RECOMMENDATION: We recommend against the routine use of Remdesivir in all out-patients with 1 risk factor with non severe COVID-19 illness. There is no mortality benefit and it does not reduce progression to intensive care. It may reduce hospitalization or medically attended visits.
DATE OF RECOMMENDATION: 04th May 2022
A conditional recommendation is one for which the panel is less confident about the balance between desirable and undesirable effects of the intervention, or other aspects, such as cost and feasibility.
Definition of mild:
• Symptomatic (any acute COVID-19 related symptoms)
• AND respiratory rate <24/min
• WITHOUT pneumonia or hypoxia
Remdesivir [RDV], a nucleotide pro-drug, an inhibitor of viral RNA polymerase was the only antiviral drug approved in the early phase of the pandemic based on reduction in time to clinical recovery, clinical improvement and faster symptom resolution in mild to severe COVID-19 illness (1). Early treatment of other acute viral infections has shown better clinical outcomes (2). Hence, it has been long suggested that being an antiviral the maximum benefit of Remdesivir would be in early illness before the hyper inflammatory phase has set in. Data available from this single trial (3) with intravenous Remdesivir in out-patients with mild COVID-19 illness did not reach its required sample size. Though this single study has shown that Remdesivir reduces COVID-19 related hospitalizations (RR 0.14; 95% CI 0.03 to 0.59) and medically attended visits (RR 0.19;95% CI; 0.07 to 0.56), the ARR is low and the NNT was 22 to reduce hospitalizations and 17 to reduce medically attended visits. This evidence is low quality at best and in addition has been done in unvaccinated individuals and in those with Omicron or Delta unrelated COVID-19 illness. Hence the generalizability of these benefits is suspect in a population like India where the vaccination coverage is greater than 75% and where the most recent wave has been caused by Omicron in which the baseline risk of hospitalisation is low, the ARR may be lower and NNT higher (4). The 3 days of intravenous infusion therapy of this expensive drug as an out-patient is not a feasible option in resource limited settings as it involves cost, facility and man-power resources to treat a large number of mild COVID-19 infections. With the above information and lack of enough evidence to support its use currently, the panel does not recommend routinely using (conditional recommendation against) using 3 days intravenous Remdesivir for mild COVID-19 with non severe COVID-19. In those with high risk of progression to severe disease an individualized decision may be made by the treating physician in discussion with the patient.
Date of latest search: 02/03/2022
Date of completion & presentation to Expert Working Group: 11/03/2022
Evidence synthesis team: Hanna Alexander, Naveena Gracelin Princy Z, Richard Kirubakaran, Priscilla Rupali and Bhagteshwar Singh
Explanations:
a. Downgraded by 1 level for serious indirectness as the study population is unvaccinated which does not reflect the current scenario
b. Downgraded by 1 level for serious imprecision as optimal information size was not met
c. Downgraded by two levels for serious imprecision as the 95% CI includes appreciable benefit and harm and optimal information size was not met
d. Downgraded by 1 level for serious imprecision as optimal information size was not met
Remdesivir is a monophosphoramidate adenosine analogue prodrug which is metabolized to an active triphosphate form that inhibits viral RNA synthesis via RNA dependent RNA polymerase (2). It was developed as a therapeutic agent for treating RNA-based viruses such as Ebola virus, MERS, SARS-CoV-1 and other zoonotic corona viruses (5). It has demonstrated in vitro and in vivo antiviral activity against SARS-CoV-2 (6). Remdesivir is widely used across the world and a number of guidelines have recommended its use in severe COVID-19 patients (7,8). The WHO living guideline provides a conditional recommendation against the usage of Remdesivir in COVID-19 irrespective of the severity of the disease (9). A number of therapeutic options are available currently for patients with mild covid-19 at high risk for progression to severe disease.
