This recommendation applies to acute COVID-19 in adults. Some of our recommendations vary according to the severity of COVID-19 illness. Definitions of the categories are based on the World Health Organization (WHO) criteria and can be viewed by clicking the plus (+) signs below.
RECOMMENDATION:
The group makes a strong recommendation against the use of convalescent for patients with COVID-19 infection. The recommendation was made across all levels of Covid-19 illness. The group is aware that trials are currently investigating its use after early exposure to infection in people at high risk.
DATE OF RECOMMENDATION: 27th October 2021
Definition of mild:
• Symptomatic (any acute COVID-19 related symptoms)
• AND respiratory rate <24/min
• WITHOUT pneumonia or hypoxia
Definition of moderate illness:
• Pneumonia (clinical or radiological) OR hypoxia (SpO2 <94% in adults with no underlying lung disease)
• AND respiratory rate ≤30/min
• AND SpO2 ≥90% on room air
Definition of Severe illness
Pneumonia with ANY ONE of the following:
• severe respiratory distress or respiratory rate >30/min
• SpO2 <90% on room air
• NO invasive or non-invasive respiratory support needed
Definition of critical:
• Requirement for high-level respiratory support: noninvasive ventilation, high-flow oxygen (≥20 litre per minute) or invasive mechanical ventilation
• OR acute respiratory distress syndrome (PaO2/FiO2 ratio of <300)
• OR sepsis
• OR shock
Convalescent plasma, from individuals who have recovered from infection and have generated an immune response, may provide passive immunity from neutralizing antibodies against SARS-Co-V2, and specialists have suggested any class of antibodies may be useful1. It is postulated that antibodies may bind to the virus, decrease viral entry into the cells and enhance viral clearance via antibody-dependent phagocytosis or antibody-dependent cellular toxicity2. The hypothesis is that convalescent plasma early in the illness could attenuate the severity of illness and prevent complications.
The group assessed the evidence from multiple randomized trials which showed that, convalescent plasma is not associated with improved outcomes for hospitalized COVID 19 patients in terms of 28-day mortality, progression to ventilatory support, need for oxygen support, and time to clinical improvement (see GRADE Table and analysis below).
Convalescent plasma is an expensive intervention and needs considerable medical resources to deliver it. This therapy carries a risk of transfusion related adverse effects, circulatory overload and possible risk of anaphylaxis.
In view of the lack of demonstrable efficacy, combined with the cost and potential to harm, the panel recommends strongly against the use of convalescent plasma.
Date of latest search: 31stJuly 2021
Date of completion & presentation to Expert Working Group: 23rd August 2021
Date of planned review: 23rd January 2022
Evidence synthesis team: Anju Susan Jacob, Amita Jacob, Bhagteshwar Singh, Richard Kirubakaran and Priscilla Rupali.
Explanations
a. Downgraded by 1 level for serious imprecision, due to wide 95% CI.
b. Downgraded by 1 level for serious ROB, due to some concern.
c. Downgraded by 1 level for serious inconsistency, the I2 is 87%.
Explanations
a. Downgrade by one level for risk of bias as the trial stopped early (only recruited 76% of its sample size.
b. Downgrade by on level for indirectness as the study included elderly >65 years of age with co-morbidities and those >75 years of age with or without co-morbidities.
c. Downgrade by 2 levels for very serious imprecision as the numbers were too small, OIS criteria were not met -single small trial. There were 2 deaths in the convalescent plasma group and 4 in the control group.
d. Downgraded by 1 level for serious imprecision as the 95% CI is wide (0.29 to 0.94)
Explanations
a. Downgrade by 2 levels for very serious imprecision as the numbers were too small, OIS criteria were not met -single small trial in 103 patients
b. Downgraded by 1 level for serious indirectness, due to early stopping at April 2020 and no usage of steroids.
c. Downgraded by 1 level for serious ROB, studies with some concerns.
d. Downgraded by 2 level for very serious imprecision as the 95% CI is wide (0.21 to 12.62)
e. Downgraded by 1 level for serious imprecision as the 95% CI is wide (1.20 to 4.34)
Convalescent plasma providing rapidly acting passive immunity as treatment of infections has been in use for more than a hundred years. Early studies in the COVID 19 pandemic provided weak data on clinical efficacy with limited side effects leading to an emergency use authorization by the FDA. Due to the lack of effective therapeutic options early in the pandemic for COVID 19, thousands of patients across the world received convalescent plasma therapy, many in an unregulated setting. Subsequent trials have shown less promising results.
To identify all available systematic reviews pertaining to our PICO question, a systematic search of PubMed, covid-nma.org, Cochrane and the Epistemonikos databases was conducted. The search strategy was designed and validated by the Group’s information specialist.
The most recent Cochrane review had not included several recent studies. All available RCTs therefore, were identified from the above databases and included in this meta-analysis.
For the risk of bias, Cochrane ROB2 tool was used and assessed by at least 2 reviewers with disagreements being settled by consensus.
Data was entered into Review manager version 5.4 for meta-analysis. The results were entered into Grade ProGDT (online), to create the summary of findings (GRADE) table. We used risk ratios (RR) for dichotomous outcomes with 95% confidence intervals (CIs).
Population – Patients in hospital with symptomatic COVID-19
Intervention – Convalescent plasma at any dose or duration
Control – Standard of care/ No convalescent plasma
Outcomes:
Primary:
- All-cause mortality
- Progression to ventilation (invasive or non-invasive) or organ support (dialysis, vasopressors/inotropes, ECMO)
Secondary:
- Time to clinical improvement (WHO ordinal scale or other definition)
- Duration of ventilatory support (invasive or non-invasive)
- Duration of vasopressor support
- Need for oxygen therapy
- Decrease in CRP at 7 days post-CP
- Decrease in IL-6 at 7 days post-CP
Adverse events:
- All
- Serious
- Transfusion-associated events:
- Fever
- Allergic reactions, especially severe
- TRALI
- Circulatory overload
The expert working group was also concerned regarding the impact of high/vs low titer antibody in the efficacy of convalescent plasma as a therapeutic option and also the category of severity that it was deployed in and hence requested a sub group analysis regarding the same. Subgroup analysis conducted were as follows
1. According to the category of severity: Mild. Severe/Critical COVID-19
2. According to high/low titer of antibody as per criteria defined per FDA and IDSA (http://www.idsociety.org)
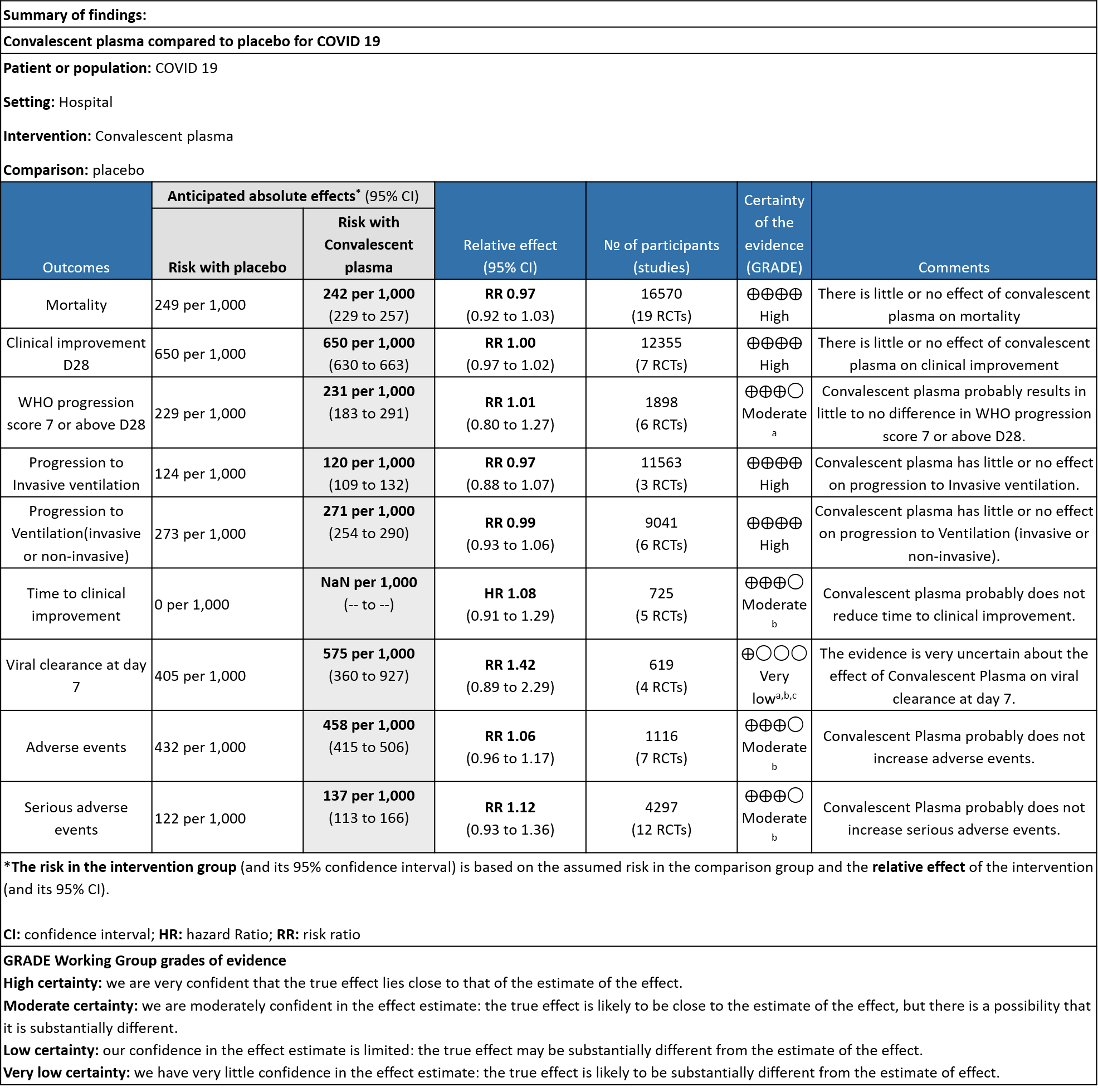
OUTCOMES: CONVALESCENT PLASMA COMPARED TO STANDARD OF CARE IN COVID-19:
The 19 RCTs differed in the categories of severity in their analysis and we evaluated the following sub group
1. Convalescent plasma compared to standard of care in COVID-19 (across all severity categories)
2. Convalescent plasma compared to standard of care in mild COVID-19
3. Convalescent plasma compared to standard of care in severe - critical COVID-19
I. CONVALESCENT PLASMA COMPARED TO STANDARD OF CARE IN COVID-19(across all severity categories):
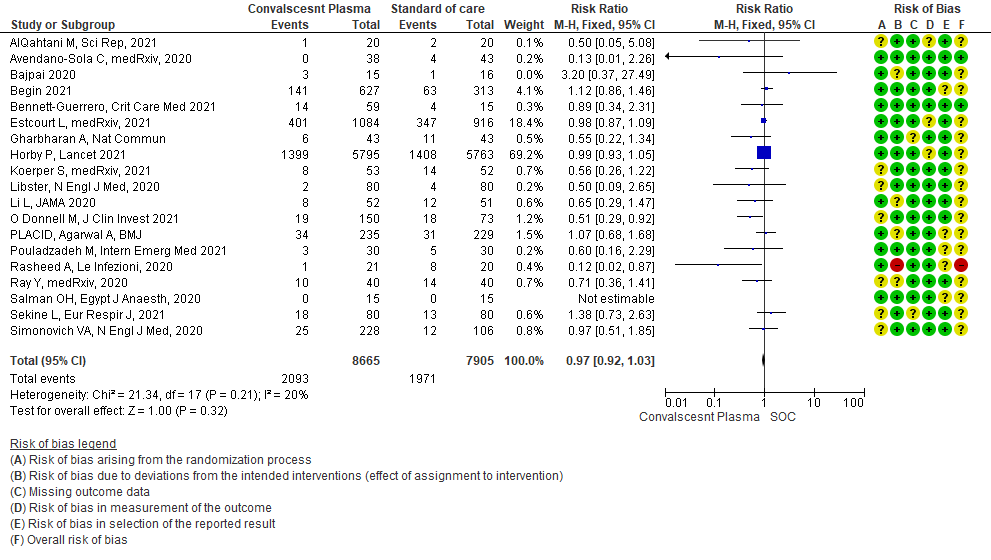
1. All-cause mortality at 28 days:
19 RCTs3-21 that included 16570 patients with COVID-19 disease indicated that there is high certainty evidence that Convalescent plasma does not reduce mortality at 28 days RR 0.97 (95% CI 0.92 to 1.03). Based on the data, we anticipate that for 1000 patients with COVID-19 disease treated with standard of care, 249 are likely to die vs 242 if Convalescent plasma is administered for COVID-19. The number needed to treat (NNT) to reduce an additional mortality is 143 patients with COVID-19.
