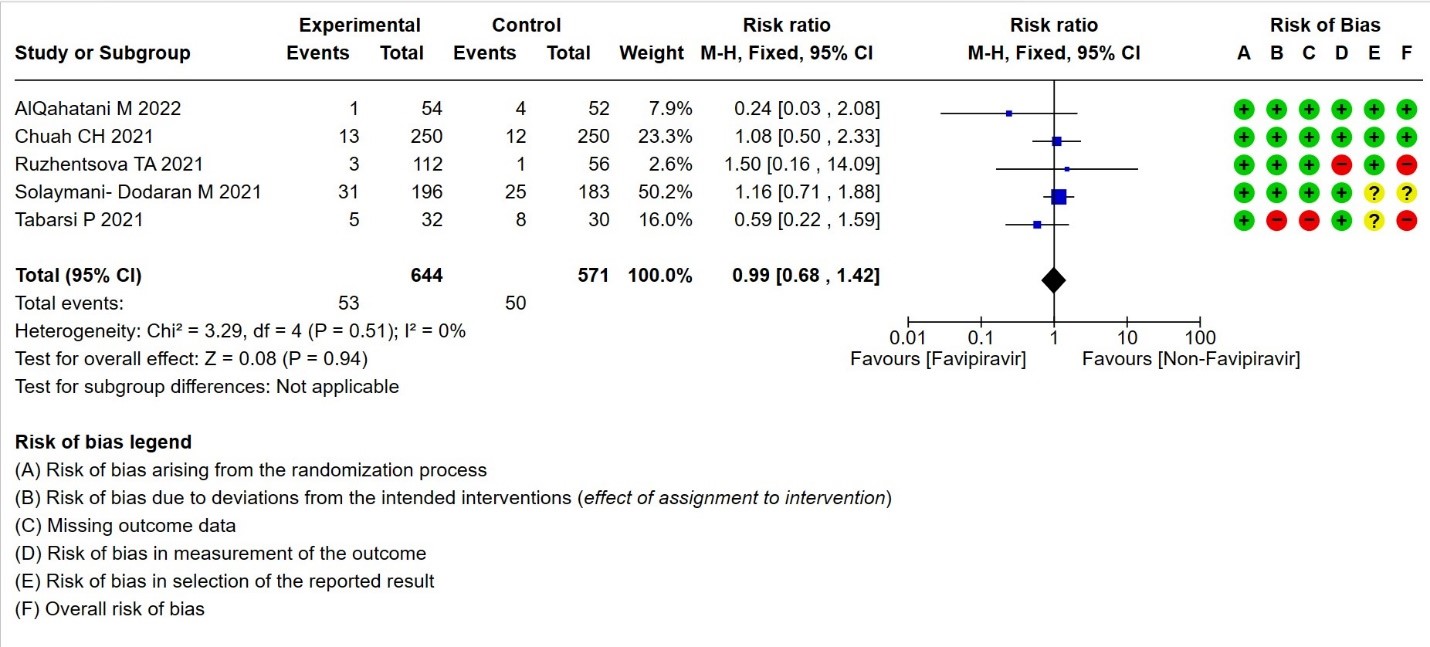
8. Duration of hospitalization

9. Time to negative PCR for SARS‐CoV‐2
10. All adverse events
11. Serious adverse events attributable to the drug
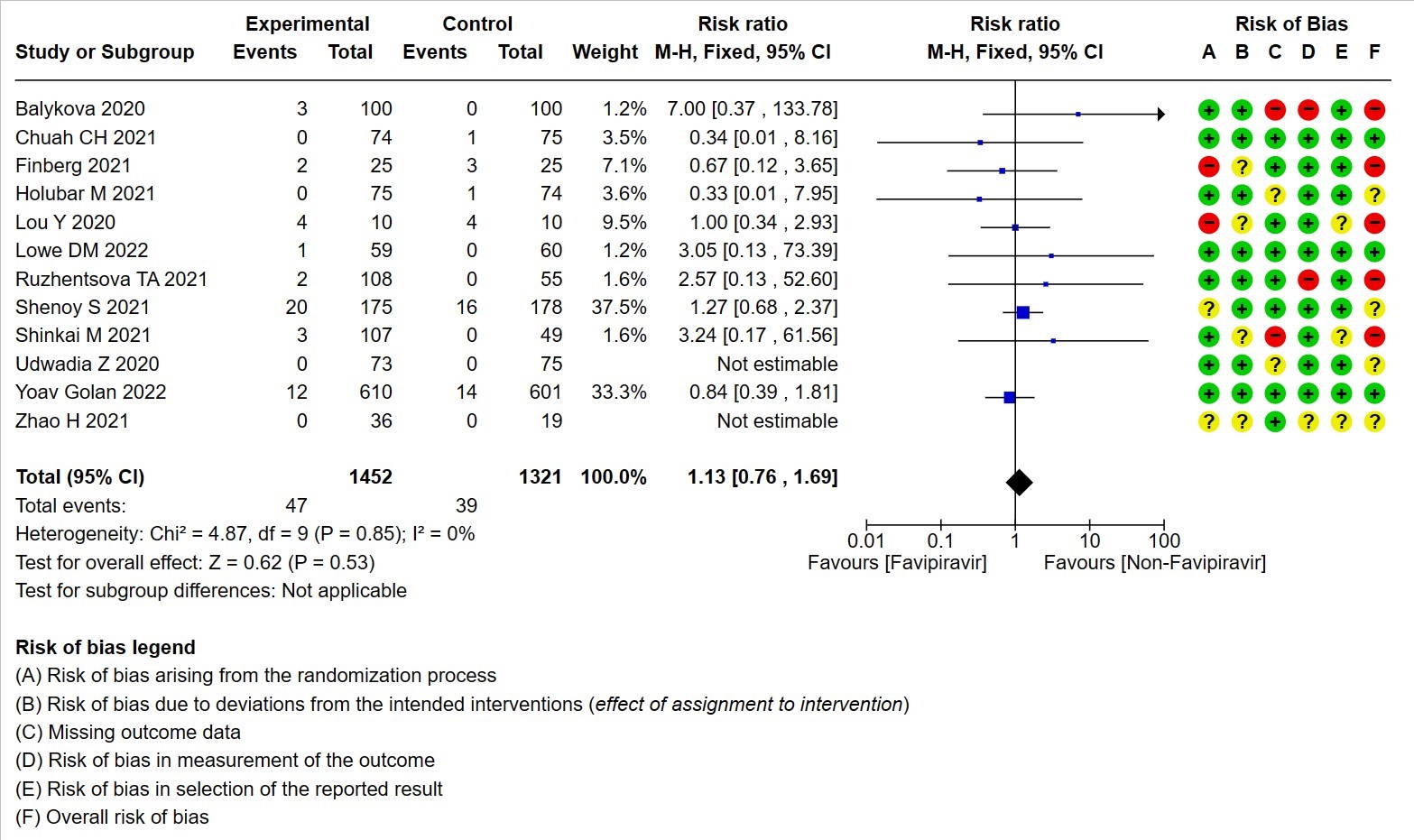
12. Hyperuricemia
Sensitivity analysis (SOC + active comparator (another antiviral) separately vs together)
13. Subgroup analysis: - All-cause Mortality
14. Subgroup analysis: - Need for Critical Care
