This recommendation applies to acute COVID-19 in adults. Some of our recommendations vary according to the severity of COVID-19 illness. Definitions of the categories are based on the World Health Organization (WHO) criteria and can be viewed by clicking the plus (+) signs below.
RECOMMENDATION: In people with COVID-19, the group recommends respiratory support measures targeting a peripheral oxygen saturation (SpO2) of 94-98%, and not a lower saturation target as there is insufficient evidence and potential harm (Conditional recommendation, very low certainty evidence).
DATE OF RECOMMENDATION: 14th February 2022
A conditional recommendation is one for which the desirable effects probably outweigh the undesirable effects (weak recommendation FOR an intervention) or undesirable effects probably outweigh the desirable effects (weak recommendation AGAINST an intervention) but appreciable uncertainty exists. This implies that not all will be best served by this recommendation and decisions can be made by the patient and caregiver based on patient values, resources and setting with a clear understanding of the ensuing harms and benefits.
Definition of mild:
• Symptomatic (any acute COVID-19 related symptoms)
• AND respiratory rate <24/min
• WITHOUT pneumonia or hypoxia
Definition of moderate illness:
• Pneumonia (clinical or radiological) OR hypoxia (SpO2 <94% in adults with no underlying lung disease)
• AND respiratory rate ≤30/min
• AND SpO2 ≥90% on room air
Definition of Severe illness
Pneumonia with ANY ONE of the following:
• severe respiratory distress or respiratory rate >30/min
• SpO2 <90% on room air
• NO invasive or non-invasive respiratory support needed
Definition of critical:
• Requirement for high-level respiratory support: noninvasive ventilation, high-flow oxygen (≥20 litres per minute) or invasive mechanical ventilation
• OR acute respiratory distress syndrome (PaO2/FiO2 ratio of <300)
• OR sepsis
• OR shock
Patients receiving respiratory support measures must have their SpO2 monitored regularly as oxygen toxicity is a risk with any person on supplementary oxygen.
The group acknowledged that some hospitals and clinicians may set a lower SpO2 target when oxygen supplies are short, but care should be taken to not set a target lower than 90% for patients without underlying risk of hypercapnic respiratory failure (e.g. those with chronic obstructive pulmonary disease & obesity hypoventilation syndrome).
There is a paucity of direct evidence for safe SpO2 targets in patients with COVID-19. In brief the group considered evidence from one systematic review and four individual randomised trials comparing higher vs. lower SpO2 targets in patients with a variety of causes of hypoxic respiratory failure. One of the trials included some patients with COVID-19, for which a post hoc subgroup analysis was conducted1. The four trials did not address the PICO question pre-defined by the group, and varied amongst each other in terms of participant characteristics (including causes of their need for oxygen or respiratory support therapy), interventions & comparator target ranges, and outcomes. They also varied substantially in terms of the methods used to monitor SpO2, including frequency of monitoring, and invasive vs. non-invasive options. Furthermore, the COVID-19 subgroup analysed separately in one of the trials was too small and infrequently monitored to be considered as direct evidence1.
Adverse event measurement and reporting was limited and varied between trials. One trial reported a significantly higher proportion of patients being diagnosed with mesenteric ischaemia in the group with the lower SpO2 target9. The trial used a lower target than that defined by the group (88-92% vs. 90-94%), and this is a life-threatening event plausibly caused by hypoxia. This raises safety concerns about routine use of lower SpO2 targets. Conversely, concerns about oxygen toxicity in other conditions was also noted.
Date of latest search: 13th October 2021.
Date of completion & presentation to Expert Working Group: 29th October 2021.
Date of planned review: 29th April 2022.
Evidence synthesis team: Jefferson Daniel J, Avinash A Nair, Richard Kirubakaran, Priscilla Rupali and Bhagteshwar Singh.
Supplemental oxygen is the key intervention for severely and critically unwell COVID-19 patients. Oxygen is often administered liberally to acutely ill adults, but the evidence of benefit vs. risk of achieving high oxygen saturations in the blood is unclear, though in some conditions risk of harm has been demonstrated. The two essential questions that needed an answer were the SpO2 cut-off point for initiation of oxygen therapy and the ideal target range of SpO2 that should be maintained while on oxygen and other respiratory support therapy. If a patient can be successfully treated at lower targets without complications, unnecessary over-oxygenation and oxygen wastage could be minimised.
