All of our recommendations, unless specified, relate to acute COVID-19 in adults.
Some of our recommendations vary according to the severity of COVID-19 illness. Definitions of the categories are based on World Health Organization (WHO) criteria and can be viewed by clicking the plus (+) signs below.
RECOMMENDATION: We strongly recommend against use of Remdesivir in all patients with mild and critical COVID-19. We recommend against routine use of Remdesivir in moderate and severe COVID-19. However, clinicians may choose to use Remdesivir in selected patients with moderate to severe illness, such as those receiving low-flow oxygen therapy.
DATE OF RECOMMENDATION: 1st June 2021
Definition of mild:
- Symptomatic (any acute COVID-19 related symptoms)
- AND respiratory rate <24/min
- WITHOUT pneumonia or hypoxia
A conditional recommendation is one for which the panel is less confident about the balance between desirable and undesirable effects of the intervention, or other aspects, such as cost and feasibility.
Definition of moderate illness:
- Pneumonia (clinical or radiological) OR hypoxia (SpO2 <94% in adults with no underlying lung disease)
- AND respiratory rate ≤30/min
- AND SpO2 ≥90% on room air
Definition of severe:
Pneumonia with ANY ONE of the following:
- respiratory rate >30/min
- severe respiratory distress
- SpO2 <90% on room air
- NO invasive or non-invasive respiratory support needed
Definition of critical:
- Requirement for high-level respiratory support: noninvasive ventilation, high-flow oxygen (≥20 litres per minute) or invasive mechanical ventilation
- OR acute respiratory distress syndrome (PaO2/FiO2 ratio of <300)
- OR sepsis
- OR shock
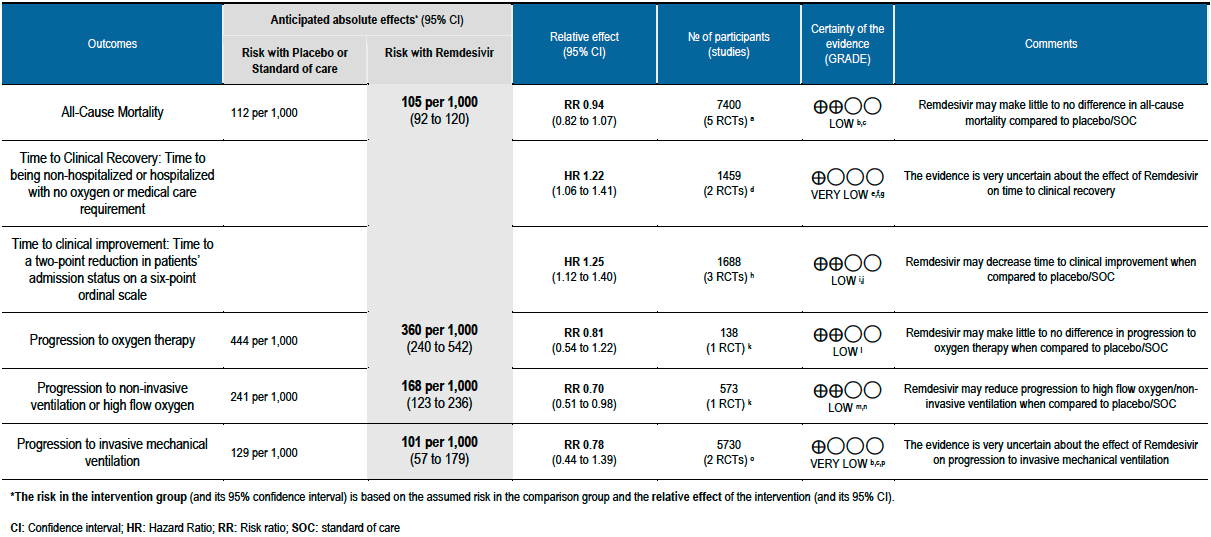
Low and very low certainty evidence from five randomized controlled trials (RCTs), indicates that Remdesivir may have little to no effect on all-cause mortality, progression to oxygen therapy, adverse events, thrombotic events and secondary infections in people hospitalized with COVID-19. Remdesivir’s effect on time to clinical recovery, progression to invasive mechanical ventilation and serious adverse events are very uncertain. It may reduce time to clinical improvement and progression to non invasive ventilation/high flow oxygen therapy, however trials which reported this benefit were conducted when steroids were not the standard of care. Hence it is uncertain as to whether Remdesivir provides a benefit over and above that clearly demonstrated by steroids. Only one study reported a possible mortality benefit when given to patients receiving low-flow oxygen, which may be biologically plausible as antivirals often have a greater effect when given earlier in a viral illness.
Overall, the benefit of Remdesivir in the treatment of COVID-19 is uncertain as there was significant heterogeneity among the trials, lower usage of steroids as compared to what would be standard of care today and wide confidence intervals in effect estimates of critical outcomes. Hence at this point there is insufficient evidence to recommend this drug routinely given the cost and availability of this drug in resource-limited settings.
Date of latest search: 15th April 2021.
Date of completion & presentation to Expert Working Group: 14th May 2021.
Date of planned review: 15th January 2022.
Evidence synthesis team: Anupa Thampy, Hanna Alexander, Naveena Princy, Richard Kirubakaran, Bhagteshwar Singh and Priscilla Rupali.
Summary of Findings Table 1 - Efficacy Outcomes:
GRADE Working Group grades of evidence
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect
Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect
Explanations:
a. Wang 2020, ACTT Beigel 2020, Spinner 2020, Solidarity 2021, Mahajan 2021
b. Downgraded by one for level serious indirectness; The usage of steroids varied across the studies
c. Downgraded by one level for serious imprecision; the 95% CI is wide
d. ACTT Beigel 2020, Spinner 2020. Pooled estimate is from 2 trials at 10 days
e. Downgraded by one level for serious risk of bias; Spinner 2020 was at high risk of bias and contributed to 36.1% of the weight in the metanalysis
f. Downgraded by one level for serious indirectness; Spinner 2020 had moderate severity patients only and ACTT Beigel 2020 had a mixture of patients of different severity.
g. Downgraded by two levels for very serious imprecision; It is difficult to determine the clinical significance of the difference in the time to clinical improvement with hazard ratios, but the 95% CI was wide and included no difference.
h. Wang 2020, ACTT Beigel 2020 , Spinner 2020. Pooled estimate from 3 trials at 10 days
i. Downgraded by one level for serious risk of bias ; Spinner 2020 was at high risk of bias and contributed 25.9% of the weight to the pooled effect estimate.
j. Downgraded by one level for serious indirectness; The three studies included patients with different grades of disease severity and Spinner 2020 included only patients with moderate severity.
k. ACTT Beigel 2020
l. Downgraded by two levels for imprecision; 95% CI is very wide
m. Downgraded by one level serious indirectness; as very few people in ACTT Beigel 2020 were given steriods
n. Downgraded by one level for serious imprecision.The data were from only one trial and the number of events were too few to satisfy the requirements for the optimal information size (OIS) [The OIS for a RRR of 2 percent needs at least 700 events for the intervention to be significant (Remdesivir arm: 52/307, placebo arm: 64/266)]. The 95% CI of the effect estimate included clinically important and potentially clinically non-appreciable benefits with Remdesivir.
o. ACTT Beigel 2020, Solidarity 2021
p. Downgraded by two levels for very serious inconsistency; the I2 is 91%.