2. Clinical improvement D28 (Clinical improvement was defined as patient discharge or a reduction of 2 points on a 6-point disease severity scale)
High certainty evidence from 7 RCTs3,7,9,10,13,20,21 that included 12355 patients with COVID-19 disease indicated that Convalescent plasma does not increase clinical improvement at day28, RR 1.00 (95% CI 0.97 to 1.02). Based on the data, we anticipate that for 1000 patients with COVID-19 disease treated with standard of care or convalescent plasma, 650 are likely to have clinical improvement by 28 days. The number needed to treat (NNT) to have an additional clinical improvement is 68 patients with COVID-19.
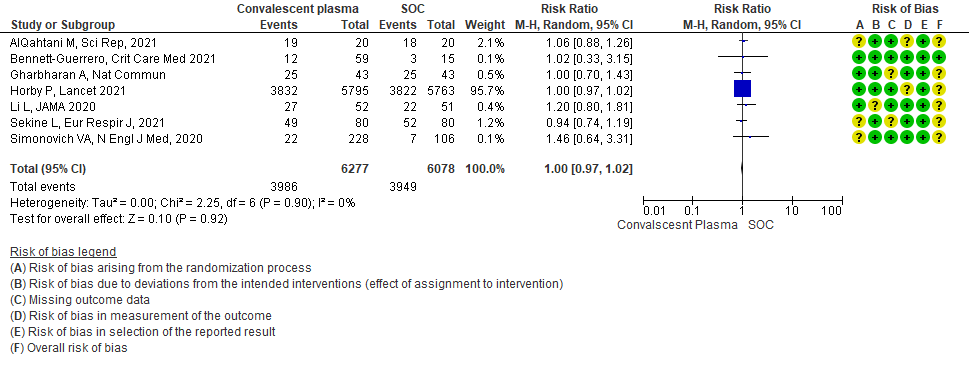
3. WHO progression score 7 or above D28:
Moderate certainty evidence from 6 RCTs4,6,12,14,20,21 that included 1898 patients with COVID-19 disease indicated that Convalescent plasma probably has no effect on WHO progression score 7 [mechanical ventilation +/- additional organ support (ECMO, HD or vasopressors) OR death] or discharge on ≥ 28 days RR 1.01 (95% CI 0.80 - 1.27) compared to standard of care. Based on the data, we anticipate that for 1000 patients with COVID-19 disease treated with standard of care 229 are likely to progress according to WHO progression score 7 or discharged on ≥ 28 days vs 231 administered convalescent plasma.
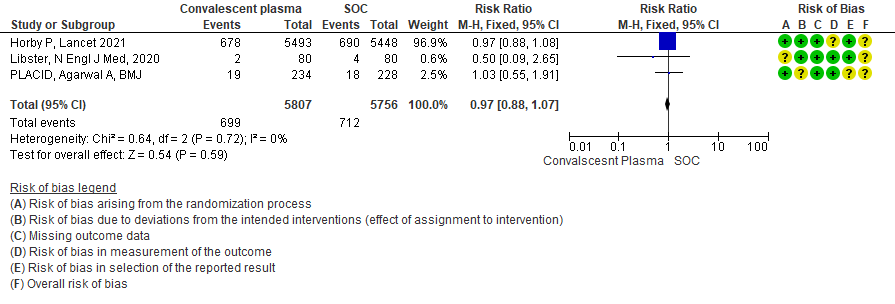
4. Progression to Invasive ventilation:
High certainty evidence from 3 RCTs10,12,15 that included 11563 patients with COVID-19 disease indicates that Convalescent Plasma results in little to no difference in progression to Invasive ventilation compared to standard of care RR 0.97 (95% CI 0.88 - 1.07). Based on the data, we anticipate that for 1000 patients with COVID-19 disease treated with standard of care 124 are likely to progress to invasive ventilation vs 120 per 1000 people if Convalescent plasma is administered for COVID-19.
5. Progression to Ventilation (invasive or non-invasive):
High certainty evidence from 6 RCTs3,4,8,10,12,15 that included 9041 patients with COVID-19 disease indicates that Convalescent Plasma results in little to no difference in progression to ventilation (invasive or non-invasive) compared to standard of care RR 0.99 (95% CI 0.93 - 1.06). Based on the data, we anticipate that for 1000 patients with COVID-19 disease treated with standard of care 273 are likely to progress to ventilation vs 271 i.e., 2 fewer (from 19 fewer to 17 more) per 1000 people if Convalescent plasma is administered for COVID-19.
6. Time to clinical improvement:
Moderate certainty evidence from 5 RCTs4,7,13,14,21 that included 815 patients with COVID-19 disease indicates that Convalescent Plasma probably results in little to no difference in time to clinical improvement, Hazard ratio 1.08 (95% CI 0.91 to 1.29).
7. Viral clearance at day 7
Very low certainty evidence from 4 RCTs13,15,19,20 that included 619 patients with COVID-19 illness indicated that Convalescent Plasma had a very uncertain effect on viral clearance at day 7, RR 1.42 (95% CI 0.79 to 2.29). Based on the data, we anticipate that for 1000 patients with COVID-19 disease treated with standard of care 405 are likely to have viral clearance at day 7 vs 575, if Convalescent plasma is administered for COVID-19. The number needed to treat (NNT) to have an additional patient attaining viral clearance at day 7 is 7.
8. All adverse events:
Moderate certainty evidence from 7 RCTs5, 11,14,20,21that included 1116 patients with COVID-19 illness indicated that Convalescent plasma probably results in little to no difference on all adverse events, RR 1.06 (95% CI 0.96 to 1.17). We anticipate that for 1000 patients with COVID-19 disease treated with standard of care 432 adverse events are likely vs 458 (from 17 fewer to 74 more) in those administered convalescent plasma for COVID-19.
9. Serious adverse events:
Moderate certainty evidence from 12 RCTs4-9, 11,14,20,21 that included 4297 patients with COVID-19 illness indicated that Convalescent Plasma probably results in little to no difference in serious adverse events RR 1.12 (0.93 to 1.36) compared to standard of care. Based on the data, we anticipate that for 1000 patients with COVID-19 treated with standard of care 122 serious adverse events are likely vs 107 (from 9 fewer to 44 more) in those given convalescent plasma.
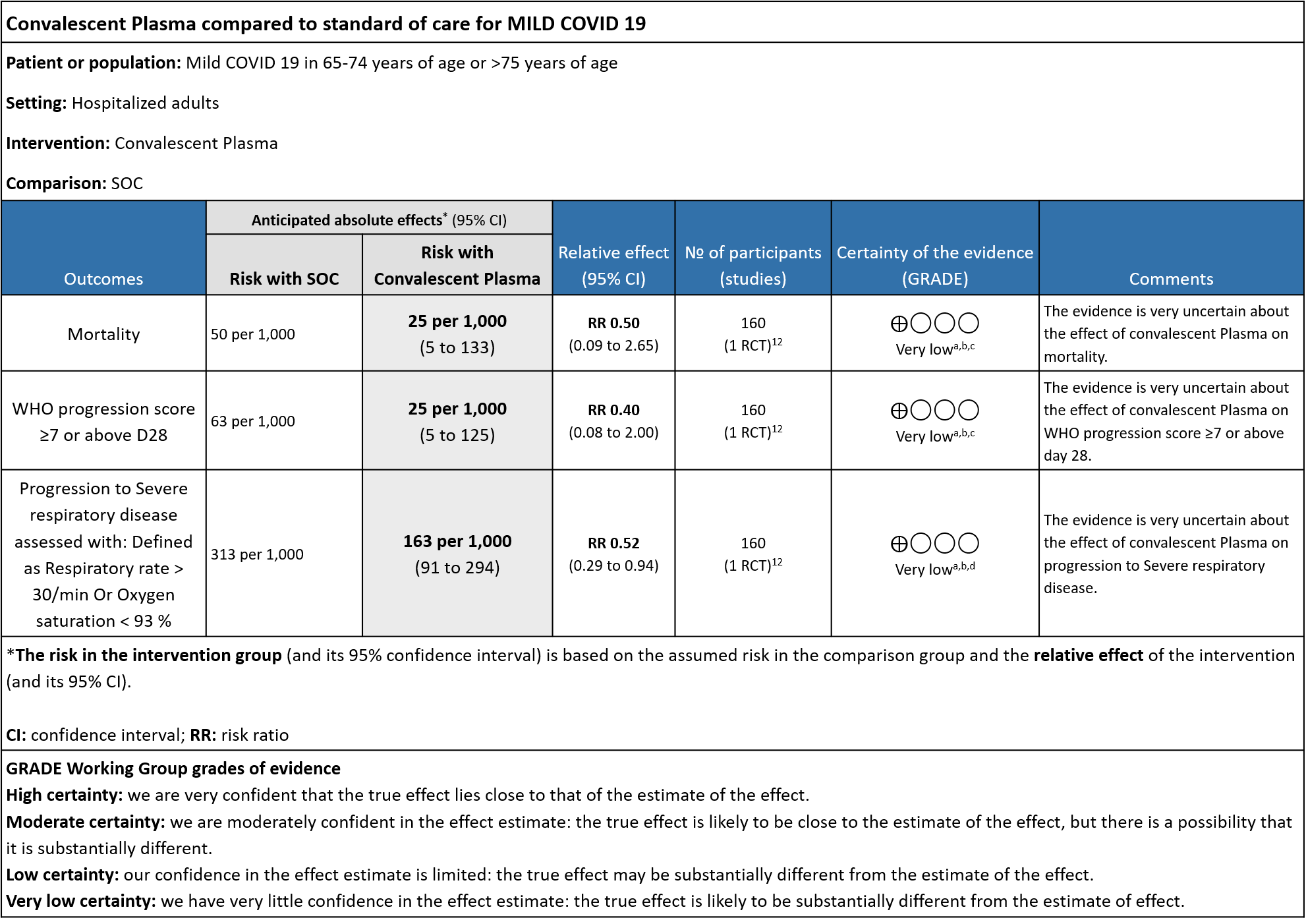
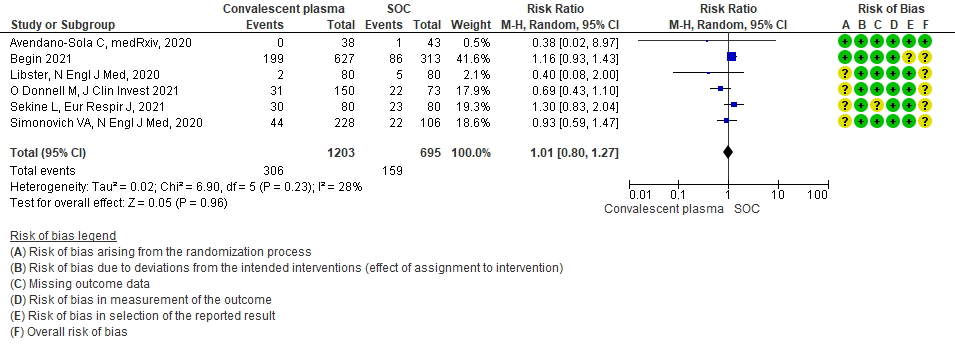
II. CONVALESCENT PLASMA COMPARED TO STANDARD OF CARE IN MILD COVID-19:
The group noted that there were not too many trials measuring outcomes exclusively in early disease and mild category. Most studies seemed to include all categories of severity and we were unable to tease out disaggregated data for mild or severe infections alone. There was only 1 trial in this category but it included adults 65-74 years of age with comorbidities and those > 75 years of age irrespective of comorbidities. This trial was stopped early after only 76% of sample size was attained.