15. Subgroup analysis :- Time to negative PCR
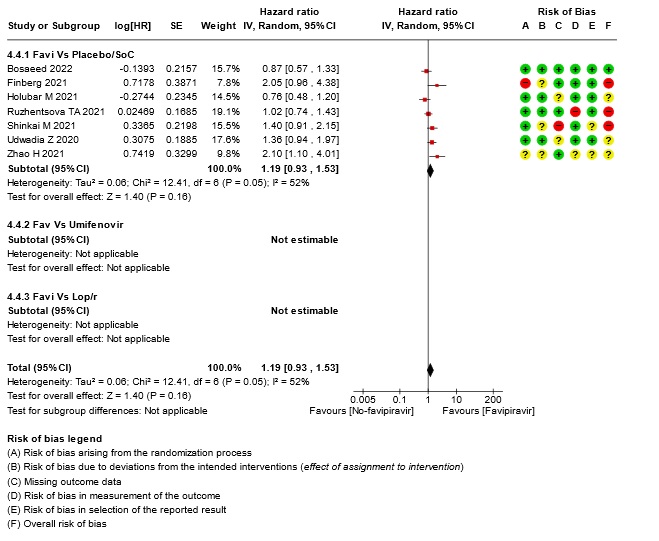
16. Subgroup analysis: - Time to Clinical Improvement
17. Subgroup analysis: - All-Adverse Events
18. Subgroup analysis: - Hyperuricemia
The Antiviral Expert Working Group met on 13th June 2021 to consider Favipiravir as a treatment for COVID-19. Conflict of interest declarations were reviewed by the Steering Committee; none were found to be relevant to Favipiravir.