Summary of Findings Table 2 - Complications & Safety Outcomes:
GRADE Working Group grades of evidence
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect
Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect
Explanations:
a. ACTT Beigel 2020, Wang 2020
b. Not downgraded for indirectness: Wang 2020 and ACCT Beigel 2020 included similar proportions of patients with severe disease and the incidence of adverse events was unlikely to have been confounded by severity.
c. Downgraded by two levels for very serious imprecision; the number of events were few and the 95% CI of the effect estimate included clinically important reductions and increases in thrombotic events with Remdesivir.
d. Downgraded by one level for serious inconsistency; the I2 is 65%
e. Downgraded by one level for serious imprecision: The number of events were few and the 95% CI of the effect estimate included a substantial reduction as well as a modest increase in the incidence of infections with Remdesivir compared to placebo/SOC.
f. Wang 2020, Spinner 2020
g. Downgraded by one level for serious risk of bias; Spinner 2020 was judged to be at high risk of bias and contributed 56.5 % of weight in the metanalysis
h. ACTT Beigel 2020 , Wang 2020, Spinner 2020
i. Downgraded by one level serious inconsistency; as I2 is 76%
j. Downgraded by two levels very serious imprecision; the 95% CI is very wide
Remdesivir is a monophosphoramidate adenosine analogue prodrug which is metabolized to an active triphosphate form that inhibits viral RNA synthesis via RNA dependent RNA polymerase [1]. It was developed as a therapeutic agent for treating RNA-based viruses such as Ebola virus, MERS, SARS-CoV-1 and other zoonotic coronaviruses [2]. It has demonstrated in vitro and in vivo antiviral activity against SARS-CoV-2 [3].
Remdesivir is widely used across the world and a number of guidelines have recommended its use in severe COVID-19 patients [4;5]. The recent WHO living guideline provides a conditional recommendation against the usage of Remdesivir in COVID-19 irrespective of the severity of the disease [6].
Since Remdesivir is currently the only antiviral drug recommended for severe COVID-19 by the Ministry of Health & Family Welfare guidelines, and appears to have a relatively good safety profile, it has been in high demand. Its current limited availability in India has led to a crisis involving desperate crowds flouting Covid-19 restrictions to avail the drug.
This review aims to provide a summary of the available evidence from randomized clinical trials of Remdesivir for treatment of acute COVID-19, for any duration, which could guide clinicians and researchers regarding the appropriate use of this drug in the future.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language. We also reviewed reference lists of systematic reviews and included studies. We performed all searches up to 15 April 2021.
We included randomized controlled trials (RCTs) testing Remdesivir in people with COVID‐19, and extracted data for the following pre-defined outcomes:
- Critical (primary for this review):
- Mortality (all-cause): at 28-30 days, or in-hospital
- Progression to:
- Oxygen therapy
- Ventilation: non-invasive or invasive
- Critical/Intensive care (any reason) - Time to clinical improvement
- Time to clinical recovery
- Adverse events: all and serious
- Important (secondary):
- Time to negative PCR for SARS-CoV-2
- Duration of hospitalization
- Complications of COVID-19:
- Thrombotic events
- Pulmonary function/fibrosis
- Long COVID/post-acute sequelae COVID
- Secondary infections
Two reviewers independently assessed eligibility of search results. One reviewer extracted data from each included study, and assessed risk of bias using the Cochrane Risk of bias (RoB) v2.0 tool. Data and RoB assessments were checked by the second reviewer. The entire RoB assessment was scrutinized by the whole team for this review, to reach consensus.
We used RevMan 5.4 to perform meta‐analysis using a random‐effects model for outcomes where pooling of effect estimates was appropriate. We used risk ratios (RR) for dichotomous outcomes and hazard ratios for continuous outcomes, with 95% confidence intervals (CIs). We performed a subgroup analysis to explore the effect on mortality stratified by different oxygen and ventilation requirements at baseline.
We used the I2 statistic to measure residual heterogeneity. We used GRADE methodology to assess the certainty in the evidence, and documented this in a ‘Summary of findings’ table using GradeProGDT.
We included five trials involving 7452 participants, all of whom were adults, and 3886 of whom received Remdesivir [7-11]. Two trials reported each from a single country: China [7] and India [10]; two from multiple countries in North America, Europe and Asia [8;11]; one recruited worldwide [9]. All trials were done in hospitalized patients. Disease severity, prevalence of comorbidities, and use of co‐interventions varied substantially between trials. Out of the five trials three [7;9;11] were found to have low risk of bias across all domains for all outcomes and two [8;10] had high risk of bias across multiple domains. Risk of bias for each domain per trial is displayed alongside the forest plots below.
The following comparisons were investigated in the trials (we compared outcomes for arms randomized to Remdesivir vs. outcomes for arms randomized to standard of care or placebo):
- Two trials [7;11] compared Remdesivir with placebo (1299 participants)
- Three trials [8-10] compared Remdesivir with standard of care (6153 participants). Of these, one trial [8] had two arms with different durations of Remdesivir (5 days arm and 10 days arm) compared with a standard of care arm (596 participants).
Our expert working group classified progression to oxygen, non-invasive ventilation (NIV) and invasive mechanical ventilation (IMV) as critical outcomes and mortality, time to clinical improvement and thrombotic or secondary infections as important outcomes. However, as the situation in the country evolved, the guidelines group upgraded mortality, time to clinical recovery and time to clinical improvement as critical outcomes, as an effect on those could reduce pressure on the overwhelmed health system.
Critical (primary) Outcomes
As presented in the ‘Summary of findings’ table, the evidence is of low or very low certainty about the effect of Remdesivir on mortality, progression to non-invasive ventilation or high flow oxygen, progression to invasive mechanical ventilation, time to clinical recovery (not requiring oxygen or hospitalised care) and adverse events leading to drug discontinuation.
(a) All-cause mortality: Low certainty of evidence in 7400 patients in five RCTs [7-11] found little or no difference between Remdesivir and placebo or standard of care (RR 0.94; 95% CI 0.82 to 1.07).There were no significant differences observed even when trials were stratified by severity, risk of bias or steroid usage.