1. All-cause mortality at 28 days:
Very low certainty evidence from 1 RCT12 that included 160 patients with mild COVID-19 disease indicated that Convalescent Plasma may reduce mortality at 28 days RR 0.50 (95% CI 0.09 - 2.65). Based on the data, we anticipate that for 1000 patients with mild COVID-19 disease treated with standard of care, 50 are likely to die vs 25 (from 45 fewer to 83 more) if Convalescent plasma is administered for mild COVID-19. The number needed to treat (NNT) to reduce an additional mortality is 40 patients with mild COVID-19.
2. WHO progression score 7 or above D28:
Very low certainty evidence from 1 RCT12 that included 160 patients with mild COVID-19 disease indicated that Convalescent Plasma may reduce progression to WHO progression score 7 and above [mechanical ventilation +/- additional organ support (ECMO, HD or vasopressors) OR death] at day 28, RR 0.40 (95% CI 0.08 to 2.00) compared to standard of care. Based on the data, we anticipate that for 1000 patients with mild COVID-19 disease treated with standard of care 63 are likely to progress beyond WHO progression score 7 or discharge ≥ 28 days vs 25 (from 58 fewer to 62 more) patients per 1000 people if Convalescent plasma is administered for mild COVID-19. The number needed to treat (NNT) to reduce an additional progression to WHO progression score 7 or discharge above day 28 is 26.
3. Progression to Severe respiratory disease (Defined as Respiratory rate > 30/min Or Oxygen Saturation < 93 %)
Very low certainty evidence from 1 RCT12 that included 160 patients with mild COVID-19 disease indicated that convalescent plasma may reduce progression to severe respiratory disease (defined as respiratory rate >30/min or oxygen saturation <93%), RR 0.52 (95% CI 0.29 to 0.94) compared to standard of care. Based on the data, we anticipated that for 1000 patients with mild COVID-19 disease treated with standard of care, 313 are likely to progress to severe respiratory disease vs 163 patients per 100 patients if convalescent plasma is administered for mild COVID 19.
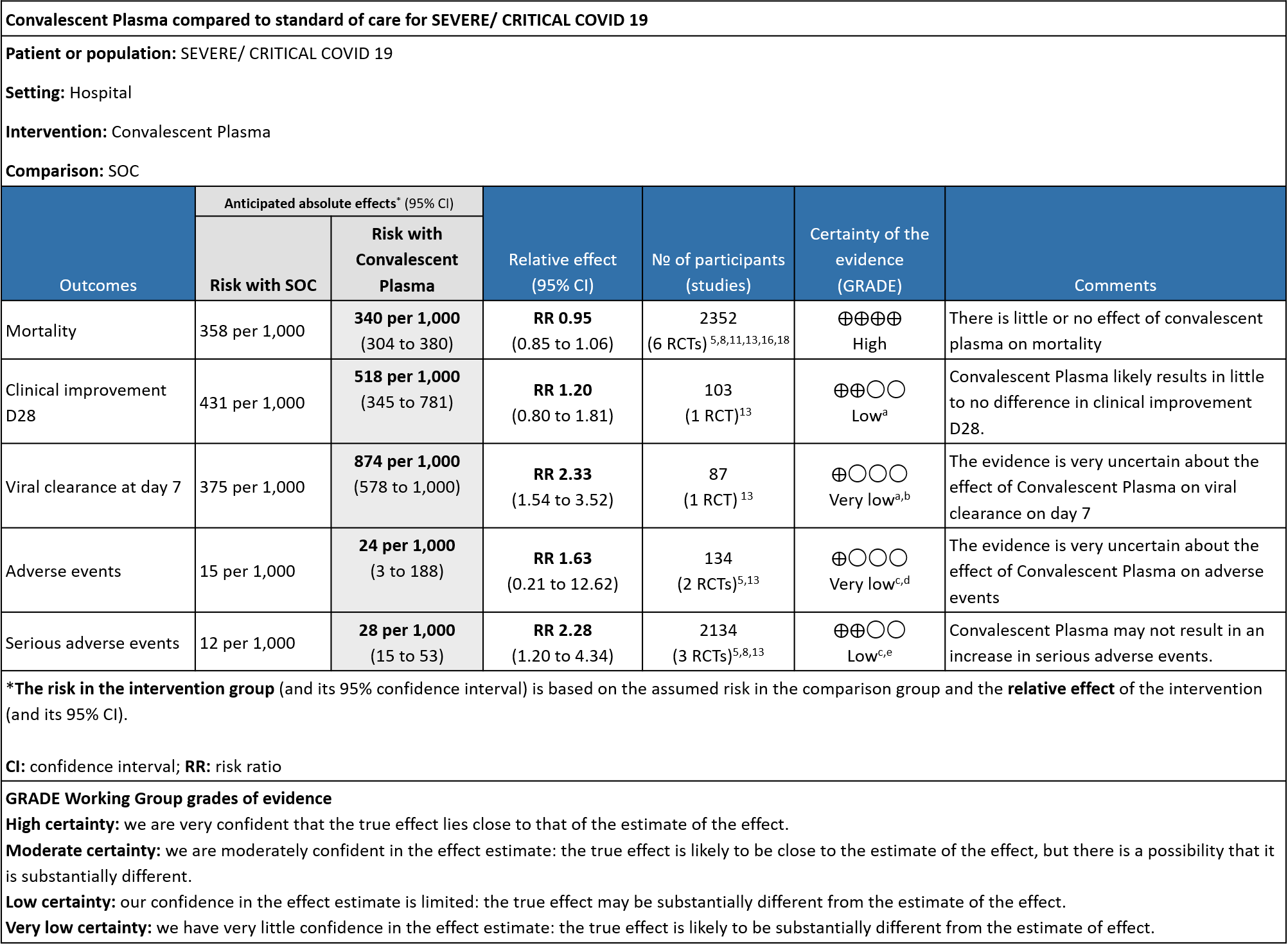
III. CONVALESCENT PLASMA COMPARED TO STANDARD OF CARE IN SEVERE-CRITICAL COVID-19:
The group noted that there were not too many trials measuring outcomes exclusively in severe/critical category. Most studies seemed to include all categories of severity and we were unable to tease out disaggregated data for mild or severe infections alone.
1. All-cause mortality at 28 days:
High certainty evidence from 6 RCTs5,8,11,13,16,18 that included 2352 patients with severe-critical COVID-19 disease indicated that Convalescent Plasma results in little to no difference in mortality at 28 days, RR 0.95 (95% CI 0.85 to 1.06). We anticipate that for 1000 patients with severe-critical COVID-19 disease treated with standard of care, 358 are likely to die vs 340 people if Convalescent plasma is administered for severe-critical COVID-19. The number needed to treat (NNT) to reduce an additional mortality is 71 patients with severe-critical COVID-19.
2. Clinical improvement D28 (Clinical improvement was defined as patient discharge or a reduction of 2 points on a 6-point disease severity scale):
Low certainty evidence from 1 RCT13 that included 103 patients with severe-critical COVID-19 disease indicated that Convalescent Plasma may result in a slight increase in clinical improvement by 28 days RR 1.20 (95% CI 0.80 to 1.81). We anticipate that for 1000 patients with severe-critical COVID-19 disease treated with standard of care, 431 are likely to have clinical improvement by 28 days vs 518 (from 86 fewer to 350 more) if Convalescent plasma is administered.
3. Time to clinical improvement:
Very low certainty evidence from 1 RCT13 that included patients with severe-critical COVID-19 disease revealed a very uncertain effect of Convalescent Plasma on time to clinical improvement, HR 2.56 (95% CI 1.80 to 3.63)
4. Viral clearance at day 7:
Very low certainty evidence from 1 RCT13 that included 87 patients with severe-critical COVID-19 disease revealed that the effect of Convalescent Plasma on viral clearance at day 7 is uncertain RR 2.33 (95% CI 1.54 to 3.52).
5. All adverse events:
Very low certainty evidence from 2 RCTs5,13 that included 134 patients with severe-critical COVID-19 disease revealed an uncertain effect of Convalescent plasma on all adverse events RR 1.63 (95% CI 0.21 to 12.62).
6. Serious adverse events:
Low certainty evidence from 3 RCTs5,8,13 that included 2134 patients with COVID-19 disease revealed that Convalescent plasma may increase serious adverse events RR 2.28 (95%CI 1.20 to 4.34) compared to standard of care. Based on the data, we anticipate that for 1000 patients with severe-critical COVID-19 treated with standard of care 12 serious adverse events are likely vs 28 if convalescent plasma is administered for patients with severe-critical COVID-19. The number needed to harm (NNH) to result in one additional serious adverse event is 53 patients with severe-critical COVID-19.
