RECOMMENDATION: We recommend against using Interferons for the treatment of COVID-19 illness. Results of trials do not show benefit, but they show probable harm.
DATE OF RECOMMENDATION: 21st March 2022
Definition of mild:
- Symptomatic (any acute COVID-19 related symptoms)
- AND respiratory rate <24/min
- WITHOUT pneumonia or hypoxia
Definition of moderate illness:
- Pneumonia (clinical or radiological) OR hypoxia (SpO2 <94% in adults with no underlying lung disease)
- AND respiratory rate ≤30/min
- AND SpO2 ≥90% on room air
Definition of Severe illness
Pneumonia with ANY ONE of the following:
- Severe respiratory distress or respiratory rate >30/min
- SpO2 <90% on room air
- NO invasive or non-invasive respiratory support needed
Definition of critical:
- Requirement for high-level respiratory support: noninvasive ventilation, high-flow oxygen (≥20 litres per minute) or invasive mechanical ventilation
- OR acute respiratory distress syndrome (PaO2/FiO2 ratio of <300)
- OR sepsis
- OR shock
Interferons exhibit direct inhibitory effects on viral replication and potentiate immune responses involved in clearing of viral infections by multiple pathways. Interferons were one of the many repurposed drugs tried in the treatment of COVID.
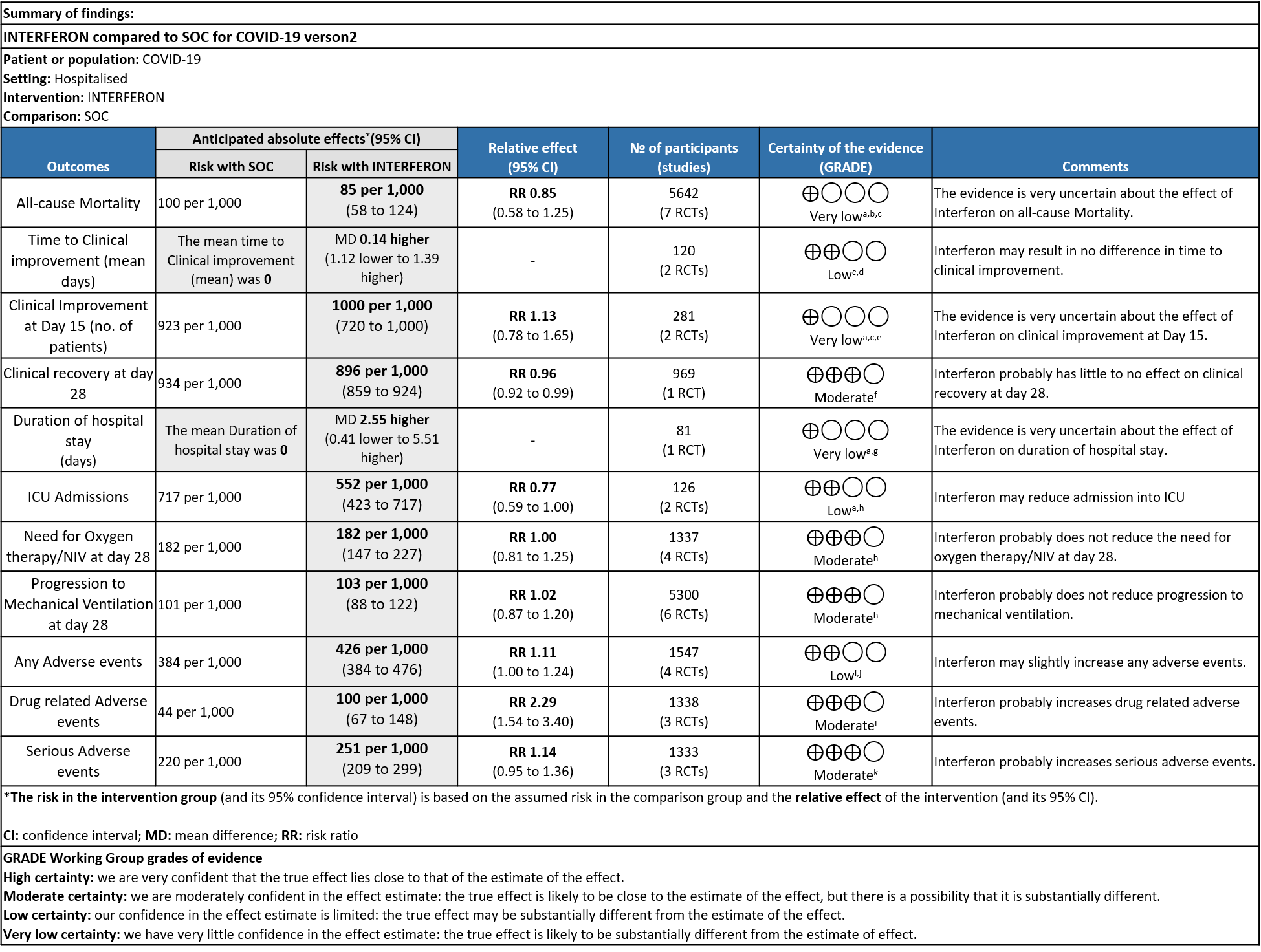
Low to moderate certainty evidence reveals that Interferons do not impact any clinically important outcome in COVID-19 i.e., time to clinical improvement, duration of hospital stay, need for oxygen therapy, ICU admission, need for mechanical ventilation and most importantly, all cause mortality. It should however, be noted that majority of the published studies are relatively old and included interferons as a therapeutic modality in the initial few months of the pandemic. The current day antivirals were not available then and the vast majority of these studies had small sample sizes (the largest study has about 80 patients overall). These factors lead to the conclusion that Interferons were not beneficial in the treatment of COVID-19.