On January 21, 2022, FDA approved a supplemental application for Remdesivir use in adults and children >12 years of age in out-patients with mild to moderate COVID-19 illness at high risk of progression to severe COVID-19[10]. Remdesivir, as a short duration treatment has recently been studied in outpatients with mild covid-19 based on the hypothesis that early treatment for other viral infections have shown to improve clinical outcomes and reduce mortality (12,13,14).
This review aims to provide a summary of the available evidence from randomized clinical trials of Remdesivir for treatment of outpatients with mild COVID-19 for any duration.
We searched PubMed, Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language. We also reviewed reference lists of systematic reviews and included studies. We performed all searches up to 01/03/ 2022.
We searched the above databases and found 31 records. After removing duplicates and excluding that did not match our PICO question, 1 RCTs were included for meta-analysis.
We extracted data for the following outcomes, pre-defined by the Expert Working Group:
- Critical (primary for this review):
- Admission into hospital (covid-19 related)
- Covid-19 related medical visits
- Progression to severe disease- requiring O2, mechanical ventilation or ICU care
- Important (secondary):
- Duration of hospitalisation
- Mortality all cause(28-30 days)
- Time to clinical recovery
- Time to resolution of covid-19 signs/symptoms
- Time to viral clearance(negative PCR)
- Complications of covid-19
- Thrombotic events
- Pulmonary function/ Fibrosis
- Secondary Infections
- Adverse events: All and Serious
Two reviewers independently assessed eligibility of search results using the online Rayyan tool. Data extraction was performed by one reviewer, and checked by another, using a piloted data extraction tool on Microsoft Word. Risk of bias (RoB) was assessed using the Cochrane RoB v2.0 tool, with review by a second reviewer and compared with covid-nma database. If there was a difference in more than one domain it was assessed by a third independent author. We planned to use risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). We planned to meta-analyze if appropriate, i.e., if outcomes were measured and reported in a similar way for similar populations in each trial, using random-effects models with the assumption of substantial clinical heterogeneity (the appropriateness of which would be checked by the Methodology Committee), and the I2 test to calculate residual statistical heterogeneity, using Review Manager (RevMan) v5.4. We used GRADE methodology to make summary of findings tables on GRADEPro GDT.
We found 1 RCT that matched the PICO question as pre-defined by the expert working group.
This double blind randomized controlled trial included a total of 562 participants, of which 30.2% were ≥60 years and 1.4% was below the age of 18 (3). The trial was done across 64 centres in Denmark, Spain, UK and USA from September 18, 2020, through April 8, 2021 and was funded by Gilead Sciences. It compared Remdesivir with placebo in unvaccinated patients with mild Covid-19 and at least one pre-existing risk factor for progression to severe Covid-19 or were 60 years of age or older, regardless of whether they had other risk factor. Remdesivir was administered for a period of 3 days.
The included trial and its results are described below; characteristics of the trials are summarised in the Summary of characteristics of included trials table. Risk of bias for each domain per trial is displayed alongside the forest plots below.
The following comparisons were investigated in the trial (we compared outcomes for arms randomized to Remdesivir vs. standard of care or placebo).
Our expert working group classified admission into hospital (Covid-19 related), Covid-19 related medical visits, progression to severe disease- requiring O2, mechanical ventilation or ICU care as critical outcomes. Duration of hospitalisation, all cause mortality at 28-30 days, time to clinical recovery, time to resolution of Covid-19 signs/symptoms, time to viral clearance (negative PCR), complications of Covid-19: thrombotic events, pulmonary fibrosis, secondary Infections and adverse events: all and serious were designated as important outcomes.