Subgroup analysis
1. High vs low titer antibody levels
It has been postulated that convalescent plasma with high antibody titer levels is likely to be more effective as a therapeutic option vs low antibody titer. Hence a subgroup analysis was done. The cut off was based on a cut-off of a high neutralizing antibody titer of >1:250 as per Federal Drug Administration (FDA) or Infectious Diseases Society of America (IDSA) 24, 25
• Mortality in high vs low titer antibody group: 14 studies3,5,6,8,9,11,13-20 where low titer antibody donor convalescent plasma was administered, did not show any impact on mortality RR 0.95 (95% CI 0.86-1.04). 5 studies4, 7,10,12,21 with high titer antibody suggested similarly that convalescent plasma does not impact mortality RR 0.98 (95% CI 0.92-1.05). A test of subgroup differences revealed an I2 =0% suggesting that there is no difference between the subgroups.
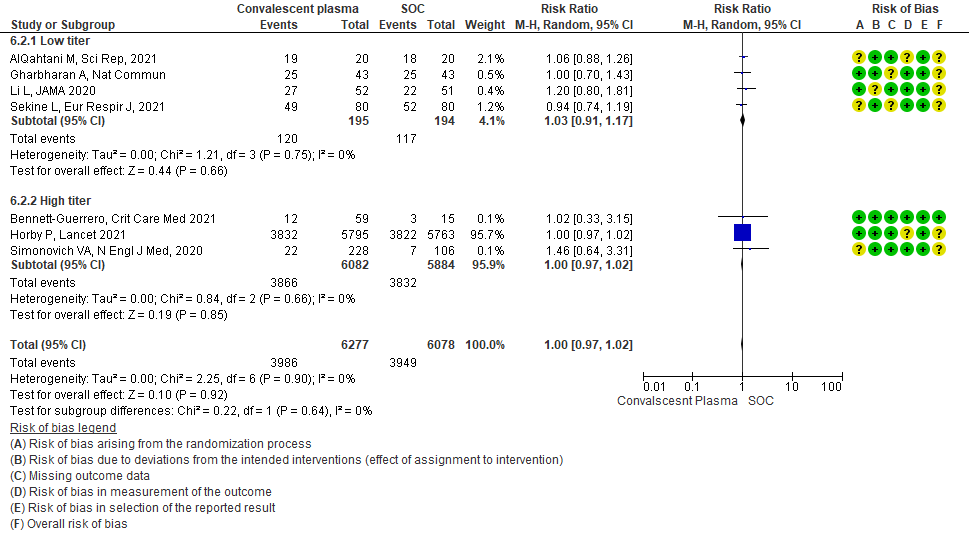
• Clinical improvement: 4 studies3, 9, 13, 20 where a low titer antibody donor convalescent plasma was administered did not impact clinical improvement RR 1.03 (95% CI 0.91-1.17). 3 studies7,10,21 with high titer donor convalescent plasma also showed similar results RR 1.00 (95% CI 0.97-1.02). Test for subgroup differences revealed an I2 = 0% suggesting there was no difference between the two groups.
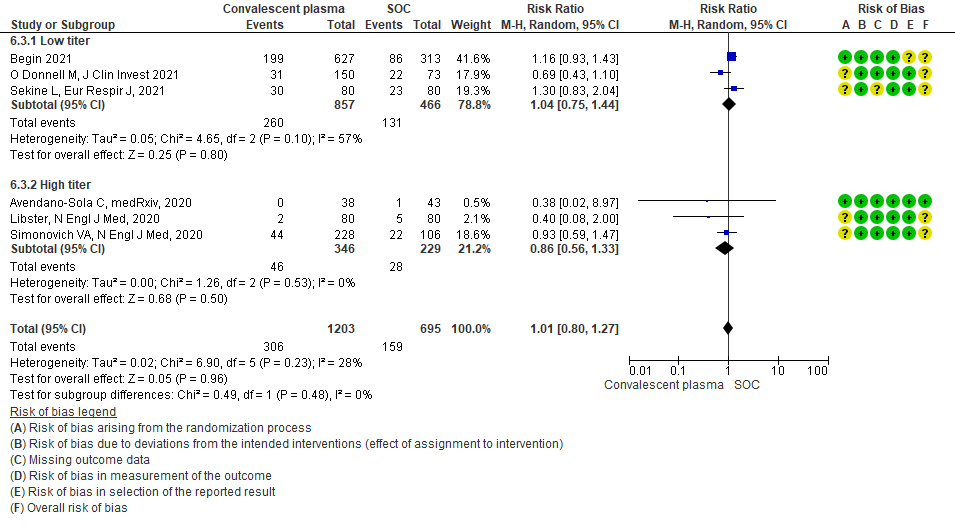
• Progression to WHO progression score ≥ 7 or discharge at day 28 or later: 3 studies6,14,20 where a low titer antibody donor convalescent plasma was administered did not reduce progression to WHO progression score >7 or lead to early discharge RR 1.04 (95% CI 0.75-1.44). 3 studies4,12,21 where a high titer donor convalescent plasma was administered showed similar results RR 0.86 (95% CI 0.56-1.33). Test for subgroup differences revealed an I2 = 0% suggesting there was no difference between the two groups.
• Progression to invasive ventilation: 1 study15 where a low titer antibody donor convalescent plasma was administered did not reduce progression to invasive ventilation RR 1.03 (95% CI 0.55-1.91). 2 studies10,12 where a high titer donor convalescent plasma was administered showed similar results RR 0.97 (95% CI 0.88-1.07). Test for subgroup differences revealed an I2 = 0% suggesting there was no difference between the two groups.
• Progression to non-invasive (NIV) or invasive mechanical ventilation (IMV): 3 studies3,8,15 where a low titer antibody donor convalescent plasma was administered did not reduce progression to NIV or IMV RR 1.05 (95% CI 0.94-1.18). 3 studies4,10,12 where a high titer donor convalescent plasma was administered showed similar results RR 0.96 (95% CI 0.89-1.04). Test for subgroup differences revealed an I2 = 36.3 % suggesting there was no difference between the two groups.
• Time to clinical improvement: 2 studies13,14 where a low titer antibody donor convalescent plasma was administered did not reduce time to clinical improvement RR 1.25 (95% CI 0.95-1.64). 3 studies4,7,21 where a high titer antibody donor convalescent plasma was administered showed similar results RR 0.98 (95% CI 0.77-1.23). Test for subgroup differences revealed an I2 = 46.3%.
• Viral clearance at day 7: 4 studies12,15,19,20 where a low titer antibody donor convalescent plasma was administered did not reduce viral clearance at day 7; RR 1.42 (95% CI 0.89-2.29). There were no studies with high titer convalescent plasma which measured this outcome.
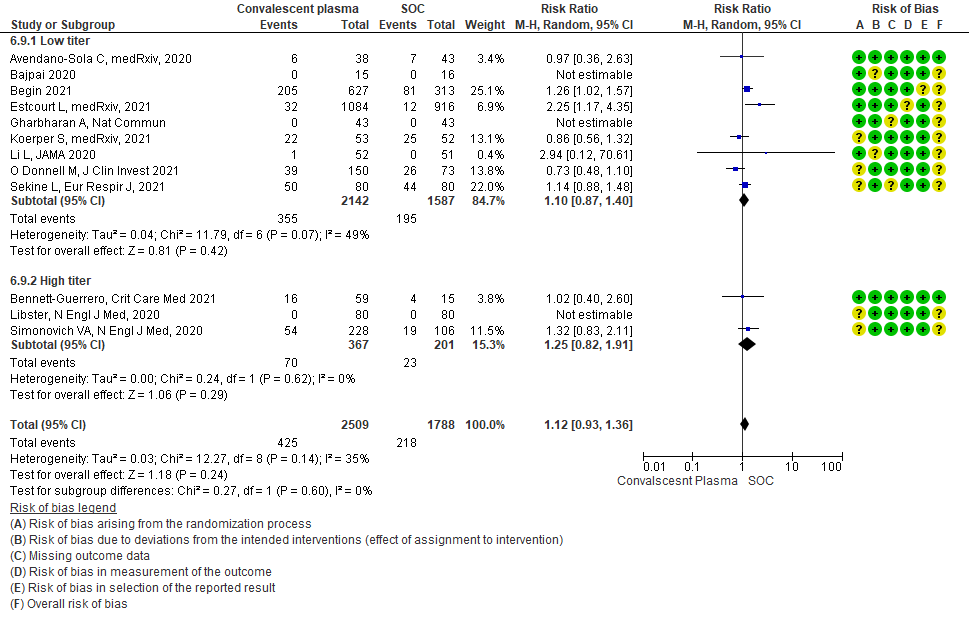
• Adverse events: 5 studies5,11,13,14,20 where a low titer antibody donor convalescent plasma was administered did not seem to cause an increase in adverse events RR 1.05 (95% CI 0.92-1.19). Similarly 2 studies12,21 with high titer antibody donor convalescent plasma also did not seem to cause an increase in adverse events RR 1.08 (95% CI 0.96-1.17). Test for subgroup differences revealed an I2 = 0% suggesting there was no difference between the two groups.
• Serious adverse events: 9 studies4-6,8,9,11,13,14,20 where a low titer antibody donor convalescent plasma was administered did not seem to cause a major increase in serious adverse events RR 1.10 (95% CI 0.87-1.40). Similarly in 3 studies7,12,21 with high titer antibody donor convalescent plasma there was no increase in serious adverse events RR 1.25 (95% CI 0.82-1.91). Test for subgroup differences revealed an I2 = 0% suggesting there was no difference between the two groups.
We included 19 RCTs4-21 from the COVID NMA data base in our analysis.
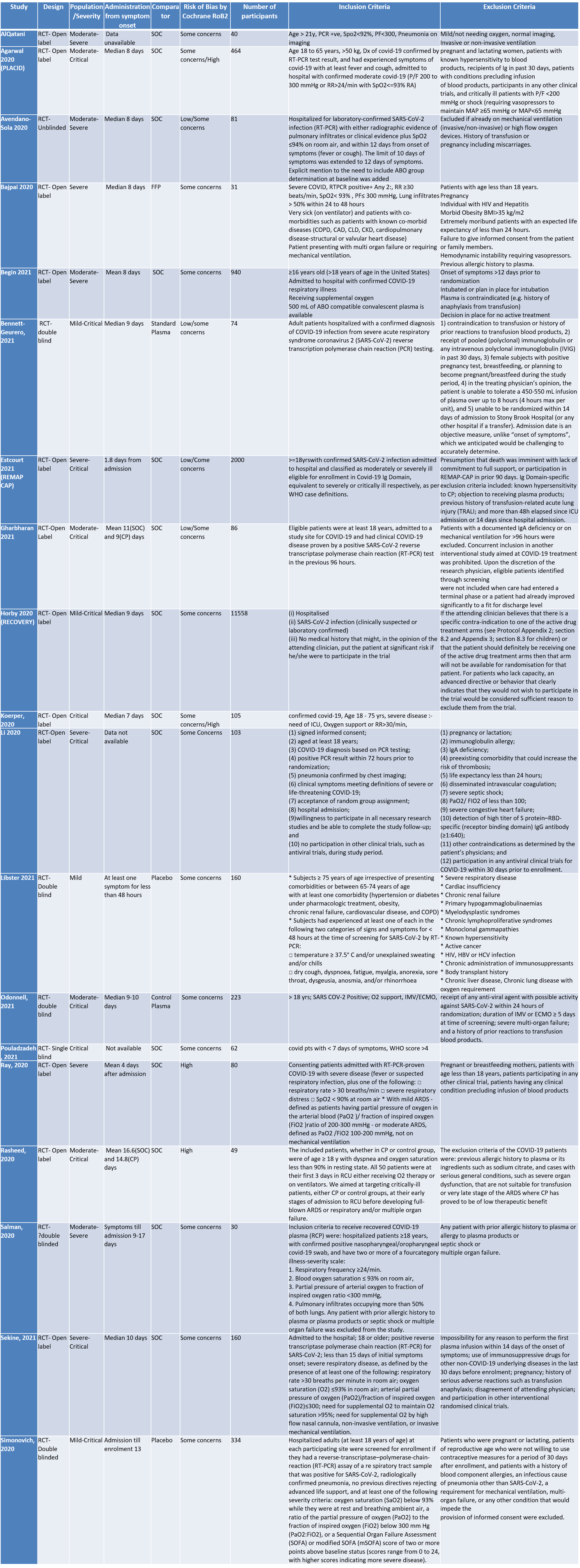
SOC=standard of care
1. CONVALESCENT PLASMA COMPARED TO PLACEBO IN PATIENTS WITH COVID 19
1.1 All-cause Mortality at 28 days in patients treated with Convalescent plasma as compared to standard of care in COVID 19 patients
1.2 Clinical improvement by day 28in patients treated with Convalescent plasma as compared to standard of care in COVID 19 patients
Clinical improvement was defined as patient discharge or a reduction of 2 points on a 6-point disease severity scale.