(b) Time to clinical recovery (time to achieve WHO score 1,2,3 or not requiring hospitalization for oxygen or medical care): In 1459 patients from two RCTs [8;11], the hazard ratio (HR) for reduction of time to clinical recovery in participants receiving Remdesivir compared with those receiving placebo or standard of care was 1.22 (95% CI 1.06 to 1.41). The evidence was of very low certainty. The median time reported in two studies ranged from 6-10 days in the Remdesivir group vs 7-15 days in the control groups (See Results Table 1 for results from each trial). A mean difference to quantify the magnitude of potential benefit was not available.
(c) Time to clinical improvement (>2 point reduction in the WHO ordinal score): Low certainty evidence from 1688 participants in three RCTs [7;8;11] suggested that Remdesivir may decrease time to clinical improvement (HR 1.25; 95% CI 1.12 to 1.40). The median and IQR ranged from 6-21 days in the Remdesivir group vs. 6-23 days in the control group. (See Results Table 1 for results from each trial). A mean difference to quantify the magnitude of potential benefit was not available.
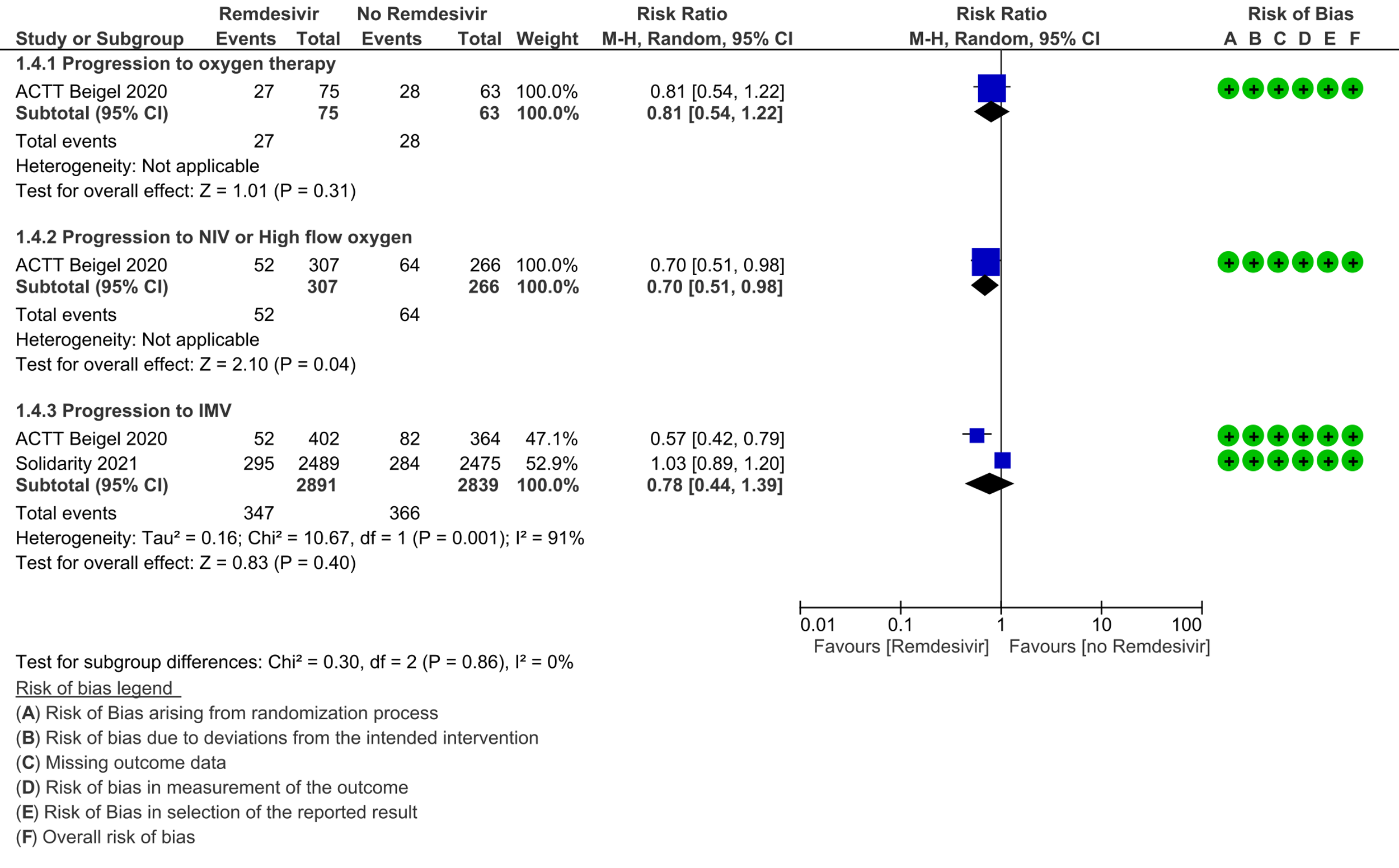
(d) Progression to oxygen therapy: Low certainty evidence in 138 patients from one RCT [11], found that Remdesivir may make little or no difference in progression to oxygen therapy vs. standard of care (SOC)/placebo (RR 0.81; 95% CI 0.54 to 1.22).
(e) Progression to non-invasive ventilation (NIV) or high flow oxygen (HFO): Evidence from 573 patients in one RCT [11] found that Remdesivir may reduce progression to NIV or HFO (RR 0.70; 95% CI 0.51 to 0.98) and varied as widely as 2% to 49% with small sample size and few events.
(f) Progression to invasive mechanical ventilation: The evidence from 5730 patients in two RCTs [9;11] found that the effect of Remdesivir on progression to invasive mechanical ventilation was very uncertain (RR 0.78; 95% CI 0.44 to 1.39).
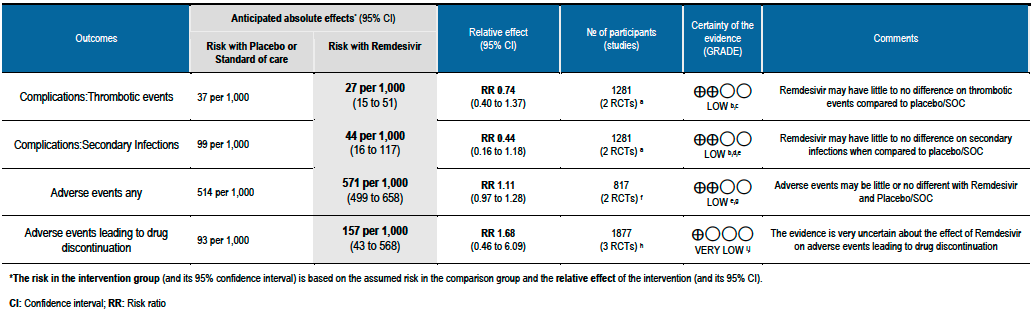
(g) Adverse events: Low certainty evidence from 817 patients in two RCTs [7;8] revealed Remdesivir may contribute to few or no additional adverse events as compared to placebo (RR 1.11; 95% CI 0.97 to 1.28). In addition, very low certainty evidence from 1877 patients in three RCTs [7;8;11] were found regarding the effects of Remdesivir on adverse effects leading to drug discontinuation (RR1.68; 95% CI 0.46 to 6.09).