A summary and then more detailed explanations of the Expert Working Group's judgements follow.
Problem
The COVID-19 pandemic in India with more than 44 million cases and over 0.53 million deaths has significantly impacted and stressed the health structure of the country. During the inception of the pandemic, with a shortage of intensive care unit beds, oxygen and trained personnel the country was facing a major health crisis. This had prompted many irrational and experimental treatment interventions like Favipiravir in all severity of patients across the country in patients hospitalized in COVID-19 without clear indication or evidence in which subgroup of population or disease severity the drug is effective for. Currently the drug is not used popularly for the treatment of covid-19. The group judged the problem to be of utmost priority.
Desirable effects
The panel agreed that the evidence suggests that Favipiravir doesn’t have a clinically significant effect on mortality, time to negative PCR, reducing progression of disease, or preventing critical or intensive care admissions. There is no significant effect in reducing the duration of hospitalization, time to clinical improvement or time to viral clearance.
Undesirable effects
The pooled data did not suggest any increase in serious or other adverse events, or adverse events leading to discontinuation when Favipiravir was added to usual care or with active comparators. However, the panel felt that hyperuricemia is a well-known adverse effect of favipiravir which is self-limiting in most cases and caution should be advised for use in renal failure and gout.
Certainty of evidence
Using GRADE methodology, the evidence synthesis team rated the certainty of evidence as very low for all-cause mortality, need for hospitalization, time to clinical improvement, need for critical or intensive care, progression to non-invasive ventilation, time to negative PCR and all adverse events. Certainty was low for progression to invasive mechanical ventilation, progression to oxygen therapy and serious adverse events attributable to the drug. The certainty of evidence was high for hyperuricemaia and moderate for duration of hospitalization. The expert working group agreed with these judgements and rated the overall certainty as very low.
Values
The EWG felt that all the outcomes including those of mortality, progression to respiratory failure, oxygen therapy or mechanical ventilation, critical or intensive care for any reason, duration of hospitalization and outcomes related to adverse events were expressed variably in the studies. However, there is probably no important uncertainty or variability on how people would value the main outcomes.
Balance of effects
The expert working group felt that the balance of effects does not favor either the intervention or the comparison. The panel felt that there is no convincing evidence to prove the benefit of this intervention, since there were different comparators and varying severity of disease across all studies. The intervention was also found to be not efficacious as per the sensitivity analysis where results were similar when we compared after splitting into 2 categories -SOC + active comparator (another antiviral) vs together.
Resources required.
The group felt that the costs were small. The erratic supply chain had led to an increased demand for this drug during the initial phase of the second wave of the pandemic despite its uncertain clinical benefits until the drug was removed from the ministry of health guidelines for COVID-19 on June 7th, 2021. The group includes clinicians in different types of Indian hospitals who have a good idea of drug and hospitalization costs.
Certainty of evidence of required resources.
No studies reporting this were reviewed by the group but the clinicians in the group were aware of the cost and hence felt that there was high certainty of evidence for required resources to implement this intervention.
Cost effectiveness
The panel discussed that even though there was no research evidence that evaluated cost of Favipiravir in an Indian context, it favors the comparison, as this intervention was not of clinical benefit and conferred a cost for implementation and would always be in addition to the standard care.