During the peak of the second wave of the SARS CoV-2 pandemic, India faced a severe oxygen shortage2-3. The government rapidly increased oxygen supply to various states4.
To preserve oxygen reserves and avoid wastage, various measures were looked at, such as:
- Timing of initiation of oxygen therapy,
- Target peripheral oxygen saturation (SpO2) range with the most clinical benefit,
- Lowest safe target oxygenation range,
- Timing of intubation and ventilation to achieve the lowest oxygen use
In COVID-19, World Health Organization (WHO) recommends that the target SpO2 should be ≥ 90%, and supplemental oxygen should be initiated below this cut-off. While an upper limit was not prescribed, it was understood that the patient should have a SpO2 of 90% or above at all times5. The US National Institutes of Health, British Thoracic Society, Italian Thoracic Society and the ICMR Covid-19 National Taskforce (India) recommend a SpO2 target of 92% to 96%6-8. This is based on the IOTA systematic review and LOCO2 randomized control trial, both based on non-COVID patients9,10. While the IOTA systematic review includes a high-quality meta-analysis, the population and interventions included in the analysis were diverse. In IOTA, substantial weight was given to trials that included patients with myocardial infarction, neurological disorders, trauma and surgery. Most studies included for analysis defined the interventions (conservative/lower SpO2 target vs liberal/higher SpO2 targets) based on a pre-fixed standard fraction of inhaled oxygen (FiO2) delivered to the patient, rather than titration of FiO2 to arrive at a target SpO2 range.
For studies on this topic, we searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Epistemonikos, and the COVID‐19‐specific resource covid19.recmap.org published up to 13th October 2021.
Our population, pre-defined by the Expert Working Group, was inpatients with confirmed or suspected COVID-19 with specific sub-populations: Pre-existing COPD, Obesity, Co-existing cardiac failure (acute/chronic) and Age >65 years.
The intervention was defined as active titration of oxygen delivery or respiratory support by measuring saturation at-least every 6 hours to target a SpO2 90-94%.
The comparison was defined as active titration of oxygen delivery or respiratory support by measuring saturation at-least every 6 hours to target a SpO2 95-100%.
We planned to extract data for the following outcomes, predefined by the Expert Working Group:
Primary outcome:
• All-cause Mortality
• Need for ventilation (IMV or NIV)
Secondary Outcomes:
• Time to clinical improvement (WHO ordinal scale or other definitions)
• Length of stay in hospital
• Duration of days free from invasive ventilation
• Length of stay in critical care
Adverse events:
1. All
2. Serious
3. Hypoxemia-related events:
-
-
- Mesenteric ischemia
- Myocardial infarction/ischemia
-
4. Others as specified by trials/reviews
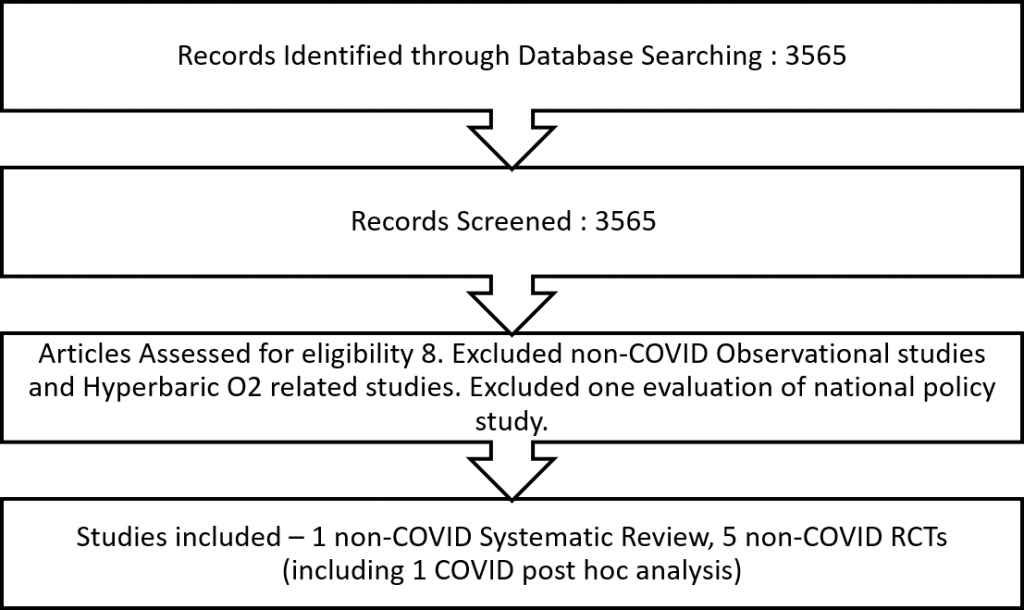
We found the non-COVID IOTA review, five non-COVID RCTs, with one of them performing a post-hoc subgroup analysis of COVID-19 patients.