Critical (primary) Outcomes
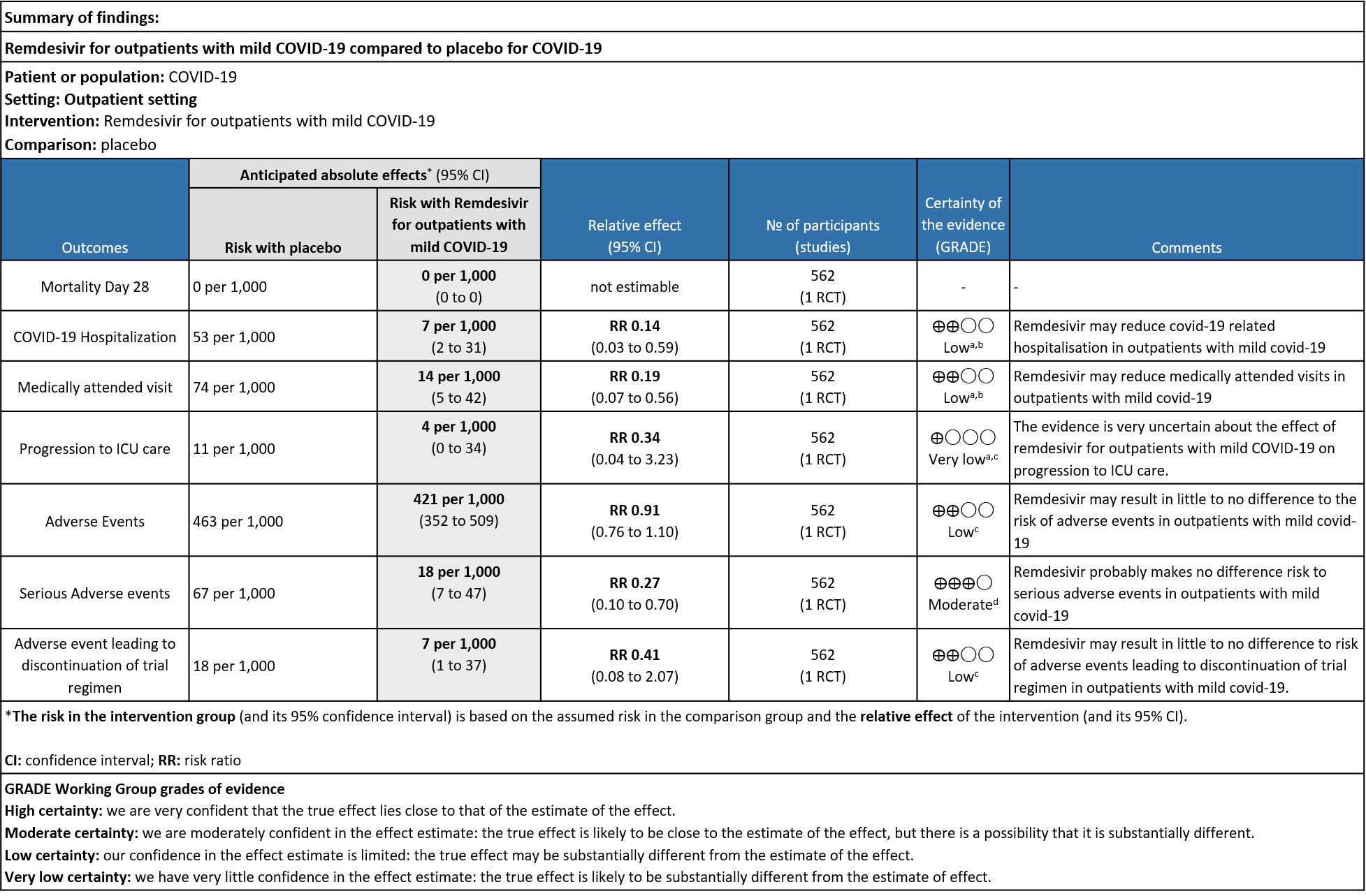
As presented in the ‘Summary of findings’ table, the evidence regarding effect of Remdesivir on covid-19 related hospitalisation, medically attended visits, progression to ICU care and adverse events leading to drug discontinuation is of low certainty but it is moderate certainty for adverse events.