Equity
At this point in time this intervention would increase equity if found efficacious as it would prevent admission into hospital. However as of now due to the lack of efficacy unnecessary addition of this drug would incur an additional cost and hence reduce equity.
Acceptability
The group felt that this intervention is likely to have wide acceptance by all the relevant stakeholders (policymakers, patients, and clinicians) if efficacious as it is an oral drug that is probably quite safe, but taking evidence and cost into account a well-informed clinician would be unlikely to use it.
Feasibility
This is a feasible intervention if found efficacious as it is easy to deliver and available easily over the counter in the country.
Currently the evidence to support using Favipiravir for the treatment of COVID-19 in any patient group is lacking, as the certainty of the evidence to date is very low. Regarding adverse effects, there is evidence to suggest that there is definite hyperuricemia when given as a treatment for COVID-19 and the benefits (or the lack thereof) right now do not outweigh the risk. It is contraindicated in pregnancy based on data from animal studies showing teratogenicity thus caution should be exercised when prescribing for patients of reproductive age. Favipiravir is an oral drug, making it suitable as an outpatient or inpatient treatment. The dose is 1800 mg twice daily on the first day, followed by 800mg twice daily up to day 14. Previously there were only 200mg and 400mg doses available making the pill burden quite high which might affect treatment adherence, however 800mg tablets are now available. A two-week treatment course cost around Rs.3600. If further evidence emerges showing Favipiravir is effective and safe for the treatment of COVID-19, it should be straightforward to implement this treatment into existing protocols.
Our conditional recommendation against the use of Favipiravir applies to all subgroups of patients with COVID-19. The group considered all trials of Favipiravir, and found no subgroups where there was benefit in clinically meaningful endpoints whether evaluated by category of severity or by co-morbidities. There are very limited data available assessing its use in patients with liver or kidney disease.
Although the evidence for discontinuation of the drug because of the undesirable effects reported with widespread use such as hyperuricemia, liver dysfunction and chest pain has so far been low, this needs careful monitoring as the potential for drug-related harm cannot be ruled out.
There is currently no evidence to support the use of Favipiravir in any patient group for COVID-19 treatment. There is a need for conduct of well-structured, adequately powered randomized controlled trials with a low risk of bias to address the following:
- Does use of Favipiravir in different subgroups of disease severity or in different special populations (children, immunosuppressed, co-morbid conditions) keep patients from getting admitted into hospital?
- What dose of Favipiravir is safe and efficacious for the treatment of COVID-19?
- Novel coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK – sixth update. 2019;28.
- Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022 Jan;23(1):3–20.
- Manabe T, Kambayashi D, Akatsu H, Kudo K. Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis. BMC Infectious Diseases. 2021 May 27;21(1):489.
- Pipeline Pharmacological Therapies in Clinical Trial for COVID-19 Pandemic: a Recent Update | SpringerLink [Internet]. [cited 2022 Sep 29]. Available from: https://link.springer.com/article/10.1007/s40495-020-00226-5
- Eroglu E, toprak OVERVIEW OF FAVIPIRAVIR AND REMDESIVIR TREATMENT FOR COVID-19. International Journal of Pharmaceutical Sciences and Research. 2021 Apr 1;12:1950–7.
- Shannon A, Le NTT, Selisko B, Eydoux C, Alvarez K, Guillemot JC, et al. Remdesivir and SARS-CoV-2: Structural requirements at both nsp12 RdRp and nsp14 Exonuclease active-sites. Antiviral Research. 2020 Jun 1;178:104793.