Two reviewers independently assessed the eligibility of search results. Since all RCTs were non-COVID and the COVID subgroup analysis from the HOT-ICU trial13 was small and underpowered, we did not perform meta-analysis, and described the findings narratively.
Comments on the non-COVID RCTs.
• While all these were ICU based trials, only CLOSE 2016, LOCO2 and ICU ROX trials included mechanically ventilated patients at recruitment. OXYGEN ICU 2016 and the HOT ICU 2021 trials included patients in the ICU irrespective of their need for ventilation.
• The population varied across the trials. The OXYGEN ICU 2016 and CLOSE 2016 trials recruited only 35% of patients with medical causes of respiratory failure, and the rest had surgical causes. ICU ROX 2020 did not mention the nature of the underlying illness and instead provided details on where the patients were brought to ICU from, for example,the emergency ward and operation theatre. 57% of the HOT ICU 2021 trial patients had pneumonia, while the rest had non-pulmonary causes.
• Trials varied in their reporting of the syndrome necessitating oxygen supplementation, such as respiratory failure and acute respiratory distress syndrome (ARDS), and their causes. Most studies had recruited patients with type 1 respiratory failure; while some had ARDS, others were respiratory failure due to other causes. The following were the proportion of patients with documented ARDS in these trials: OXYGEN ICU 2016 (did not report), CLOSE 2016 (33 - 35%), ICU ROX 2020 (67 - 71%), HOT ICU 2021 (12 - 13%), LOCO2 2020 (100%); OXYGEN ICU 2016 (did not report)
• There was indirectness of evidence due to the differences in SpO2 targets in each arm across the various RCTs. None of the trials was close to our predefined definition of intervention (conservative;SpO2 90-94%) and comparison (liberal;95-100%).
• Some studies monitored partial pressure of oxygen from blood gas analysis while other studies used a peripheral SpO2 monitor and some used both. It is well known that cold peripheries, peripheral vascular compromise, abnormal haemoglobin, shock and vasoconstriction can cause differences in correlation between SpO2 and PaO2 at a particular point, which limits comparability between trials using SpO2 and those using PaO2.
• Implemented/resultant SpO2 varied from the target in some trials. However, as the “intervention” is a package of actions to achieve a target, the effect of this is unclear on the directness of the evidence.
• Monitoring of the patients to pick up deviations from the intended interventions was not uniform across studies. While the LOCO2 2020 trial used six-hourly blood gas analysis, HOT ICU 2021 performed blood gas analysis twice-daily.
HOT ICU COVID-19 subgroup analysis.
The HOT ICU 2021 trial was recruiting patients until the latter half of 2020. They had a subgroup of confirmed COVID-19 PCR-positive patients. The authors performed a posthoc analysis of the COVID positive subgroup and published it separately. There was no difference in mortality between the arms with conservative saturation target group (PaO2 60mmHg) compared to the liberal saturation (PaO2 90mmHg) target group. However, the conservative saturation target arm had a significantly lower percentage of life support-free days than the liberal arm. This significant difference was not present when the whole HOT ICU 2021 trial data (including the COVID-19 and non-COVID-19 participants) were analysed together. Here, life support was defined as the use of invasive or non-invasive mechanical ventilation, or continuous positive airway pressure, infusion of vasopressors or inotropic agents, or any form of renal replacement therapy.
HOT ICU post-hoc COVID subgroup analysis.
This subgroup had a sample size of around 100 and was not sufficiently powered to describe the outcomes meaningfully. The oxygenation target was primarily based on twice-daily monitoring of arterial partial pressure of oxygen. Intermittent SpO2 monitoring was done as per the discretion of the treating doctor to adjust the FiO2 to reach the target PaO2 levels. The twelve-hour gap in ABG monitoring could have delayed the adjustment needed in the deviated PaO2 to attain the target range.