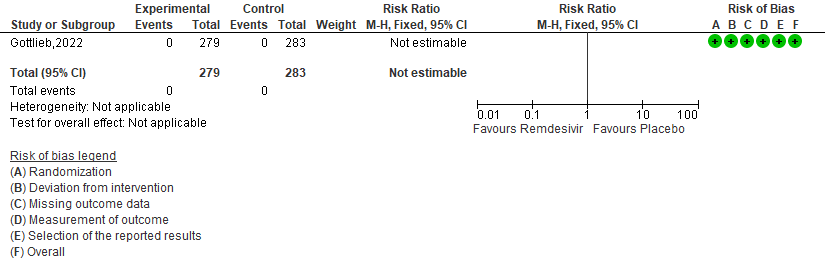
a. All-cause mortality D28: No events reported.
b. Covid-19 related hospitalisation by D28: Low certainty of evidence in 562 patients in one RCT [3] found that the Remdesivir may reduce hospitalisation by Day 28 in outpatients with mild covid-19 [RR 0.14 (95% CI 0.03 to 0.59)]
c. Medically attended visit : Low certainty of evidence in 562 patients in one RCT [3] found that the Remdesivir may reduce medically attended visits day 28 in outpatients with mild covid-19 [RR 0.19 (95% CI 0.07 to 0.56)]
d. Progression to ICU care: Very low certainty of evidence in 562 patients in one RCT [3] found that the evidence is very uncertain about the effect of remdesivir for outpatients with mild COVID-19 on progression to ICU care [RR 0.34 (95% CI 0.04 to 3.23)]
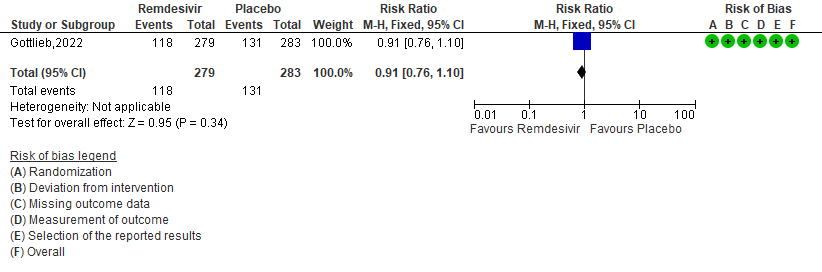
e. Adverse events: Low certainty of evidence in 562 patients in one RCT [3] found that the evidence suggests Remdesivir may result in little to no difference to the risk of adverse events in outpatients with mild covid-19 [RR 0.91 (95% CI 0.76 to 1.10)]
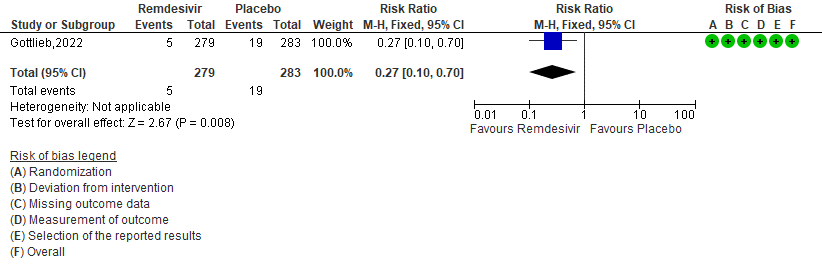
f. Serious Adverse events: Moderate certainty of evidence in 562 patients in one RCT [3] found that Remdesivir probably makes no difference risk to serious adverse events in outpatients with mild covid-19 [RR 0.27 (95% CI 0.10 to 0.70)]
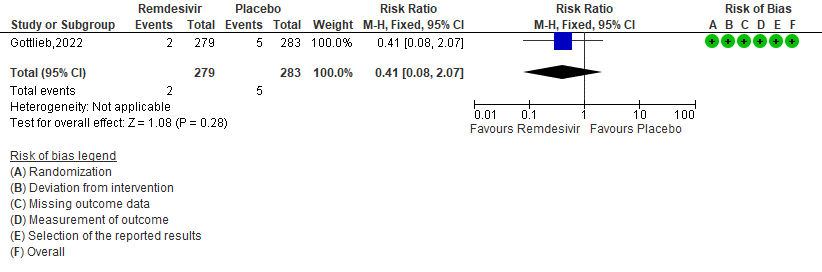
g. Adverse event leading to discontinuation of trial regimen: Low certainty of evidence in 562 patients in one RCT [3] found that the Remdesivir may result in little to no effect on progression to ICU care in outpatients with mild covid-19 [RR 0.41 (95% CI 0.08 to 2.07)]

Primary:
1. Mortality all cause by day 28[3]
2. (COVID-19) Related Hospitalization (Defined as at Least 24 Hours of Acute Care) [3].