Scale was defined as follows: 6 points, death; 5 points, hospitalization plus ECMO or invasive mechanical ventilation;
4 points, hospitalization plus noninvasive ventilation or high-flow supplemental O2;
3 points, hospitalization plus supplemental O2 (not high-flow or noninvasive ventilation);
2 points, hospitalization with no supplemental O2;
1 point, hospital discharge
1.3 WHO progression score7 or above Day 28 [mechanical ventilation +/- additional organ support (ECMO, HD or vasopressors) OR death- or discharge beyond 28 days]28in patients treated with Convalescent plasma as compared to standard of care in COVID 19 patients
1.4 Progression to Invasive ventilation in patients treated with Convalescent plasma as compared to standard of care in COVID 19 patients
1.5 Progression to Ventilation (invasive or non-invasive) in patients treated with Convalescent plasma as compared to standard of care in COVID 19 patients
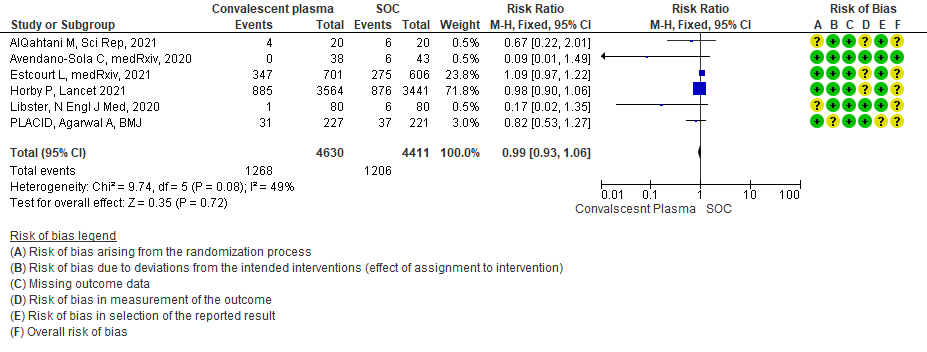
1.6 Time to clinical improvement in patients treated with Convalescent plasma as compared to standard of care in COVID 19 patients
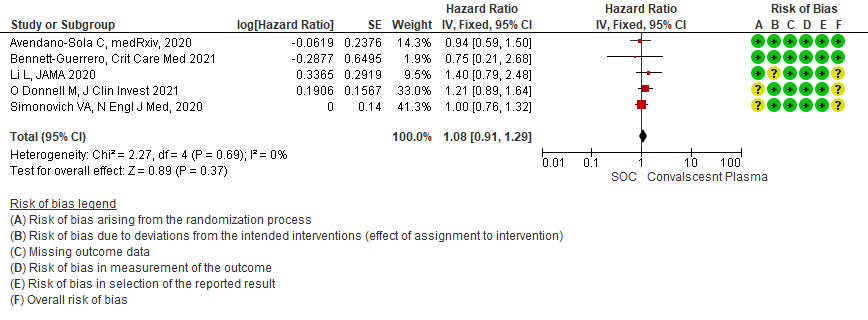
1.7 Viral clearance at day 7 in patients treated with Convalescent plasma as compared to standard of care in COVID 19 patients
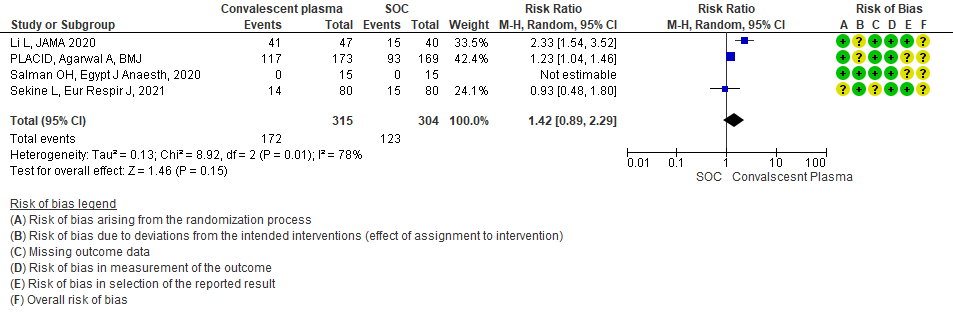
1.8 Adverse events in patients treated with Convalescent plasma as compared to standard of care in COVID 19 patients
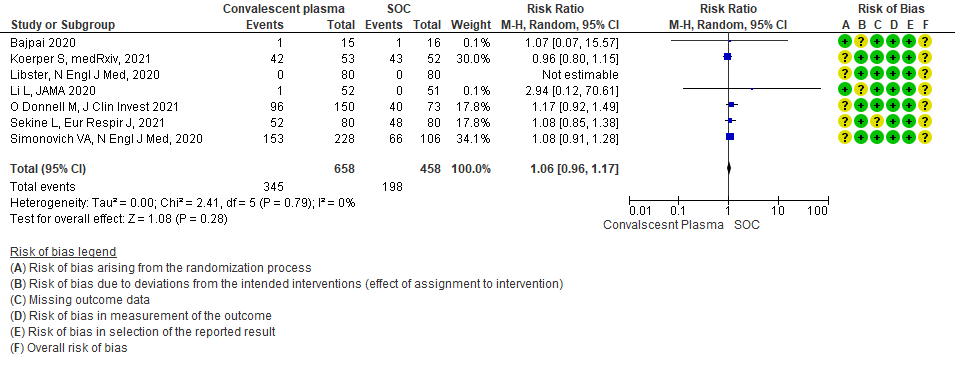
1.9 Serious adverse events in patients treated with Convalescent plasma as compared to standard of care in COVID 19 patients
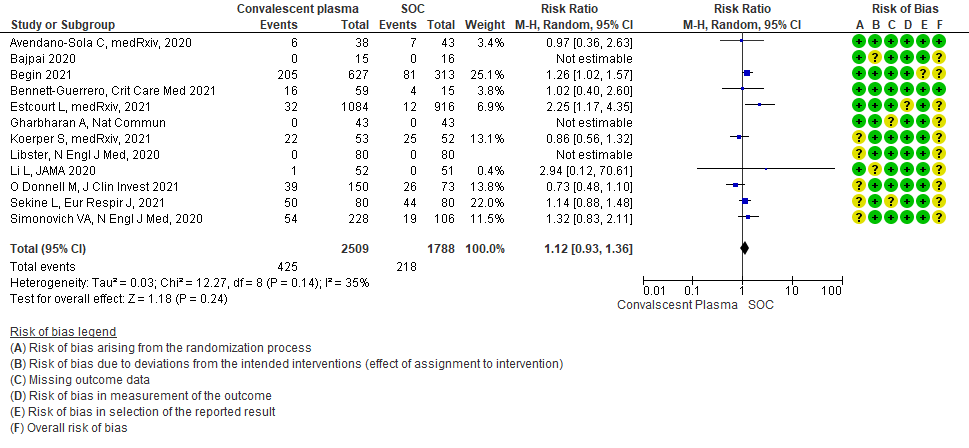
2. MILD CATEGORY – OUTCOMES
2.1 All-cause Mortality at 28 days in patients treated with Convalescent plasma as compared to standard of care in mild COVID 19 patients
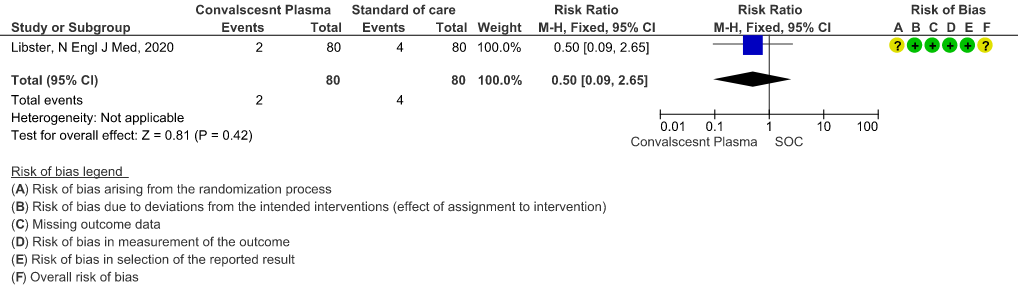
2.2 Mild - WHO progression score 7 or above D28 [mechanical ventilation +/- additional organ support (ECMO, HD or vasopressors) OR death- or discharge beyond 28 days]in patients treated with Convalescent plasma as compared to standard of care in mild COVID 19 patients
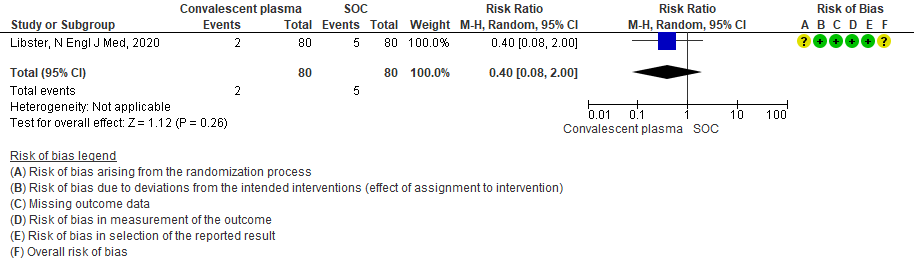