• Mortality was around 41% in both arms and was generally higher than other RCTs, however this may be explained by the mortality risk of COVID-19 ARDS earlier in the pandemic. Also, the mortality described is only available for 90 days. Whereas clinically, a one-month mortality would be more useful
• Invasive mechanical ventilation: At recruitment, 44% of the conservative arm and 55% of the liberal arm were already intubated. Around 83% of conservative target arm patients and 97% of liberal target arm group required invasive mechanical ventilation by the end of the study.
• Requirement for intubation (new): 39% of the conservative arm and 41% of the liberal arm needed intubation for the first time after trial recruitment.
Summary:
We did not consider the IOTA systematic review in the evidence contributing directly to this recommendation as the population and definition of interventions were very different from that in our PICO.
The non-COVID RCTs also provided only indirect evidence. One safety concern with lower SpO2 targets emerged from one trial, with mesenteric ischaemia being more common in the group with the lower SpO2 target, though this target (88-92%) allowed lower SpO2 than that considered in our PICO (90-94%). The HOT ICU trial was a post hoc analysis with 109 patients and the data is not powered adequately to make meaningful recommendations.
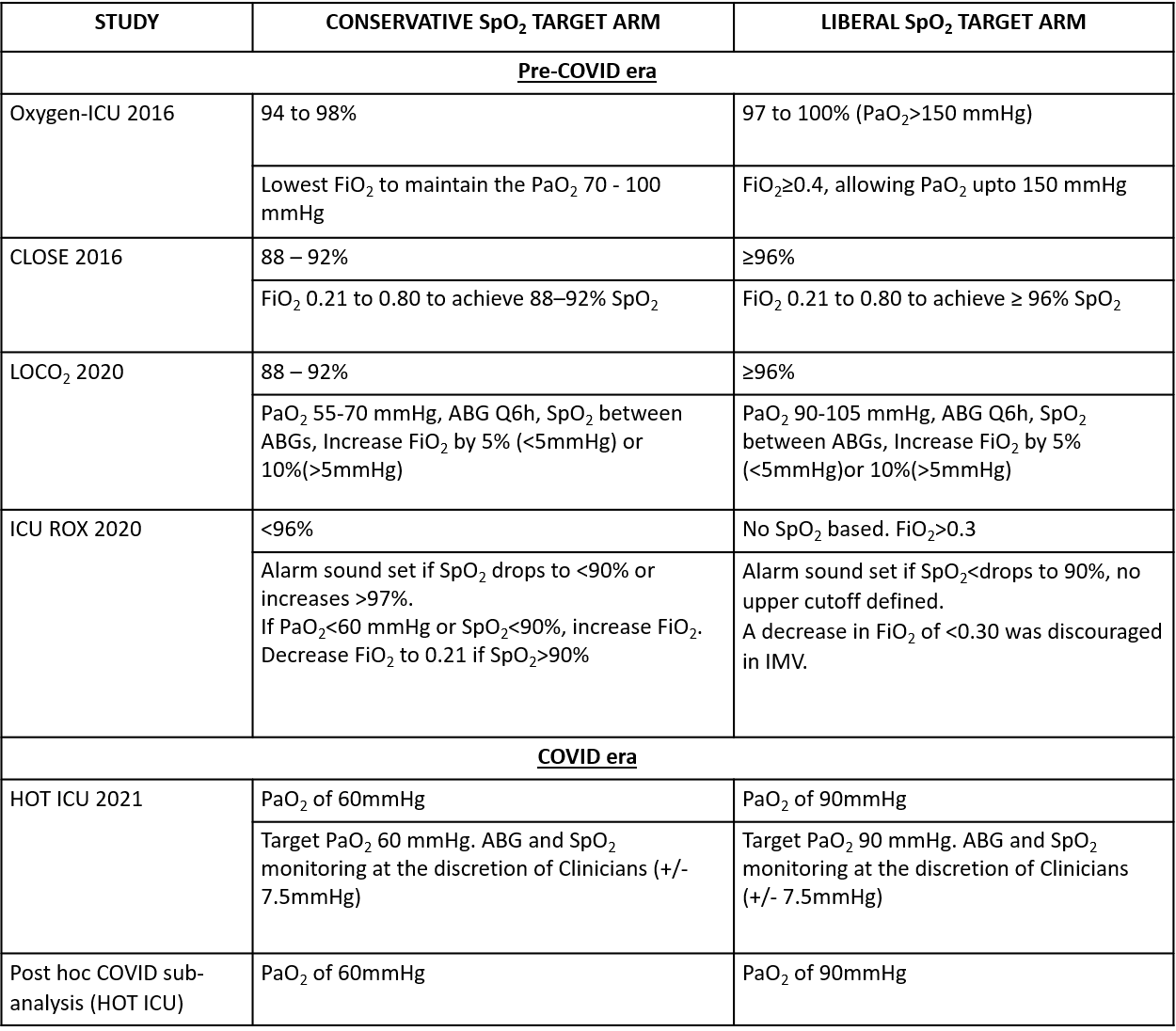
We critically looked at the five non-COVID RCTs and the post hoc subgroup analysis on COVID-19 patients. The definition of the conservative oxygen arm and the liberal oxygen arm was different across the studies (Table 2). The interventions to maintain the desired SpO2 target were also different for each arm and varied across the studies. Table 2 summarizes these essential differences.