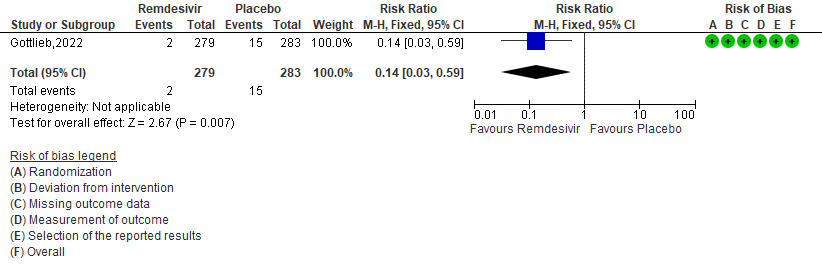
3. Medically attended visits[3]
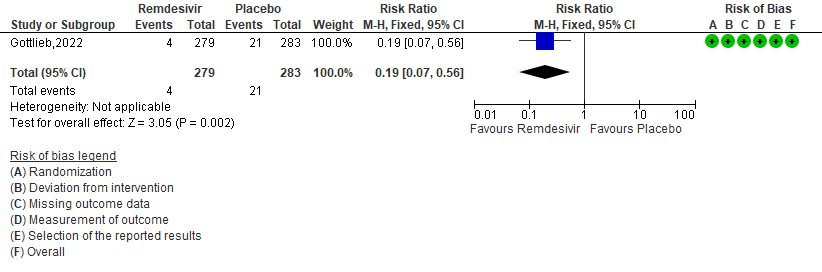
4. Progression to ICU care [3]
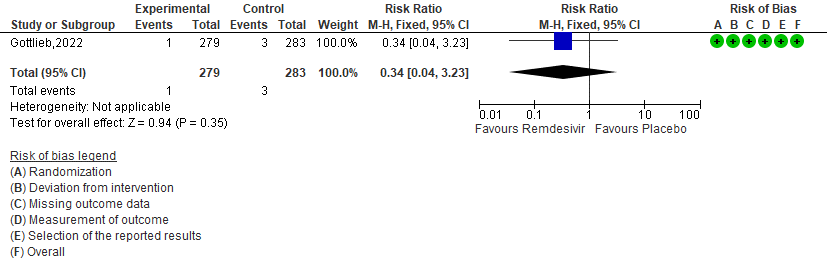
Secondary:
5. Adverse events [3]
6. Serious Adverse events [3]
7. Adverse events leading to drug interruption [3]
The Antiviral Expert Working Group met on 11th March 2022 to consider Remdesivir as a treatment in outpatients with mild COVID-19. Conflict of interest declarations were reviewed by the Steering Committee; none were found to be relevant to Remdesivir.
A summary and then more detailed explanations of the Expert Working Group's judgements follow.
Problem
The COVID-19 pandemic caused by the SARS-COV2 virus has significantly impacted India’s health structure by increasing the morbidity and mortality. Even though around 75% of the population is currently fully or partially vaccinated an effective antiviral to eliminate the virus is lacking. In the recent past, various drugs like Remdesivir were given emergency use authorization to treat COVID-19, but there was no evidence to support its use in outpatients for a shorter duration. It is also anticipated that most cases of covid-19 in the future will be mild and hence the group felt that the problem is not of utmost priority to treat the non-severe group with drugs like Remdesivir. The group voted ‘probably yes’.