(h) Duration of hospitalization: This was reported in two studies as a median and hence a meta-analysis could not be performed. Wang 2020 [7] reported a median (IQR) of 21 (12 to 31) days in the Remdesivir group vs. 21 (13.5 to 28.5) days in the SOC or placebo groups. ACTT Beigel 2020 [11] reported median (IQR) as 12 (6-28) days vs. 17 (8-28) days in the SOC/placebo groups.
Important (secondary) outcomes:
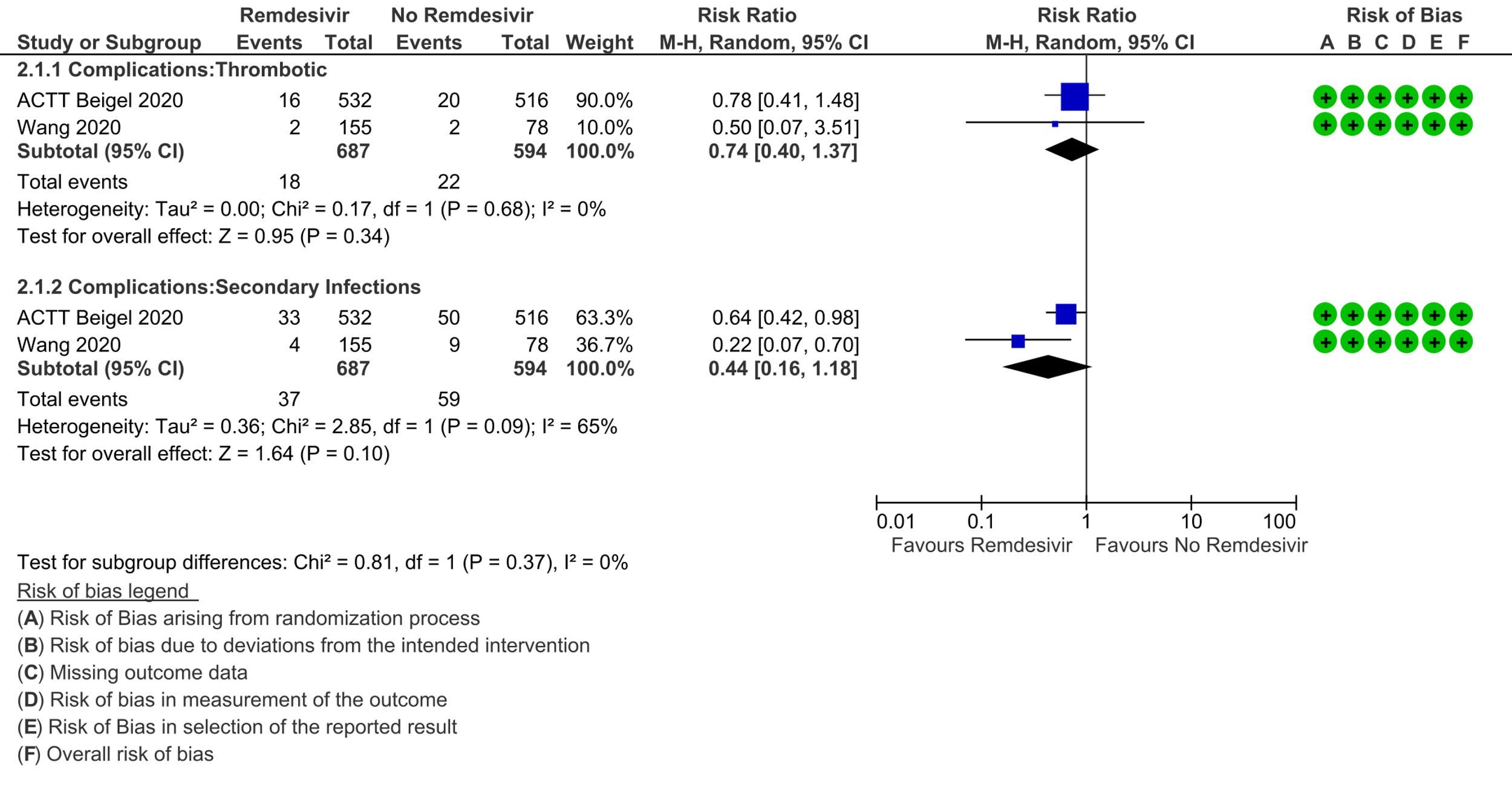
(1) Thrombotic events: Low certainty evidence from two RCTs [7;11] in 1281 participants suggests that Remdesivir may make little or no difference to occurrence of thrombotic events when compared to placebo or SOC (RR 0.74; 95% CI 0.40 to 1.37).
(2) Secondary infections: Low certainty evidence from two RCTs [7;11] in 1281 participants suggests that Remdesivir make little or no difference to occurrence of secondary infections when compared to placebo or SOC (RR 0.44; 95% CI 0.16 to 1.18).
No comparative data could be extracted for time to negative PCR; or post-acute COVID-19 pulmonary function/fibrosis or other sequelae.
Subgroup analysis
A subgroup analysis was attempted as pre-specified by the Expert Working Group.
- In two RCTs [7;9] with 1,463 participants, in the subgroup with pneumonia but no hypoxia Remdesivir did not show a mortality benefit (RR 0.85; 95% CI 0.42 to 1.72).
- In one RCT [7] Remdesivir showed no mortality benefit when participants were stratified by shorter duration of symptoms (<10 days; RR 0.76; 95% CI 0.29 to 1.95) or ≥10 days of symptoms (RR 1.48; 95% CI 0.45 to 4.88).
Disaggregated data could not be obtained for co-morbidities, inflammatory markers or different age groups. Subgroups of pregnancy, lactation, renal failure, liver failure were excluded from most trials. No data were available separately for immunocompromised individuals in any of the trials.