- Favipiravir: A new and emerging antiviral option in COVID-19 - PMC [Internet]. [cited 2022 Sep 29]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7467067/
- Kumari: Pipeline pharmacological therapies in clinical... - Google Scholar [Internet]. [cited 2022 Sep 29]. Available from: https://scholar.google.com/scholar_lookup?hl=en&volume=6&publication_year=2020&pages=228-240&issue=5&author=P+Kumari&author=K+Rawat&author=L.+Saha&title=Pipeline+pharmacological+therapies+in+clinical+trial+for+COVID-19+pandemic%3A+a+recent+update
- Favipiravir for treatment of outpatients with asymptomatic or uncomplicated COVID-19: a double-blind randomized, placebo-controlled, phase 2 trial | medRxiv [Internet]. [cited 2022 Sep 29]. Available from: https://www.medrxiv.org/content/10.1101/2021.11.22.21266690v1
- Viruses | Free Full-Text | Longitudinal Analysis of Neutralizing Potency against SARS-CoV-2 in the Recovered Patients after Treatment with or without Favipiravir [Internet]. [cited 2022 Sep 29]. Available from: https://www.mdpi.com/1999-4915/14/4/670
- AlQahtani M, Kumar N, Aljawder D, Abdulrahman A, Mohamed MW, Alnashaba F, et al. Randomized controlled trial of favipiravir, hydroxychloroquine, and standard care in patients with mild/moderate COVID-19 disease. Sci Rep. 2022 Mar 23;12(1):4925.
- Balykova LA, Pavelkina VF, Shmyreva NV, Pyataev NA, Selezneva NM, Shepeleva OI, et al. EFFICACY AND SAFETY OF SOME ETIOTROPIC THERAPEUTIC SCHEMES FOR TREATING PATIENTS WITH NOVEL CORONAVIRUS INFECTION (COVID-19). Farm farmakol (Pâtigorsk). 2020 Dec 21;8(3):150–9.
- Mahmudie B, Kamali A, Sarmadian H, et al. Evaluation of the effect of favipiravir in patients with COVID-19.J RNA Genomics 2022 Volume 18 Issue Female 21 (44.7) 21 (42) 4 2022;18(4):1-6
- Bosaeed M, Mahmoud E, Alharbi A, Altayib H, Albayat H, Alharbi F, et al. Favipiravir and Hydroxychloroquine Combination Therapy in Patients with Moderate to Severe COVID-19 (FACCT Trial): An Open-Label, Multicenter, Randomized, Controlled Trial. Infect Dis Ther. 2021 Dec 1;10(4):2291–307.
- Chen C, Zhang Y, Huang J, Yin P, Cheng Z, Wu J, et al. Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial [Internet]. medRxiv; 2020 [cited 2022 Oct 19]. p. 2020.03.17.20037432. Available from: https://www.medrxiv.org/content/10.1101/2020.03.17.20037432v4
- Chuah CH, Chow TS, Hor CP, Cheng JT, Ker HB, Lee HG, Lee KS, Nordin N, Ng TK, Zaid M, Zaidan NZ, Abdul Wahab S, Adnan NA, Nordin N, Tee TY, Ong SM, Chidambaram SK, Mustafa M; Malaysian Favipiravir Study Group. Efficacy of Early Treatment With Favipiravir on Disease Progression Among High-Risk Patients With Coronavirus Disease 2019 (COVID-19): A Randomized, Open-Label Clinical Trial. Clin Infect Dis. 2022 Aug 24;75(1):e432-e439. doi: 10.1093/cid/ciab962. PMID: 34849615.
- Promomed, LLC. Open-label Randomized Multicenter Comparative Study on the Efficacy and Safety of AREPLIVIR® (Favipiravir) for Parenteral Administration (PROMOMED RUS LLC, Russia) in Hospitalized Patients With COVID-19 [Internet]. clinicaltrials.gov; 2022 Jan [cited 2022 Nov 22]. Report No.: study/NCT05185284. Available from: https://clinicaltrials.gov/ct2/show/study/NCT05185284
- Finberg Rw AMJBAFMJGINCWJPJSBLREC. US201 Study: a Phase 2, Randomized Proof-of-Concept Trial of Favipiravir for the Treatment of COVID-19. Open forum infectious diseases. 2021;8(12):ofab563.