Desirable effects
According to the data from study, Remdesivir may reduce COVID-19 related hospitalization (RR 0.14; 95% CI 0.03 to 0.59) and medically attended visits (RR 0.19;95% CI 0.07 to 0.56) but does not prevent progression to ICU (RR 0.34; 95% CI 0.04 to 3.23). Baseline risk of hospitalisation is likely low now in Omicron variant and the group also noted that the study did not reach its required sample size and hence voted that the overall desirable effects as ‘small’.
Undesirable effects
The group noted that in the study included, participants were unvaccinated and disease was caused by a non delta non Omicron strain and hence utility is likely unknown in the current context. The group agreed that according to the data available, Remdesivir has probably has no significant adverse events (RR 0.91; 95% CI 0.76 to 1.10) or serious adverse events (RR 0.27; 95% CI 0.10 to 0.70) and for adverse events leading to discontinuation (RR 0.41; 95% CI 0.08 to 2.07). Hence the group agreed that the undesirable effects of the drug are ‘trivial’.
Certainty of Evidence
Using GRADE methodology, the evidence synthesis team rated the certainty of evidence as low for all outcomes like COVID hospitalisation, medical visit, progression to ICU, serious adverse events and adverse events leading to drug discontinuation; but rated as moderate certainty for all adverse events. The expert working group agreed with these judgments and rated the overall certainty of evidence as ‘low’.
Values
The group also felt that there is ‘probably no important uncertainty or variability’ regarding values. Though the certainty of evidence was low regarding the effect estimate, the group agreed that stakeholders (for e.g., individual patient, health care providers, public or private health systems etc.) may value the beneficial effect of Remdesivir for these outcomes differently. The group also felt that there is very little evidence available in various high risk subgroups of patients.
Balance of effects
The group felt that the even though there is limited and low certainty evidence, the balance of effects ‘probably favours the intervention’ only in this very select subgroup of unvaccinated patients with 1 or more risk factor for severe disease. The panel also commented that it might not be representative of the current scenario since >75% of the population in the country is fully or partially vaccinated and in addition the present strain producing the pandemic is the Omicron variant which mostly causes a mild illness and hence the group also felt that when a recommendation is applied across all patients with mild illness it likely favors the comparison.
Resources required
The group felt that the costs were ‘moderate’. The group includes clinicians in different types of Indian hospitals who have a good idea of drug and hospitalization costs.
Certainty of evidence of required resources
No studies reporting this were reviewed by the group but the clinicians in the group were aware of the cost and hence felt that there was ‘high’ certainty of evidence for required resources to implement this intervention.
Cost effectiveness
The panel discussed and agreed that there was ‘no included studies’ that evaluated cost-effectiveness of Remdesivir in an Indian context.
Equity
At this point in time this intervention would ‘reduce equity’ if found efficacious in Indian settings.
Acceptability
The group felt that the acceptability of this intervention ‘varies’ among relevant stakeholders (policy-makers, patients and clinicians). The group felt that it may not be easy to administer Remdesivir in a peripheral care centre but maybe possible in a hospital as an IV injection as it would depend on the skillset of the available personnel. However, it is definitely cheaper than a monoclonal antibody, more cost effective but does not prevent progression to severe disease.
Feasibility
The group felt that the intervention is ‘probably not’ feasible as an outpatient treatment since this is an IV infusion and could only be delivered by skilled healthcare personnel in an ER bay or in an outpatient infusion nursing facility.
Remdesivir has only low to modest benefit in a narrow subset of unvaccinated non-severe /mild COVID 19 patients with NO hypoxia within 7 days of illness and that too only with regard to hospital admission visits. The role of Remdesivir is also unclear when the infecting strain causes mostly mild disease. Treatment decision for this therapy may be individualized in a small subset of population based on vaccination status, immune-suppression (for which again there is lack of evidence) and based on available resources. Clinicians may still choose to use Remdesivir given the evidence has not excluded the low possibility of benefit but the possibility of harm remains. Given, low to very low certainty of evidence of benefit from Remdesivir, due consideration also needs to be given to costs incurred both for drug administration via the intravenous route either by admission in hospital or being part of a program of at home intravenous drug administration.