Results Table 1: Time to clinical improvement and recovery
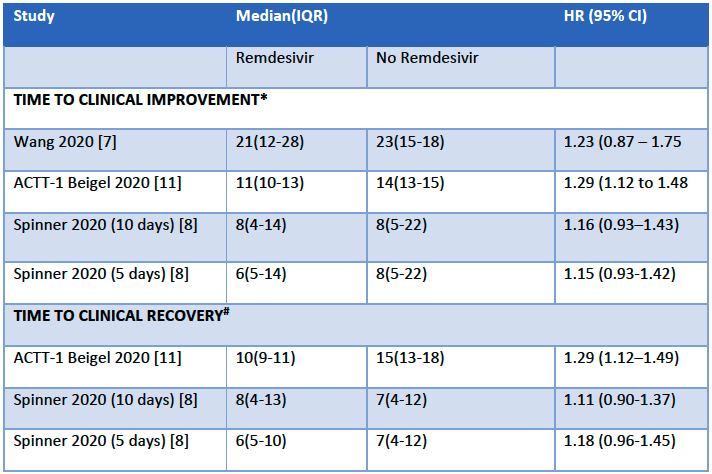
*Time to clinical improvement was defined as the time to a two-point reduction in patients’ admission status on a six-point ordinal scale
#Time to clinical recovery was defined as the time to being non-hospitalized, or hospitalized with no oxygen or medical care requirement
| Study | Intervention | Design | Location; | Age, | No | Participant |
|---|---|---|---|---|---|---|
| ACTT | Remdesivir (200 mg loading dose on | Double | USA, | Remdesivir: 58.6 Placebo: | Remdesivir: 541 Placebo: | Patients ≥18 years |
| Mahajan | Remdesivir 5 days (200 mg on day 1, 100 four | Open | India; | Remdesivir: 58.08 SOC: | Remdesivir: 10 SOC: | Patients All in the study had radiographic evidence of |
| Solidarity | Remdesivir (200 mg on day 0 and 100 | Open | Worldwide; Inpatients | Not | Remdesivir: 2750 SOC: | Patients ≥18 years old inpatients with Covid-19 |
| Spinner | Remdesivir 5-day and 10-day course (200 | Open | US, Europe and Asia; Inpatients | Remdesivir 10-day arm: 56 Remdesivir 5-day arm: 58 SOC: | Remdesivir 10-day arm: 197 Remdesivir 5-day arm: 199 SOC: | Patients Hospitalized patients>12 years with |
| Wang | Remdesivir (200 mg on day 1 | Double | China; | Remdesivir: 66·0 Placebo: | Remdesivir: 158 Placebo: | Patients ≥18 |
1. Mortality, all-cause: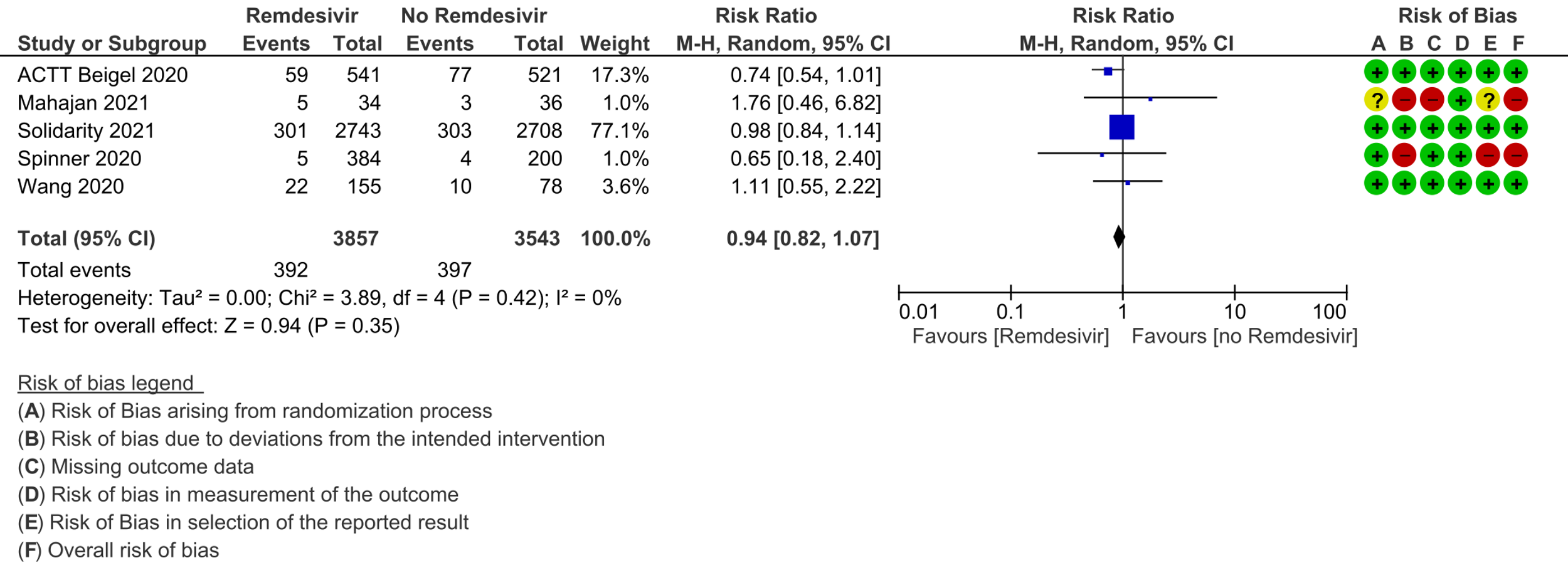
2. Time to clinical recovery:
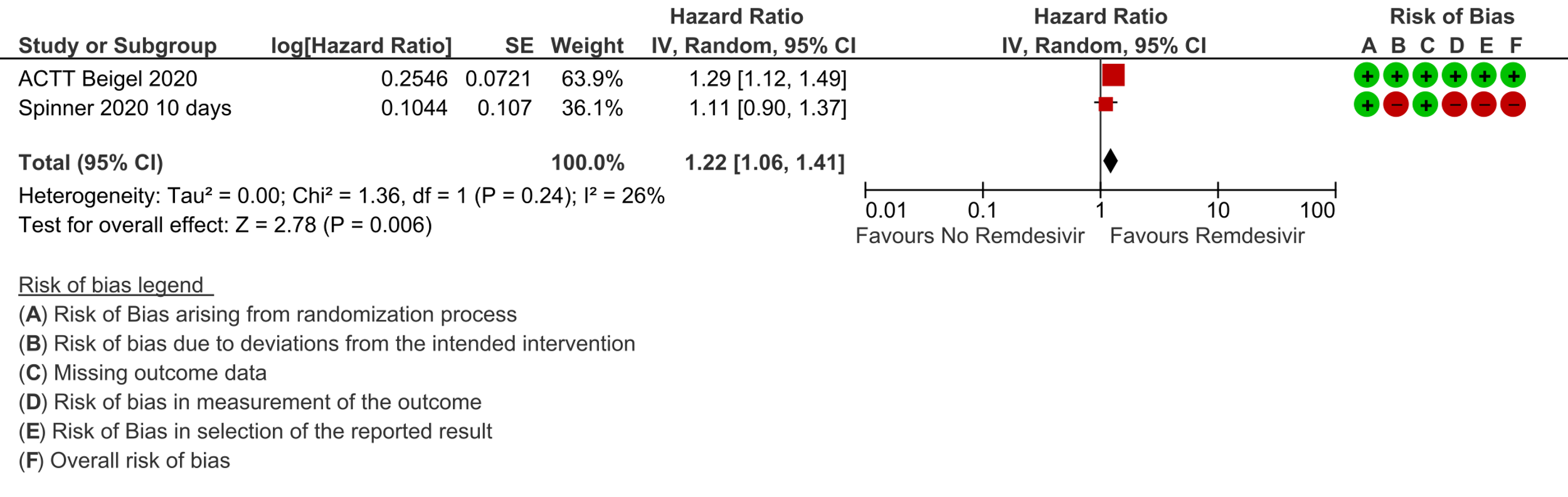
3. Time to clinical improvement:
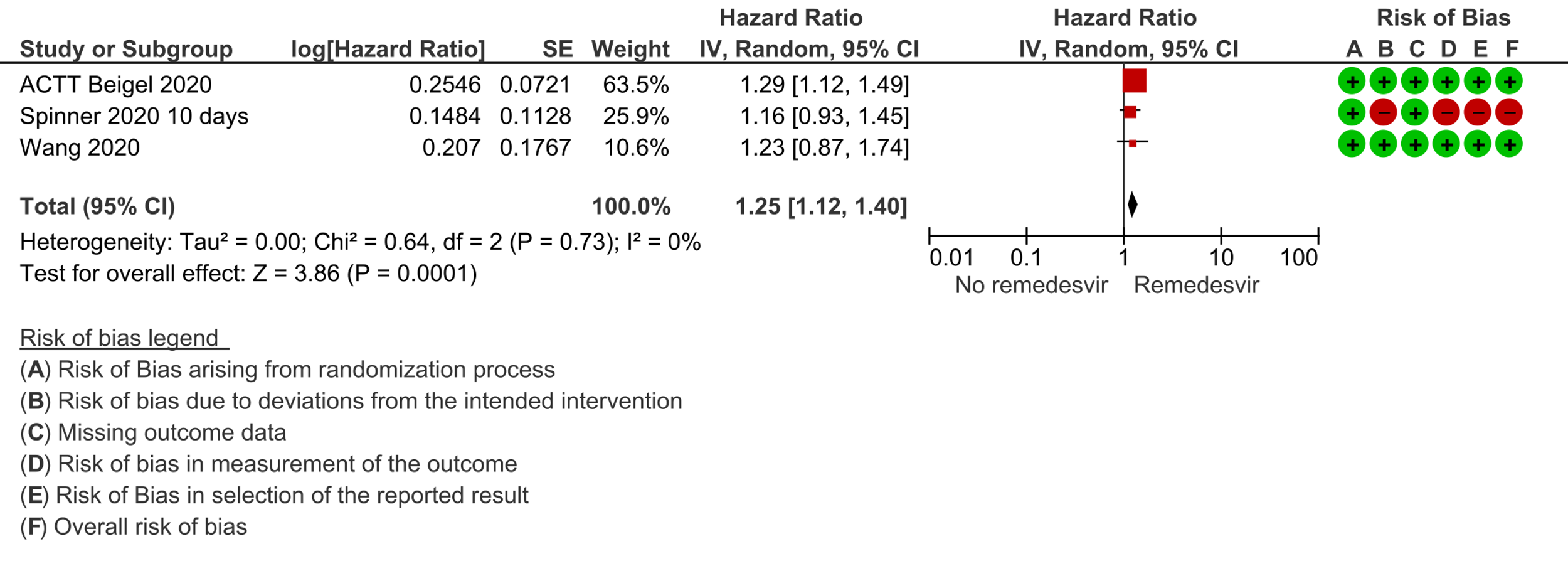
4. Progression to oxygen therapy / 5. Progression to non-invasive ventilation/high-flow oxygen / 6. Progression to invasive mechnical ventilation:
7. Complications: thrombotic events / 8. Complications: secondary infections:
9. Any adverse events:
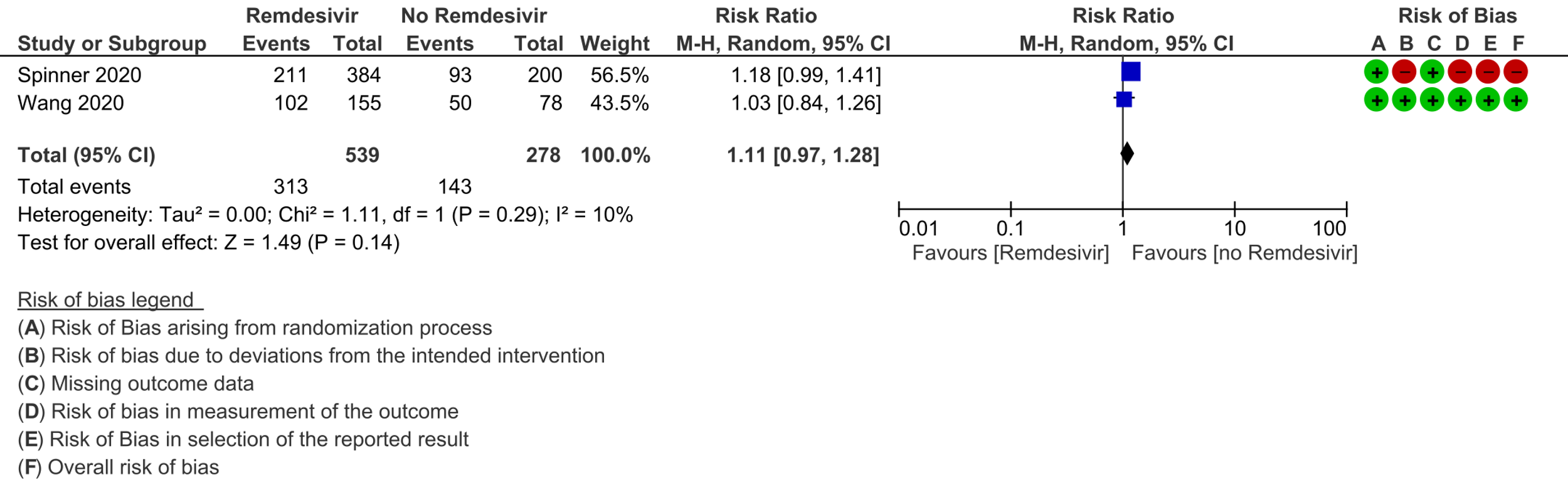