- Holubar M, Subramanian A, Purington N, Hedlin H, Bunning B, Walter KS, et al. Favipiravir for Treatment of Outpatients With Asymptomatic or Uncomplicated Coronavirus Disease 2019 (COVID-19): A Double-Blind, Randomized, Placebo-Controlled, Phase 2 Trial. Clinical Infectious Diseases. 2022 Apr 21;ciac312.
- Ivashchenko AA, Dmitriev KA, Vostokova NV, Azarova VN, Blinow AA, Egorova AN, et al. AVIFAVIR for Treatment of Patients with Moderate COVID-19: Interim Results of a Phase II/III Multicenter Randomized Clinical Trial. Clin Infect Dis Off Publ Infect Dis Soc Am [Internet]. [cited 2021 Jun 14]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7454388/
- Lou Y, Liu L, Yao H, Hu X, Su J, Xu K, et al. Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients: An Exploratory Randomized, Controlled Trial. Eur J Pharm Sci. 2021 Feb 1;157:105631.
- Lowe D M BLAKCKDSYPIFNASDLARAAAACALNDHMFNBJSJFFI. Favipiravir, lopinavir-ritonavir or combination therapy (FLARE): a randomised, double blind, 2x2 factorial placebo-controlled trial of early antiviral therapy in COVID-19. Medrxiv [Internet]. 2022; Available from: https://www.medrxiv.org/content/early/2022/02/15/2022.02.11.22270775
- Ruzhentsova TA, Chukhliaev PV, Khavkina DA, Garbuzov AA, Oseshnyuk RA, Soluyanova TN, et al. Phase 3 Trial of Coronavir (Favipiravir) in patients with mild to moderate COVID-19. :19.
- Shenoy S, Munjal S, Youha SA, Alghounaim M, Almazeedi S, Alshamali Y, et al. Favipiravir In Adults with Moderate to Severe COVID-19: A Phase 3 Multicentre, Randomized, Double-Blinded, Placebo-Controlled Trial [Internet]. medRxiv; 2021 [cited 2022 Oct 19]. p. 2021.11.08.21265884. Available from: https://www.medrxiv.org/content/10.1101/2021.11.08.21265884
- Shinkai M, Tsushima K, Tanaka S, Hagiwara E, Tarumoto N, Kawada I, et al. Efficacy and Safety of Favipiravir in Moderate COVID-19 Pneumonia Patients without Oxygen Therapy: A Randomized, Phase III Clinical Trial. Infect Dis Ther. 2021 Dec 1;10(4):2489–509.
- Solaymani-Dodaran M, Ghanei M, Bagheri M, Qazvini A, Vahedi E, Hassan Saadat S, et al. Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia. Int Immunopharmacol. 2021 Jun;95:107522.
- Tabarsi P, Vahidi H, Saffaei A, Hashemian SMR, Jammati H, Daraei B, et al. Favipiravir Effects on the Control of Clinical Symptoms of Hospitalized COVID-19 Cases: An Experience with Iranian Formulated Dosage Form. Iran J Pharm Res. 2021;20(4):1–8.
- Udwadia ZF, Singh P, Barkate H, Patil S, Rangwala S, Pendse A, et al. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial. International Journal of Infectious Diseases. 2021 Feb 1;103:62–71.
- Golan Y, Campos JAS, Woolson R, Cilla D, Hanabergh R, Gonzales-Rojas Y, Lopez R, Finberg R, Balboni A. Favipiravir in patients with early mild-to-moderate COVID-19: a randomized controlled trial. Clin Infect Dis. 2022 Sep 6:ciac712. doi: 10.1093/cid/ciac712. Epub ahead of print. PMID: 36065065; PMCID: PMC9494366.
- Zhao H, Zhang C, Zhu Q, Chen X, Chen G, Sun W, et al. Favipiravir in the treatment of patients with SARS-CoV-2 RNA recurrent positive after discharge: A multicenter, open-label, randomized trial. International Immunopharmacology. 2021 Aug 1;97:107702.
Covid Management Guidelines India Group - Anti-viral Working Group. Favipiravir. Covid Guidelines India; Published online on July 19, 2021; URL: https://indiacovidguidelines.org/favipiravir-recommendation-page/ (accessed ).