Remdesivir may have benefit in reducing hospital admission and visits in unvaccinated mild COVID-19patients with 1 or more risk factors for progression to severe disease. Its benefit in vaccinated young individuals is less clear. Though it stands to reason that it may be beneficial in immunosuppressed individuals this group was underrepresented in the study and it would be difficult to presume benefit here. Hence clinicians may exercise clinical judgement with regard to utility of the same when deciding to use Remdesivir in mild COVID-19 illness.
Where Remdesivir is used, there should be monitoring for undesirable effects which may only be apparent with widespread use. The certainty of evidence for adverse events was low, so potential drug-related harm is not fully resolved, though this appears to be trivial from evidence so far. The current recommendation is based on the evidence to date and will be updated as new evidence emerges.
There is a paucity of evidence on efficacy, safety and cost-effectiveness of Remdesivir to treat mild COVID-19 with the aim of preventing more severe illness. More research is needed to identify which sub-groups of patients with mild disease would benefit the most from treatment e.g. unvaccinated, those with other comorbidities, immunosuppressed etc. The parenteral formulation is a barrier to widespread implementation. Development of an oral formulation would facilitate increased access to Remdesivir for patients with mild disease.
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh M, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC [2020] Remdesivir for the Treatment of Covid-19 — Final Report. N Engl J Med 383:1813–1826. https://doi.org/10.1056/NEJMoa2007764
- Eastman RT, Roth JS, Brimacombe KR, Simeonov A, Shen M, Patnaik S, et al. Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19. ACS Cent Sci. 2020 May 27;6(5):672–83.
- Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. New England Journal of Medicine. 2022 Jan 27;386(4):305–15.
- Nyberg, T., Ferguson, N. M., Nash, S. G., Webster, H. H., Flaxman, S., Andrews, N., Hinsley, W., Bernal, J. L., Kall, M., Bhatt, S., Blomquist, P., Zaidi, A., Volz, E., Aziz, N. A., Harman, K., Funk, S., Abbott, S., Hope, R., Charlett, A., … Thelwall, S. (2022). Comparative analysis of the risks of hospitalisation and death associated with SARS-COV-2 omicron (b.1.1.529) and delta (b.1.617.2) variants in England: A cohort study. The Lancet, 399(10332), 1303–1312. https://doi.org/10.1016/s0140-6736(22)00462-7
- Frediansyah A, Nainu F, Dhama K, Mudatsir M, Harapan H. Remdesivir and its antiviral activity against COVID-19: A systematic review. ClinEpidemiol Glob Health. 2021;9:123–7.
- Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Research. 2020 Mar;30(3):269–71.
- Rochwerg B, Agarwal A, Zeng L, Leo Y-S, Appiah JA, Agoritsas T, et al. Remdesivir for severe covid-19: a clinical practice guideline. BMJ. 2020 Jul 30;370:m2924.
- COVID-19 Treatment Guidelines Panel: Coronavirus disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health 2020.
- Organization WH. Therapeutics and COVID-19: living guideline, 31 March 2021. World Health Organization; 2021.
- Commissioner O of the. FDA Takes Actions to Expand Use of Treatment for Outpatients with Mild-to-Moderate COVID-19 [Internet]. FDA. FDA; 2022 [cited 2022 Mar 15]. Available from: https://www.fda.gov/news-events/press-announcements/fda-takes-actions-expand-use-treatment-outpatients-mild-moderate-covid-19.
- Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med 2020;383:1827-1837
- Mulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med 2019;381:2293-2303
- Muthuri SG, Venkatesan S, Myles PR, et al. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med 2014;2:395-404.
- Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet 2000;355:1845-1850.
Covid Management Guidelines India Group - Anti-viral Working Group. Remdesivir. Covid Guidelines India; Published online on May 4, 2022; URL: https://indiacovidguidelines.org/remdesivir-mild-outpatients/ <date>