2.3 Progression to “Severe respiratory disease”in patients treated with Convalescent plasma as compared to standard of care in mild COVID 19 patients [Severe respiratory disease defined as Respiratory rate > 30/min Or Oxygen saturation < 93%]
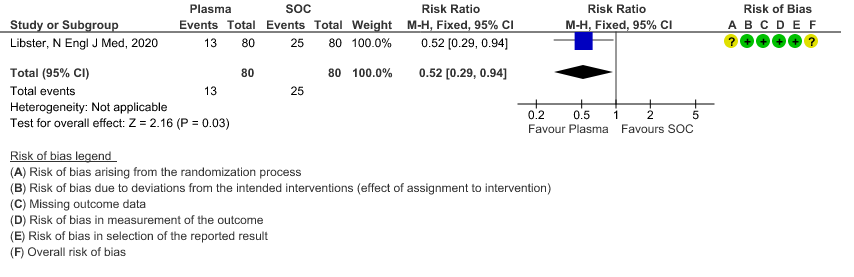
3. SEVERE/CRITICAL CATEGORY-OUTCOMES
3.1 All-cause Mortality at 28 days in patients treated with Convalescent plasma as compared to standard of care in severe-critical COVID 19 patients
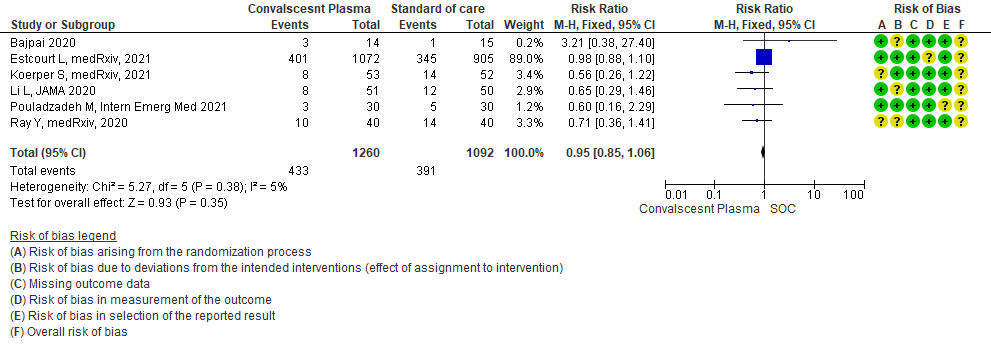
3.2 Clinical improvement by 28 days in patients treated with Convalescent plasma as compared to standard of care in severe-critical COVID 19 patients
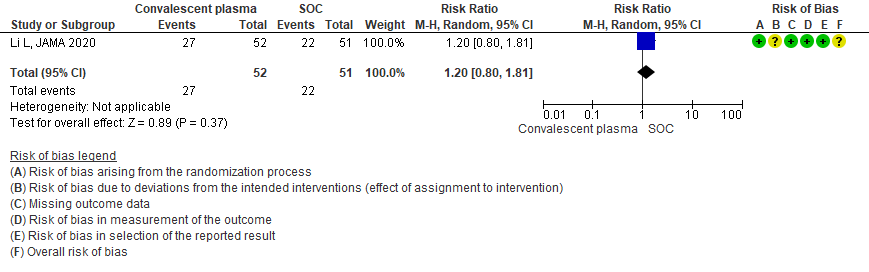
3.3 Viral clearance at day 7in patients treated with Convalescent plasma as compared to standard of care in severe-critical COVID 19 patients
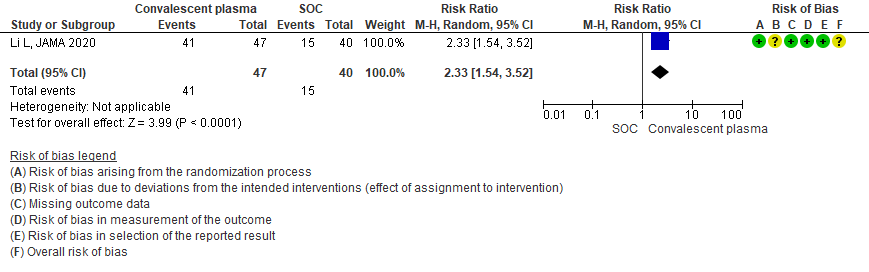
3.4 Adverse events in patients treated with Convalescent plasma as compared to standard of care in severe-critical COVID 19 patients
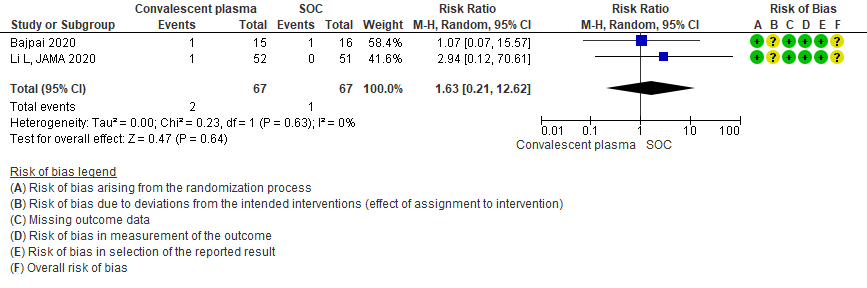
3.5 Serious adverse events in patients treated with Convalescent plasma as compared to standard of care in severe-critical COVID 19 patients
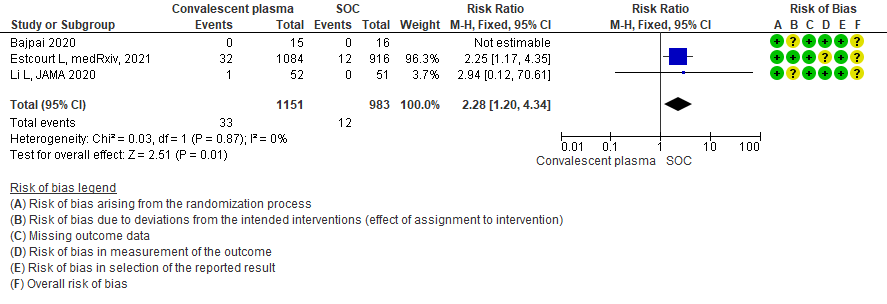
4. Subgroup-based on Antibody Titer of donor plasma
4.1 All-cause Mortality at 28 days in subgroup of patients treated with Convalescent plasma based on Antibody Titer of donor plasma
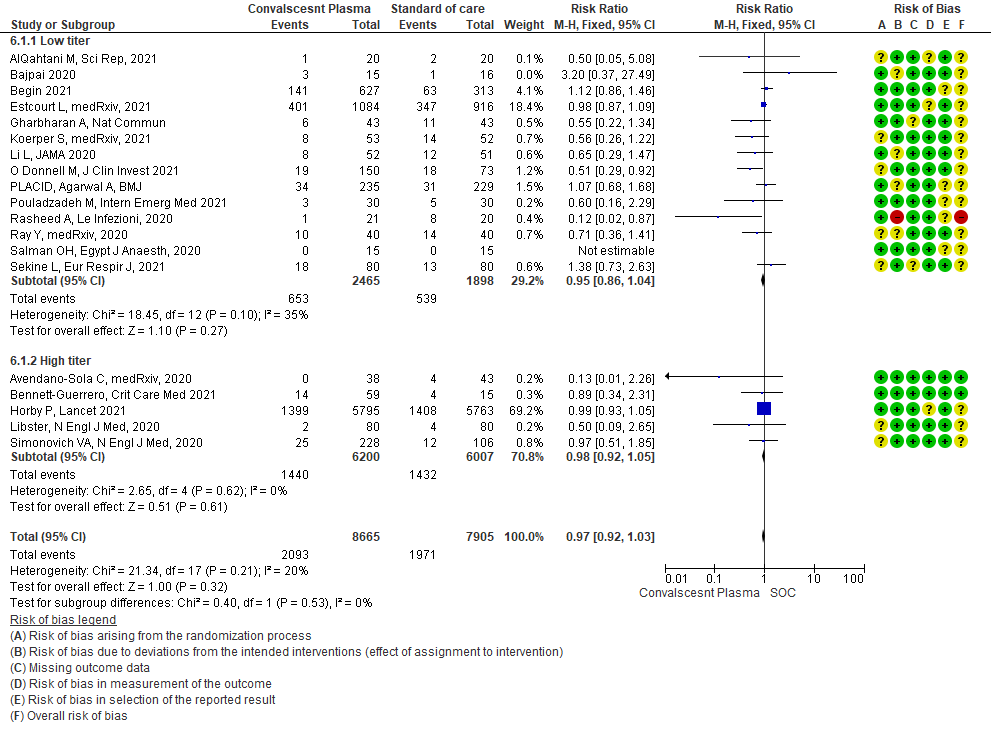
4.2 Clinical improvement at 28 days in subgroup of patients treated with Convalescent plasma based on Antibody Titer of donor plasma
4.3 WHO progression score 7 or above D28[mechanical ventilation +/- additional organ support (ECMO, HD or vasopressors) OR death- or discharge beyond 28 days]in subgroup of patients treated with Convalescent plasma based on Antibody Titer of donor plasma
4.4 Progression to invasive ventilationin subgroup of patients treated with Convalescent plasma based on Antibody Titer of donor plasma
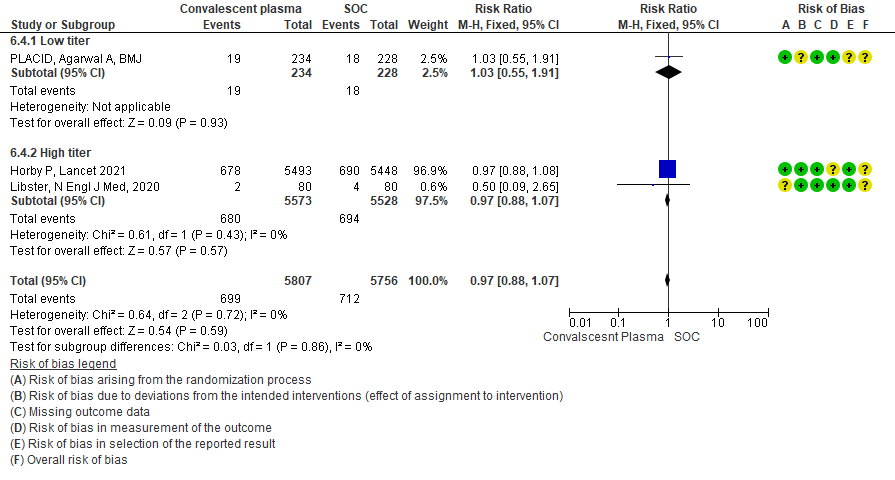
4.5 Progression to ventilation[invasive or non-invasive]in subgroup of patients treated with Convalescent plasma based on Antibody Titer of donor plasma
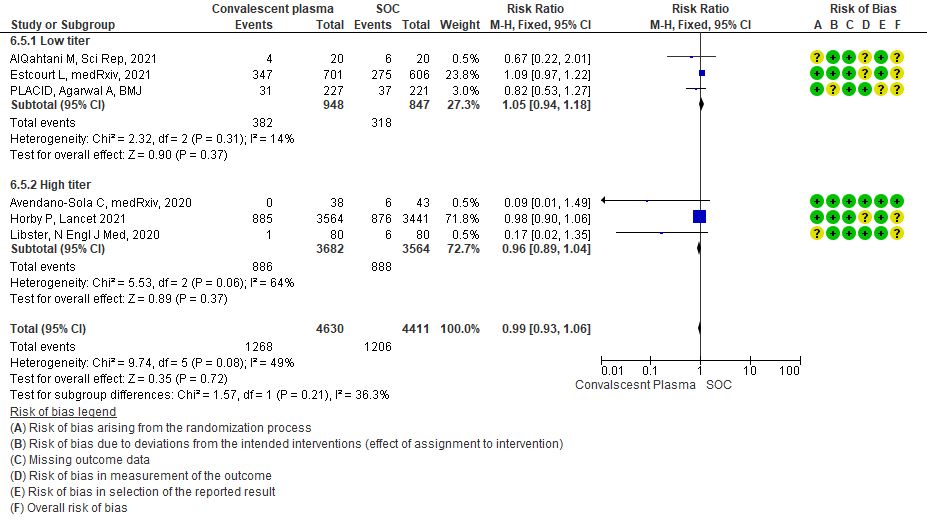
4.6 Time to clinical improvementin subgroup of patients treated with Convalescent plasma based on Antibody Titer of donor plasma