10. Adverse drug reactions leading to drug discontinuation:
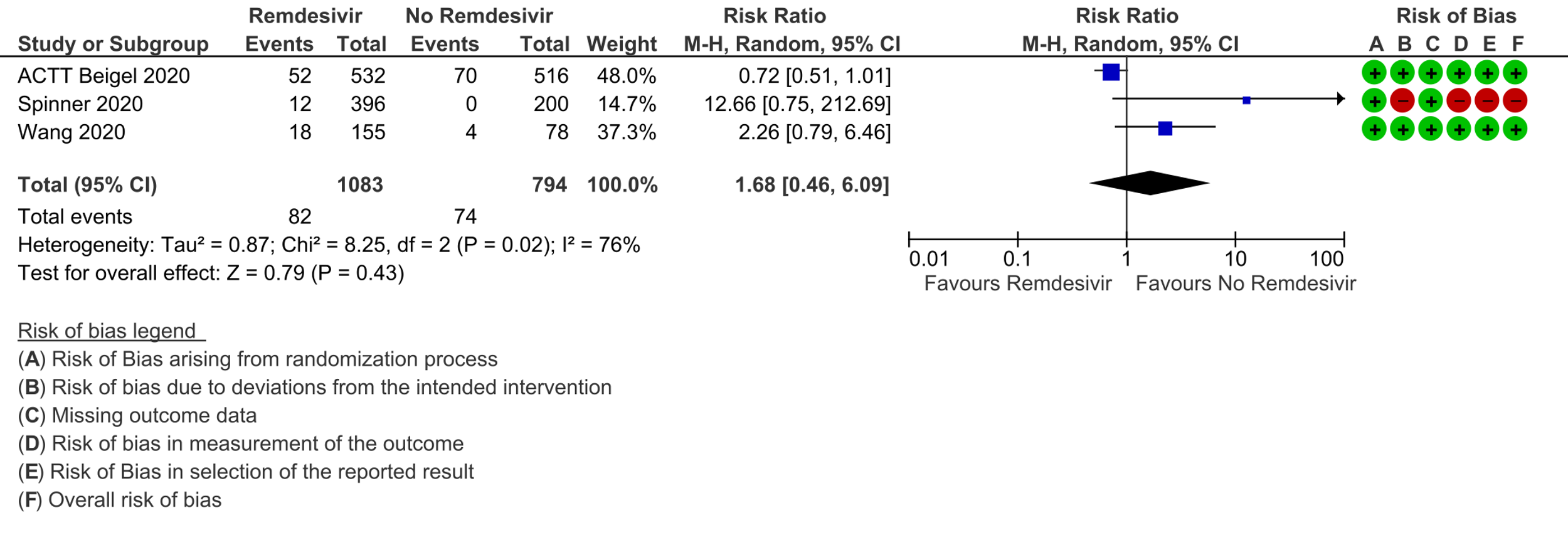
The Antiviral Expert Working Group met on 14th May 2021 to consider remdesivir as a treatment for COVID-19. Conflict of interest declarations were reviewed by the Steering Committee; none were found to be relevant to remdesivir.
A summary and then more detailed explanations of the Expert Working Group's judgements follow.
Summary of judgements
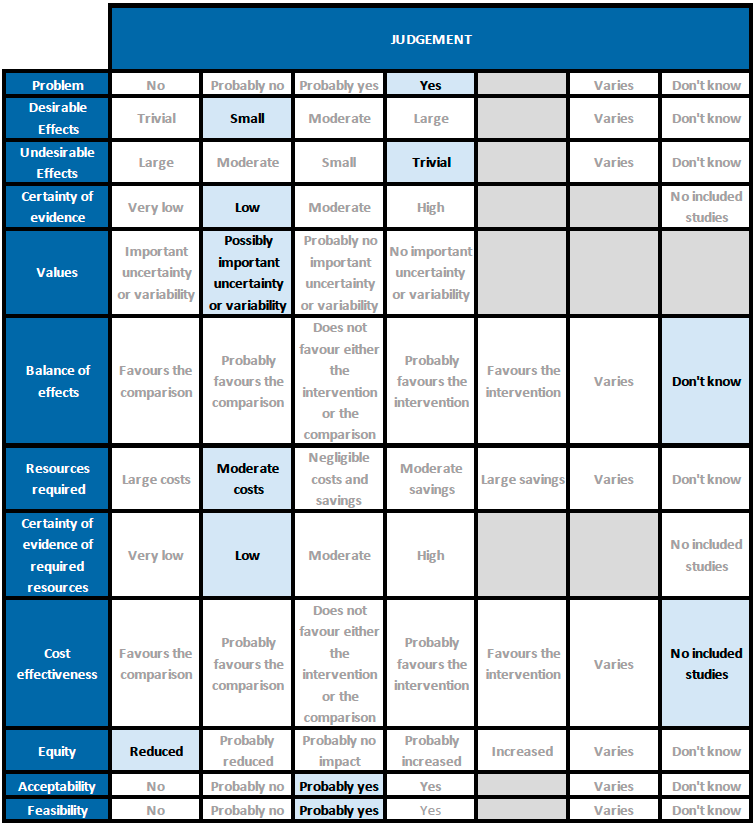
Problem
The COVID-19 pandemic in India with more than 25 million cases and over 0.3 million deaths has significantly impacted and stressed the health structure of the country. With a shortage of intensive care unit beds, oxygen and trained personnel the country is facing a major health crisis. This has prompted many irrational treatment interventions which are neither beneficial nor cost effective. Remdesivir, though touted as an antiviral with possible benefit in the early viral replication phase of the disease, has not lived up to its promise. However, it continues to be prescribed widely and often at exorbitant costs due to lack of availability. The group judged the problem to be of utmost priority.
Desirable effects
The group agreed that Remdesivir may have no effect on mortality and progression to oxygen therapy or invasive mechanical ventilation. They noted that it may reduce progression to NIV/high flow oxygen. The group agreed that that the hazard ratio for the time to clinical improvement (2 point reduction in WHO ordinal scale) may favour Remdesivir over placebo/SOC, however the magnitude of this reduction in days was unclear. The effect of Remdesivir on time to clinical recovery (time to discharge or not requiring oxygen/medical care) was very uncertain.