1. Intensive care unit (ICU) Mortality
The Oxygen ICU Trial 2016 reported that patients in the arm with a conservative SpO2 target of 94-98% had lower ICU mortality compared to a more liberal target of 97% - 100% SpO2. The CLOSE 2016 trial and the LOCO2 trial also reported ICU mortality, which did not significantly differ between the two arms.
2. 90-day Mortality
The LOCO2 trial 2020 showed that a conservative SpO2 target of 88-92% had higher 90-day mortality than a liberal target of > 97%. CLOSE 2016, ICU ROX 2020 trial and Oxygen ICU 2021 also reported 90-day mortality, which did not significantly differ between the two arms.
3. Length of stay in hospital and ICU
Only OXYGEN ICU 2016 and CLOSE 2016 trials reported length of stay in hospital and length of stay in ICU. Both these outcomes were not significantly different between the two arms.
4. Invasive Mechanical Ventilation-free days
The CLOSE 2016 and the ICU ROX 2020 trials reported the number of invasive mechanical ventilation-free days. Neither trial reported a significant difference between the two arms.
5. Adverse Events
The LOCO2 trial 2020 was prematurely stopped due to mesenteric ischaemia in the conservative (lower) oxygen target arm; 5.1% (1.7%–11.4%) vs 0. The prevalence of arrhythmias, seizures and cerebrovascular events (reported to be stroke) were also higher in the conservative oxygen therapy arm. The HOT ICU 2021 trial also reported mesenteric ischaemia, arrhythmias, seizures and strokes, but they were not significantly different between the two arms.
Table 1: List of RCTs included in evidence synthesis.
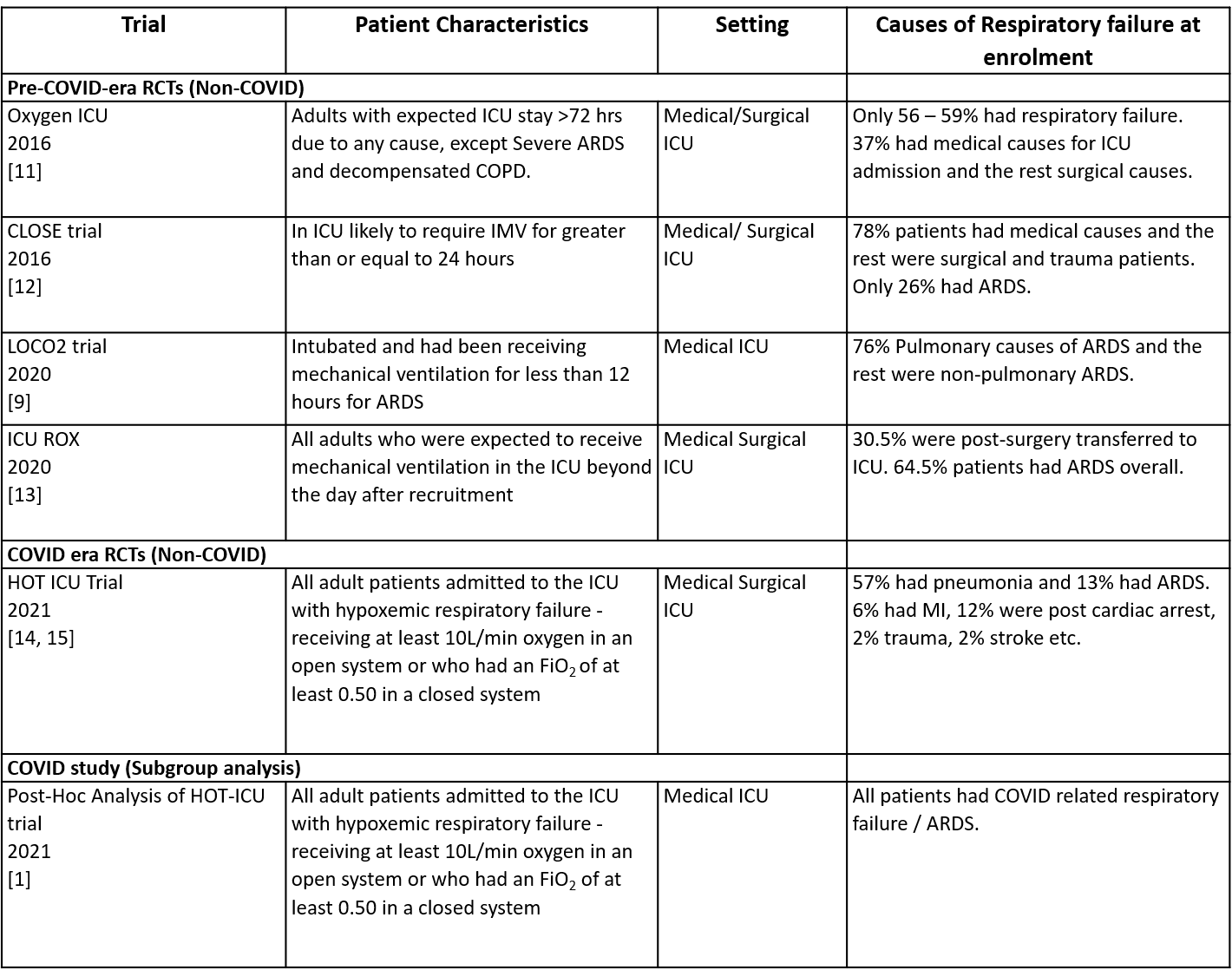
ARDS, acute respiratory distress syndrome; FiO2, fraction of inspired oxygen; ICU, intensive care unit; IMV, invasive mechanical ventilation.
Table 2: Summary of interventions and saturation targets of the RCTs.
The evidence was deemed to be too indirect to populate an ‘evidence to decision’ template for this intervention, but the group applied ‘evidence to decision’ framework principles to the available evidence and the Indian healthcare context to provide a recommendation. Other existing guidelines and the group’s own expertise were considered to formulate the consensus good practice statements.