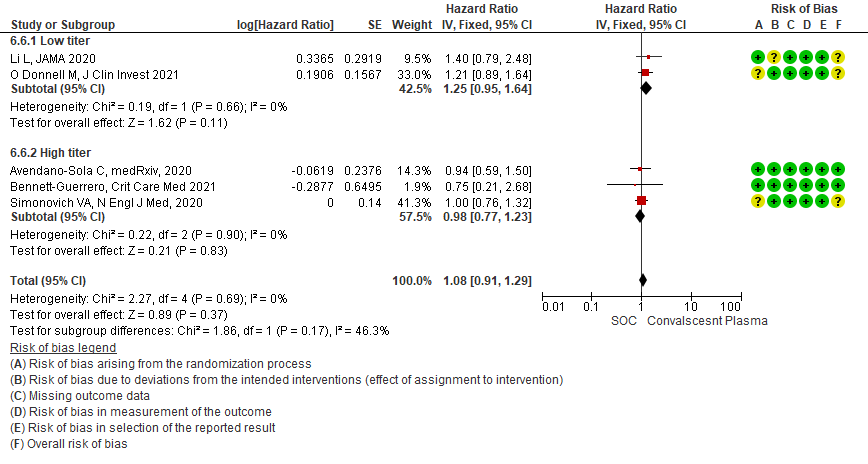
4.7 Viral clearance at day 7in subgroup of patients treated with Convalescent plasma based on Antibody Titer of donor plasma
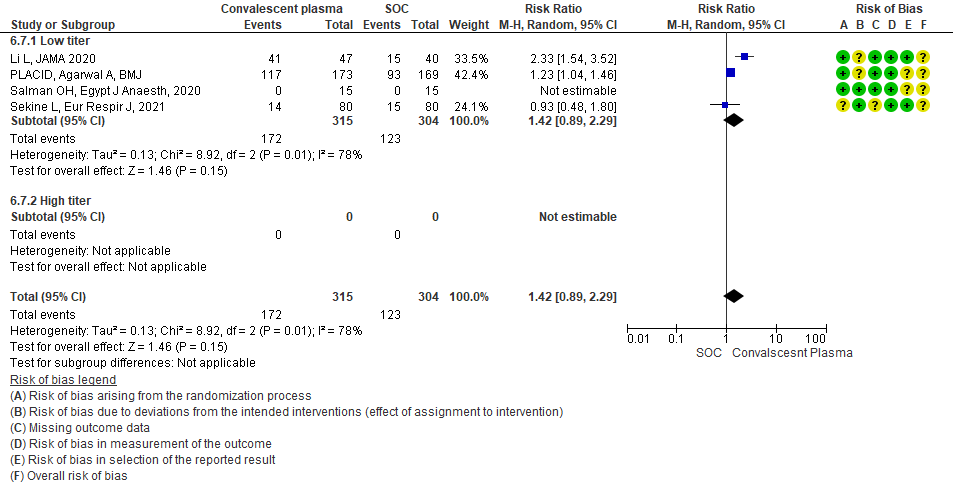
4.8 Adverse eventsin subgroup of patients treated with Convalescent plasma based on Antibody Titer of donor plasma
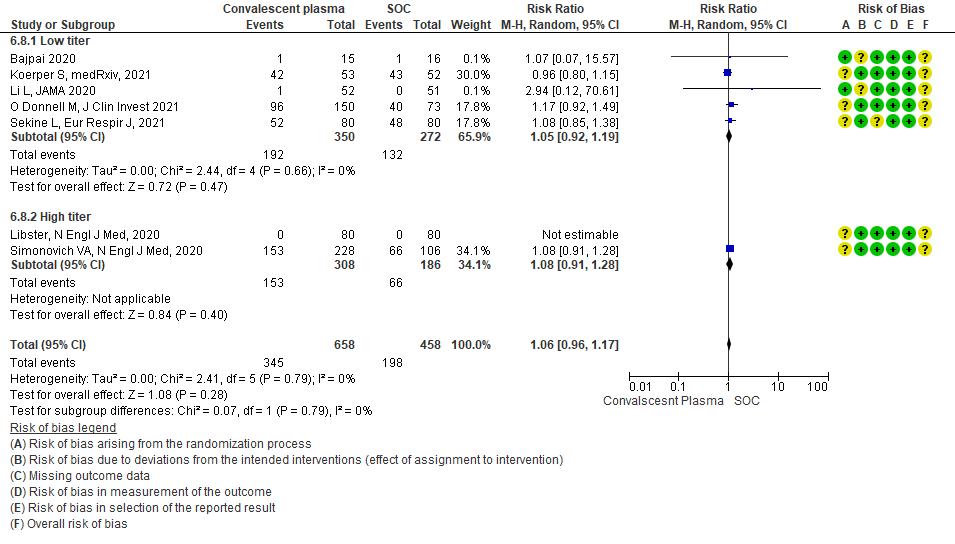
4.9 Serious adverse eventsin subgroup of patients treated with Convalescent plasma based on Antibody Titer of donor plasma
The Anti-inflammatory Expert Working Group met on 23rd August 2021 to consider Convalescent Plasma as a treatment for COVID-19. Conflict of interest declarations were reviewed by the Steering Committee; none were found to be relevant to Colchicine
A summary and then more detailed explanations of the Expert Working Group's judgements follow.
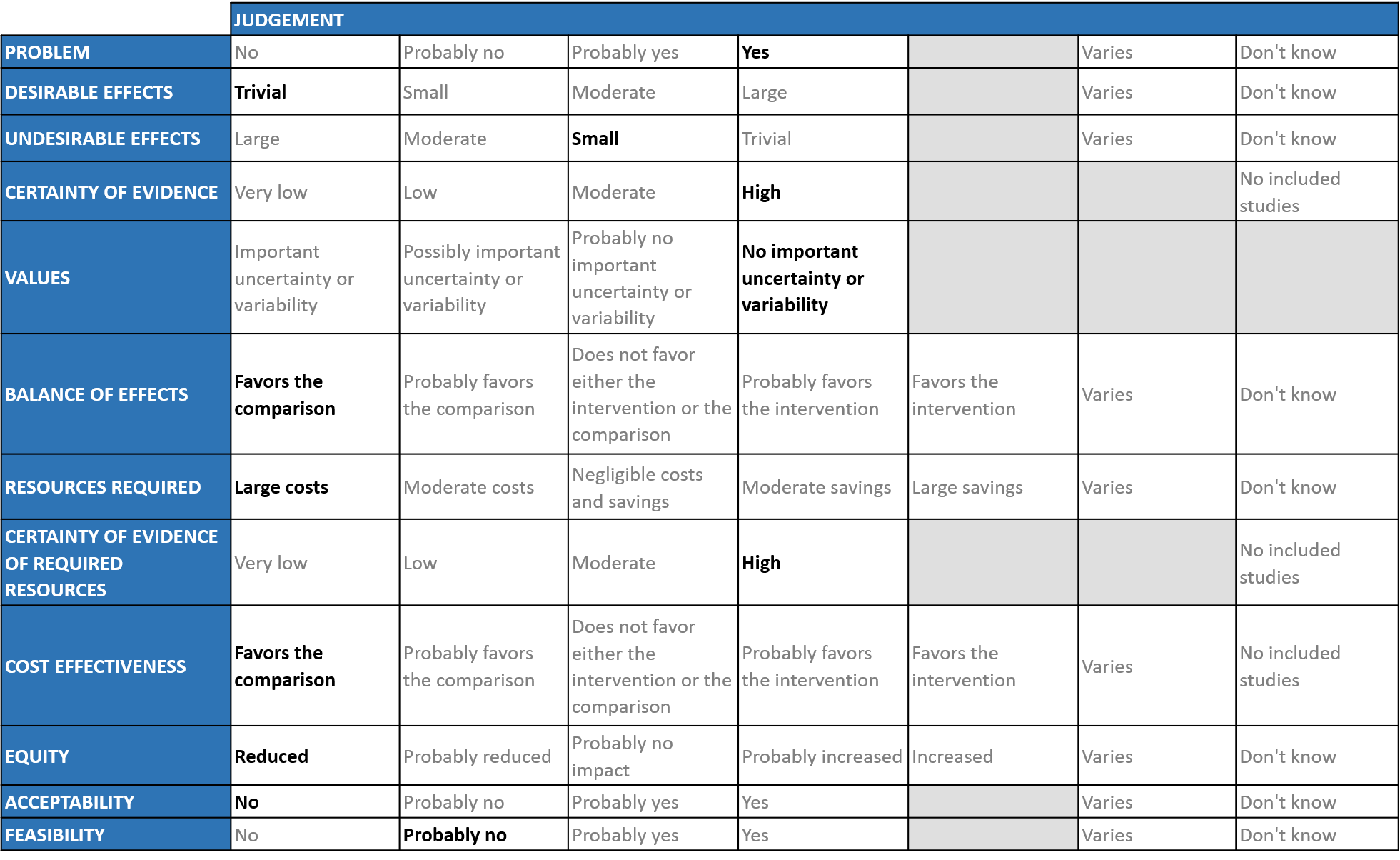
Problem
The COVID-19 pandemic in India with more than 30 million cases and over 0.39 million deaths has significantly impacted and stressed the health structure of the country. With a shortage of intensive care unit beds, oxygen and trained personnel the country is facing a major health crisis. Patients dying of COVID-19 are a problem. Any therapeutic intervention which prevents patients progressing to severe to critical disease would be a boon.
Desirable effects
The group agreed that the evidence suggests that Convalescent Plasma has no clinically significant effect on mortality, WHO progression score 7 or above, progression to ventilation, viral clearance at day 7, time to clinical improvement and clinical improvement at day 28 across all categories of severity. It was also noted that there was no effect on time to clinical recovery or length of hospital stay. The expert working group (EWG) noted that the certainty of evidence for all the outcomes and concluded that the desirable effect is trivial.
Undesirable effects
We would expect that the major adverse events are TRALI, Circulatory overload, Anaphylaxis may occur, however from the pooled data it seemed that convalescent plasma has little to no effect on adverse events. Hence the group concluded that the undesirable anticipated effects is small.