The group also noted that the early trials did not use steroids as much as they are used now. In addition, the median duration of oxygen requirement was around 3 weeks in the ACTT-1 trial (11), which is quite prolonged compared to what we have seen more recently suggesting that treatment protocols have since evolved. They also noted that there were some potential differences in subgroups of oxygen/respiratory support within a single trial but this was not reflected in the pooled subgroup analyses. Overall the benefit provided by Remdesivir over and above steroids was unclear and indicates a possible niche for further trials. It may reduce time to clinical improvement which does have implications for a health system which is overwhelmed with a shortage of supplies and hospital beds. Taking these factors into consideration, overall the group members suggested a small benefit for Remdesivir.
Undesirable effects
Adverse events were not different between groups, nor were there adverse events leading to discontinuation, suggesting this drug may be safe. The group noted evidence was lacking in pregnancy, renal and liver disease and overall judged the undesirable effects as trivial.
Certainty of evidence
The group felt that there was low to very low certainty in the evidence for all outcomes, including those judged as critical.
Values
The group felt that there is possibly important uncertainty or variability regarding values. The time to clinical improvement and progression to NIV/high flow oxygen were the only outcomes that favored Remdesivir over placebo/SOC. Though the certainty of evidence was low regarding the effect estimate, the group agreed that stakeholders (for e.g., individual patient, health care providers, public or private health systems etc.) may value the beneficial effect of Remdesivir on these outcomes differently.