As mentioned in the Good Practice Statements, most critical care situations will require an oxygen saturation target of 92-96% to ensure adequate tissue oxygenation and prevent oxygen toxicity. In some subgroups like those with chronic obstructive pulmonary disease or obesity hypoventilation syndromes a lower saturation target may be necessitated. Some guidelines including WHO advise targets lower than 94-98%, keeping in mind possible the global oxygen shortage. On synthesis of available evidence, increased adverse events were only seen in the trial with a substantially lower SpO2 target of 88-92%. The group acknowledged that a lower SpO2 target may be applied temporarily in a given facility constrained by the availability of oxygen. However, a target range should not include SpO2 less than 90%, as this is the lowest advised by any of the guidelines reviewed by the group, and as informed by the group’s own expertise. Any lower target must be reviewed regularly (see Monitoring and Evaluation section), and clinicians should be encouraged to assess patients for hypoxia related events and ensure individual safety regardless of this target.
Regarding monitoring, while measuring SpO2 using a fingertip probe is quick, easy, and more widely accessible than invasive measurements; various factors may influence the SpO2 reading, including peripheral vasoconstriction due to any cause, colouring of fingers or nails, the equipment and the method used. Therefore, measurements may need to be re-checked to determine accuracy and a dynamic trend rather than one static reading should inform management decisions. This is especially important when SpO2 readings are not consistent with the clinical picture.
The SpO2 target range provided in the recommendation, as with all of our recommendations, is for non pregnant adults, and should be applied to individual patients after clinical consideration of whether it is applicable in their situation. Target SpO2 ranges vary for some subgroups of people for physiological reasons, none of whom were specifically studied as subgroups in the trials, and most trials excluded some or all of them:
• Pregnancy: Pregnant women require higher targets than non-pregnant adults, with several guidelines recommending maintenance of SpO2 of ≥ 94-95% in women with COVID-19.