Certainty of evidence
Using GRADE methodology, the evidence synthesis team rated certainty of evidence to be moderate for - mortality, clinical improvement at day 28, progression to invasive and non-invasive ventilation whereas low for - time to clinical improvement and very low for - WHO progression score 7 or above. Certainty of evidence was found to be low for adverse events and serious adverse events in overall category.
In mild category, certainty of evidence was found to be low for mortality and WHO progression ≥ 7 at day 28. In severe, certainty of evidence was found to be high for mortality moderate for clinical improvement at day 28 and very low for time to clinical improvement and low for viral clearance. Certainty of evidence was found to be very low for adverse events and moderate for serious adverse events.
The expert working agreed with these judgements and rated the overall certainty of evidence as high for overall category. The group noted that in early disease and mild category there were not too many trials measuring these exclusively, most studies seemed to include all categories of severity and we were unable to tease out disaggregated data for mild or severe infections alone. Studies where convalescent plasma is given in early/mild disease and compared with standard of care are essential for us to make a decision as to the utility of convalescent plasma as a therapeutic option. Hence due to the prevailing uncertainty if given at all, it should begiven in the context of a RCT.
Values
The group felt that all the outcomes including those of mortality, WHO progression score 7 or above, progression to ventilation, viral clearance at day 7, time to clinical improvement and clinical improvement in day 28 were expressed variably in the studies. However, there is no important uncertainty or variability in how people would value the main outcomes.
Balance of effects
The expert working group felt that Antibody status of the patients was not available in all the trials. They felt that it was important to have a high titer of antibody, recommended donor antibody titers were 1:180 on PRNT and IgG >1:640 on CLIA. The group deliberated extensively and concluded that the balance of effects favors the comparison, rather than the intervention.
Resources required
The group considered the following - finding donors, setting up bleeding, testing and storage facilities all of which indicated huge infrastructure costs for the intervention. The group discussed and concluded that the requirement of resources included large costs. The group includes clinicians in different types of Indian hospitals who have a good idea of drug and hospitalization costs.
Certainty of evidence of required resources
No studies reporting this were reviewed by the group but the clinicians in the group were aware of the cost and hence felt that there was high certainty of evidence for required resources to implement this intervention.
Cost effectiveness
The group discussed that this is an expensive intervention with no clear-cut beneficial effects. So, the group concluded that the cost effectiveness favors the comparison. The evidence was less clear in the mild category but was moderate in the severe to critical category.
Equity
At this point in time this intervention would definitely reduce equity due to the costs, availability and access considerations.
Acceptability
The group felt that this was not an acceptable intervention as there is no clear-cut benefit and it was resource intensive which precluded its widespread implementation.
Feasibility
The group concluded that it was not feasible to implement this intervention widely due to the resources that were required for the same both in terms of trained manpower, infrastructure (good lab with blood banking facilities) or access/availability issues.
The provision of safe donor convalescent plasma parallels the criteria for safe blood with the following being mandatory: the availability of licensed blood center, a program to reach out to prior patients who will need to qualify to be usual donors with high titers of neutralizing antibodies (as per prevailing regulations and guidelines), while maintaining confidentiality25. First essential step is identification of eligible suitable donors by standard screening to ensure blood is safe and free from blood borne pathogens and of the correct blood group. Second step would be tests to ensure for the requisite titer of COVID antibodies. Third step would be maintenance and storage at -40 deg C or less with administration only within in a hospital setting with supervision of skilled health care workers. All this adds to the cost of the intervention. Further, the non-trivial transfusion related adverse events like Transfusion related hypersensitivity reactions, fever, circulatory overload including exposure to potential window period donors for other blood borne infections, should also be taken into consideration while using this therapeutic measure.
Ensuring adequate and suitable Antibody titers in the donor convalescent plasma is critical to the efficacy of this intervention. However, whether appropriate standardized assays were used to measure neutralizing antibodies or a suitable cut off of titer was present in all the patients in the clinical trials, in the intervention arm is unclear.
Disaggregated data was not obtained in those with various co-morbidities. There is absence of adequate evidence in immunocompromised patients, pregnancy and in mild disease.
There is good quality evidence to recommend against use of convalescent plasma in patients with moderate/ severe and critical disease and it is unlikely that new evidence in this area will emerge. However new evidence relating to use of convalescent plasma in patients with mild disease especially those who are immunocompromised or have other risk factors for progression may emerge. The committee will continue to monitor this and update recommendations if necessary.
There is a need for more studies specifically addressing the following:
1. Timing of administration of convalescent plasma in relationship to the onset of symptoms, dose required for efficacy and potential benefit of preventing progression to ventilation or critical care especially in immunocompromised and in those with additional risk factors.
2. There is very little evidence addressing the use of convalescent plasma in early mild COVID19 disease.
3. Many of the currently available studies were conducted prior to easy access to appropriate serological tests and widespread vaccine availability. Hence the response of convalescent plasma in seropositive recipients either due to prior infection or vaccination is a crucial area of study.
- Wu F, Liu M, Wang A et al. Evaluating the Association of Clinical Characteristics With Neutralizing Antibody Levels in Patients Who Have Recovered From Mild COVID-19 in Shanghai, China. JAMA Intern Med. 2020;180(10):1356.
- Ewan M Bloch. COVID-19: Convalescent plasma and hyperimmune globulin. In: UpToDate, Kleinman Steven (Ed), UpToDate, Waltham, MA. (Accessed on October 12, 2021.)
- AlQahtani M, Abdulrahman A, Almadani A, Alali SY, Al Zamrooni AM, Hejab AH, et al. Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease. Sci Rep. 2021 May 11;11:9927.
- Avendaño-Solà C, Ramos-Martínez A, Muñez-Rubio E, Ruiz-Antorán B, de Molina RM, Torres F, et al. Convalescent Plasma for COVID-19: A multicenter, randomized clinical trial [Internet]. Infectious Diseases (except HIV/AIDS); 2020 Sep [cited 2021 Sep 29]. Available from: http://medrxiv.org/lookup/doi/10.1101/2020.08.26.20182444
- Bajpai M, Kumar S, Maheshwari A, Chhabra K, kale P, Gupta A, et al. Efficacy of Convalescent Plasma Therapy compared to Fresh Frozen Plasma in Severely ill COVID-19 Patients: A Pilot Randomized Controlled Trial [Internet]. Infectious Diseases (except HIV/AIDS); 2020 Oct [cited 2021 Sep 29]. Available from: http://medrxiv.org/lookup/doi/10.1101/2020.10.25.20219337
- Bégin P, Callum J, Jamula E, Cook R, Heddle NM, Tinmouth A, et al. Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial. Nat Med [Internet]. 2021 Sep 9 [cited 2021 Sep 29]; Available from: https://www.nature.com/articles/s41591-021-01488-2
- Bennett-Guerrero E, Romeiser JL, Talbot LR, Ahmed T, Mamone LJ, Singh SM, et al. Severe Acute Respiratory Syndrome Coronavirus 2 Convalescent Plasma Versus Standard Plasma in Coronavirus Disease 2019 Infected Hospitalized Patients in New York: A Double-Blind Randomized Trial*. Critical Care Medicine. 2021 Jul;49(7):1015–25.
- The REMAP-CAP Investigators, Estcourt LJ. Convalescent Plasma in Critically ill Patients with Covid-19 [Internet]. Intensive Care and Critical Care Medicine; 2021 Jun [cited 2021 Sep 29]. Available from: http://medrxiv.org/lookup/doi/10.1101/2021.06.11.21258760
- Gharbharan A, Jordans CCE, GeurtsvanKessel C, den Hollander JG, Karim F, Mollema FPN, et al. Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection. Nat Commun. 2021 May 27;12(1):3189.
- Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021 May 29;397(10289):2049–59.
- Körper S, Weiss M, Zickler D, Wiesmann T, Zacharowski K, M.Corman V, et al. High Dose Convalescent Plasma in COVID-19: Results from the Randomized Trial CAPSID [Internet]. Infectious Diseases (except HIV/AIDS); 2021 May [cited 2021 Sep 29]. Available from: http://medrxiv.org/lookup/doi/10.1101/2021.05.10.21256192
- Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, et al. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N Engl J Med. 2021 Feb 18;384(7):610–8.
- Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA. 2020 Aug 4;324(5):460–70.
- O’Donnell MR, Grinsztejn B, Cummings MJ, Justman JE, Lamb MR, Eckhardt CM, et al. A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19 [Internet]. American Society for Clinical Investigation; 2021 [cited 2021 Sep 29]. Available from: https://www.jci.org/articles/view/150646/pdf
- Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentrerandomised controlled trial (PLACID Trial). BMJ. 2020 Oct 22;371:m3939.
- Pouladzadeh M, Safdarian M, Eshghi P, Abolghasemi H, bavani AG, Sheibani B, et al. A randomized clinical trial evaluating the immunomodulatory effect of convalescent plasma on COVID-19-related cytokine storm. Intern Emerg Med. 2021 Apr 10;1–11.
- Rasheed AM, Fatak DF, Hashim HA, Maulood MF, Kabah KK, Almusawi YA, et al. The therapeutic potential of convalescent plasma therapy on treating critically-ill COVID-19 patients residing in respiratory care units in hospitals in Baghdad, Iraq. :10.
- Ray Y, Paul SR, Bandopadhyay P, D’Rozario R, Sarif J, Lahiri A, et al. Clinical and immunological benefits of convalescent plasma therapy in severe COVID-19: insights from a single center open label randomised control trial [Internet]. Infectious Diseases (except HIV/AIDS); 2020 Nov [cited 2021 Sep 29]. Available from: http://medrxiv.org/lookup/doi/10.1101/2020.11.25.20237883
- Hamdy Salman O, Ail Mohamed HS. Efficacy and safety of transfusing plasma from COVID-19 survivors to COVID-19 victims with severe illness. A double-blinded controlled preliminary study. Egyptian Journal of Anaesthesia. 2020 Jan 1;36(1):264–72.
- Sekine L, Arns B, Fabro BR, Cipolatt MM, Machado RRG, Durigon EL, et al. Convalescent plasma for COVID-19 in hospitalised patients: an open-label, randomised clinical trial. EurRespir J. 2021 Jul 8;2101471.
- Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, et al. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N Engl J Med. 2021 Feb 18;384(7):619–29.
- Piechotta V, Iannizzi C, Chai KL, Valk SJ, Kimber C, Dorando E, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a living systematic review. Cochrane Database of Systematic Reviews [Internet]. 2021 [cited 2021 Sep 29];2021(5). Available from: https://www.readcube.com/articles/10.1002%2F14651858.CD013600.pub4
- https://www.idsociety.org/covid-19-real-time-learning-network/therapeutics-and-interventions/convalescent-plasma/#Guidelines
- https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-applications-inds-cber-regulated-products/recommendations-investigational-covid-19-convalescent-plasma
- https://www.icmr.gov.in/pdf/covid/techdoc/ICMR_ADVISORY_Convalescent_plasma_17112020_v1.pdf).
Covid Management Guidelines India Group – Antibody working Group – Convalescent Plasma. Covid Guidelines India; Published online on 27th October 2021; URL: https://indiacovidguidelines.org/convalescent-plasma/ (accessed )