Balance of effects
The group discussed at length regarding the balance of effects and judged that at present with the evidence available the balance of effects was unknown. The benefit extended only to time to clinical improvement but even the effect size was small with an unclear magnitude of benefit with very low to low certainty of evidence. Panel members were clear they were not worried about adverse events. It is quite safe and has been extensively used in the country with no signal of harm. They felt the reduction of time to clinical improvement could have indirect benefits as it could free up the resources like hospital beds, oxygen supplies and also indirectly impact patients' risk of nosocomial infections and other complications. However all these benefits need to be weighed against the presence of very low evidence of clinical benefit.
Resources required
The group felt that the costs were moderate. The erratic supply chain has led to an increased demand for this drug with uncertain clinical benefit thus driving up costs. In addition being an intravenous drug and mostly advised for those with moderate to severe disease it has led to a situation where people need to be admitted to be given this drug thus adding hospitalization costs. The group includes clinicians in different types of Indian hospitals who have a good idea of drug and hospitalization costs.
Certainty of evidence of required resources
No studies reporting this were reviewed by the group.
Cost effectiveness
The group noted the lack of data from available cost effectiveness studies relevant to the Indian context. There are only benefits in time to clinical improvement and progression to NIV/high flow oxygen which may result in early discharge and save hospital beds, supplies and costs; however this was low certainty evidence. This highlights the uncertainty regarding cost effectiveness that requires evidence from new clinical studies specific to the Indian context.
Equity
At this point in time this intervention reduces equity due to cost and need for hospitalization. In addition, availability is currently an issue, which forces people to obtain this drug via illegal means and further reduces equity for a drug of questionable benefit. It is possible that in future cost or availability may not be an issue.
Acceptability
The group felt that this intervention is likely to have wide acceptance by all the relevant stakeholders (policy-makers, patients and clinicians), if further research proves its efficacy in the absence of increased adverse events.
Feasibility
This is a feasible intervention in the moderate to severe groups requiring oxygen as they are likely to need hospitalization by virtue of oxygen support. However, feasibility could be impacted significantly by availability.
Remdesivir should ONLY be considered in a narrow subset of patients requiring low-flow oxygen as it may reduce time to clinical improvement saving hospital resources for sicker patients. The signals of benefit in terms of reducing length of illness are modest, and these trials were carried out before corticosteroids became standard of care, therefore we do not know whether this small benefit of Remdesivir persists when patients requiring oxygen are also given steroids.
Clinicians may still choose to use Remdesivir given the evidence has not excluded the possibility of benefit but the possibility of harm remains. Given, low to very low certainty of evidence of benefit from Remdesivir, due consideration needs to be given to costs incurred and deviation of attention from the interventions and supportive care which have proven mortality benefit in the management of severe COVID-19 for an intervention of uncertain benefit. Additionally, patients require admission to hospital for administration of this intravenous drug.
The current recommendation is based on the evidence to date and will be updated as new evidence emerges.
The group carefully considered a potential subgroup effect across patients with different levels of oxygen and ventilation requirement (i.e. measures of severity), early administration of Remdesivir and utility in non-severe patients.
(a) Oxygen requirement: For this analysis, the participants were stratified as: no supplemental oxygen requirement, low flow oxygen, high flow oxygen and non-invasive ventilation and invasive mechanical ventilation. A single trial [11] in which fewer than 25% of participants were on steroids reported a mortality benefit in those on low-flow oxygen with Remdesivir. However, this apparent benefit from a subgroup analysis, could be the result of chance, as the meta-analysis showed no significant difference in mortality across all subgroups. Since the overall certainty of evidence (for any level of severity) was low, the effect of Remdesivir in this subgroup remains very uncertain.
(b) Early administration: Subgroup analysis to determine if Remdesivir benefits those patients who present early (<10 days) was considered, however only one study [7] addressed this and no mortality benefit was reported.
(c) Non severe patients: Two studies [9;11] had included patients with no oxygen requirements which could be extrapolated to a non-severe group; however, there was no mortality benefit noted.
The committee had a priori requested analyses of other important subgroups of patients including children, age > 50 years and those with co-morbidities, but unfortunately there were no data to address outcomes in these subgroups specifically.
The conditional recommendation against the use of Remdesivir will be revisited as new evidence emerges. Along with efficacy data, evidence of undesirable effects should be monitored with widespread use, such as bradycardia, hypotension and liver dysfunction. The certainty of evidence for any adverse events was low and for adverse events leading to drug discontinuation was very low, thus potential drug-related harm has still not been ruled out.
There is considerable uncertainty surrounding the benefit of Remdesivir in moderate to severe COVID-19. Hence there is a need for conduct of well-structured, adequately powered randomized controlled trials to address the following:
- Does Remdesivir have a benefit over and above that conferred by steroids in those requiring oxygen or respiratory support?
- Does Remdesivir offer a benefit in those with moderate COVID-19 not on oxygen/respiratory support but who are at high risk of progression?
- What are the benefits or harms of using Remdesivir in pre-specified subgroups such as immunocompromised, renal failure patients on dialysis, pregnant women and children?
- Eastman RT, Roth JS, Brimacombe KR, Simeonov A, Shen M, Patnaik S, et al. Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19. ACS Cent Sci. 2020 May 27;6(5):672–83.
- Frediansyah A, Nainu F, Dhama K, Mudatsir M, Harapan H. Remdesivir and its antiviral activity against COVID-19: A systematic review. Clin Epidemiol Glob Health. 2021;9:123–7.
- Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Research. 2020 Mar;30(3):269–71.
- Rochwerg B, Agarwal A, Zeng L, Leo Y-S, Appiah JA, Agoritsas T, et al. Remdesivir for severe covid-19: a clinical practice guideline. BMJ. 2020 Jul 30;370:m2924.
- COVID-19 Treatment Guidelines Panel: Coronavirus disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health 2020.
- Organization WH. Therapeutics and COVID-19: living guideline, 31 March 2021. World Health Organization; 2021.
- Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet. 2020 May 16;395(10236):1569–78.
- Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA. 2020 Sep 15;324(11):1048.
- Repurposed Antiviral Drugs for Covid-19 — Interim WHO Solidarity Trial Results. New England Journal of Medicine. 2021 Feb 11;384(6):497–511.
- Mahajan L, Singh AP, Gifty. Clinical outcomes of using remdesivir in patients with moderate to severe COVID-19: A prospective randomised study. Indian J Anaesth. 2021 Mar;65(Suppl 1):S41–6.
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19 — Final Report. N Engl J Med [Internet]. 2020 Oct 8 [cited 2021 May 12]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7262788/
Covid Management Guidelines India Group - Anti-viral Working Group. Remdesivir. Covid Guidelines India; Published online on June 1, 2021; URL: https://indiacovidguidelines.org/remdesivir/ (accessed ).