• Children: There is a lack of evidence regarding SpO2 targets in children, and while targets are often the same as those for adults, a clinician familiar with managing children should assess and decide the appropriate target for each child.
• Conditions predisposing to hypercapnic respiratory failure: SpO2 targets are often lower in chronic obstructive pulmonary disease (COPD), obesity hypoventilation syndrome, and with various causes of respiratory depression such as neuromuscular disease and acute intoxication with some drugs, due to the risk of reducing ‘hypoxic drive’ leading to CO2 retention. Additionally, older adults may have lower baseline SpO2, so the target SpO2 range may need to take that into consideration based on a primary clinical assessment.
Despite evidence of harm with very high or very low SpO2 across a broad range of conditions, the incidence of adverse events at different SpO2 targets in patients with COVID-19 is not well established. The group recommends a target range which avoid extremes and provides a clinician or hospital the option on deciding on an alternative target range in situations of short oxygen supply keeping individual safety of patient paramount. These patients should be monitored closely for adverse events concerning hypoxia and/or oxygen toxicity.
As found in the evidence review for this recommendation, there is a paucity of trials comparing SpO2 targets in patients with COVID-19, and there is an urgent need to conduct new or report results of ongoing trials. Outside of COVID-19, key subgroups as mentioned in the subgroup considerations section are not recruited in most trials, so future trials should attempt to include these special populations. Additionally, pre-COVID-19 trials included patients with a variety of causes, and ensuring a well powered subgroup of patients with key causes of ARDS, such as pneumonia, may provide more direct evidence for COVID-19 and potential future respiratory infection epidemics.
- BS Rasmussen, TL Klitgaard, A Perner, et al. Oxygenation targets in ICU patients with COVID-19: A post hoc subgroup analysis of the HOT-ICU trial. Acta Anaesthesiol Scand. 2022;66(1):76-84.
- A Thadhani. Preventing a Repeat of the COVID-19 Second-Wave Oxygen Crisis in India | ORF. 2022.
- M Krishnan. COVID: Why is India facing an oxygen shortage? | DW | 04.05.2021. dwcom. 2022.
- N Sharma. Fearing shortage, people rush to buy oxygen now. ET Bureau. 2022.
- COVID-19 Clinical management: living guidance.
- A Machado, Jr., B Finkmoore, K Emodi, et al. Risk factors for failure to complete a course of latent tuberculosis infection treatment in Salvador, Brazil. Int J Tuberc Lung Dis. 2009;13(6):719-25.
- BT Society. BTS Guidance: Respiratory support of patients on medical wards.
- NIO Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. NIH; 2022.
- L Vettoretti, JM Constantin, G Capellier, et al. Liberal or Conservative Oxygen Therapy for Acute Respiratory Distress Syndrome. The New England journal of medicine. 2020;382(11).
- CD K., LH Kim, PJ Young, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet (London, England). 2018;391(10131).
- G M., S Busani, E Damiani, et al. Effect of Conservative vs Conventional Oxygen Therapy on Mortality Among Patients in an Intensive Care Unit: The Oxygen-ICU Randomized Clinical Trial. JAMA. 2016;316(15).
- P R., M Hardie, R Bellomo, et al. Conservative versus Liberal Oxygenation Targets for Mechanically Ventilated Patients. A Pilot Multicenter Randomized Controlled Trial. American journal of respiratory and critical care medicine. 2016;193(1).
- D Mackle, R Bellomo, M Bailey, et al. Conservative Oxygen Therapy during Mechanical Ventilation in the ICU. The New England journal of medicine. 2020;382(11).
- TL Klitgaard, OL Schjørring, T Lange, et al. Lower versus higher oxygenation targets in critically ill patients with severe hypoxaemia: secondary Bayesian analysis to explore heterogeneous treatment effects in the Handling Oxygenation Targets in the Intensive Care Unit (HOT-ICU) trial. British Journal of Anaesthesia. 2022;128(1):55-64.
- Rosborg, B Jannie, ESE Annette, et al. Lower or Higher Oxygenation Targets for Acute Hypoxemic Respiratory Failure. https://doiorg/101056/NEJMoa2032510. 2021.
Covid Management Guidelines India Group - Respiratory Therapy Working Group. Oxygen Saturation targets. Covid Guidelines India; Published online on February 14th, 2022; URL :- Oxygen Saturation Targets – Covid Guidelines India (indiacovidguidelines.org) (<date> ).