Interferons are relatively expensive with a need for multiple subcutaneous injections. Conflicting evidence exists about the adverse events associated with Interferons. Some studies report a significantly increased risk of adverse and serious adverse events, whereas others do not. In general, interferons have moderate to severe adverse effects which we also know from prior studies in treatment of other viral infections like Hepatitis B and C.1
Therefore, although the level of evidence is low to moderate, given the highly doubtful and inconsistent benefit and the potential for drug related adverse effects, the panel strongly recommends against the use of Interferons in the treatment of COVID 19.
Date of latest search: 01st October 2021
Date of completion & presentation to Expert Working Group: 10th February 2022
Date of planned review: 10th July 2022
Evidence synthesis team: Rini Bandyopadhyay, Selwyn Selvakumar, Jane Miracline, Richard Kirubarakan, Priscilla Rupali.
Explanations:
a. Downgraded by one level serious bias; high ROB
b. Downgraded by one level for serious inconsistency: as I square is 59%
c. Downgraded by one level serious imprecision; wide CI and clinically non significant
d. Downgraded by one level for serious RoB in two studies
e. Downgraded by one level serious inconsistency; I square is 82%
f. Downgraded by one level for serious imprecision; Lower limit of CI overlaps the line of no difference
g. Downgraded by two levels very serious imprecision; Three studies reporting median and IQR with favoring intervention group, Single study reporting Mean and SD favoring control group.
h. Downgraded by one level serious imprecision; wide CI and OIS criteria was not met
i. Downgraded by one level serious inconsistency; I square is 69%
j. Downgraded by one level serious imprecision; Lower limit of CI overlaps the line of no difference
k. Downgraded by one level serious imprecision; wide CI
Interferons are a biological molecule secreted by immune and infected epithelial cells in response to cell injury, viral infections and tumors. It plays a key role as a part of the innate immune response.2-4 They have immunomodulatory and antiviral effects and have been used for the treatment of Multiple sclerosis and Hepatitis C infection.5 Interferons are classified on the basis of receptors as Class I, II and III. With the start of COVID-19 pandemic, multiple repurposed drugs have been tested for its efficacy and Interferons were one of the potential agents in this category.6 In the pre COVID -19 pandemic era, studies have shown the antiviral effects of Interferons on Rhino virus, Respiratory syncytial virus and Influenza virus infections.7 This review aims to provide a summary of the available evidence from randomized clinical trials of Interferons for treatment of COVID-19, which could guide clinicians and researchers regarding the appropriate use of this drug in COVID-19 in the future.
We searched PubMed, Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language. We also reviewed reference lists of systematic reviews and included studies. We performed all searches up to 10th December 2021.
We searched the above databases and found 43 records.
After removing duplicates and excluding that which did not match our PICO question, 12 RCTs were screened and 10 studies assessed for eligibility. One meta-analysis was reviewed; however it included RCTs which were already selected by our search. A total of 9 RCTs were included for meta-analysis.
We extracted data for the following outcomes, as pre-defined by the Expert Working Group:
Primary outcome:
a) All-cause mortality
b) Time to clinical improvement
Secondary outcomes
a) Duration of hospital stay
b) Progression to ICU
c) Progression to O2 therapy/Ventilator
d) Adverse events
Two reviewers independently assessed eligibility of search results using the online Rayyan tool. Data extraction was performed by one reviewer, and checked by another, using a piloted data extraction tool on Microsoft Word. Risk of bias (RoB) was assessed using the Cochrane RoB v2.0 tool, with review by a second reviewer and compared with covidnma database . If there was a difference in more than one domain it was assessed by a third independent author. We planned to use risk ratios (RR) fordichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). We planned to meta-analyze if appropriate, i.e., if outcomes were measured and reported in a similar way for similar populations in each trial, using random-effects models with the assumption of substantial clinical heterogeneity (the appropriateness of which would be checked by the Methodology Committee), and the I2 statistic to calculate residual statistical heterogeneity, using Review Manager (RevMan) v5.4. We used GRADE methodology to make summary of findings tables on GRADEPro GDT.
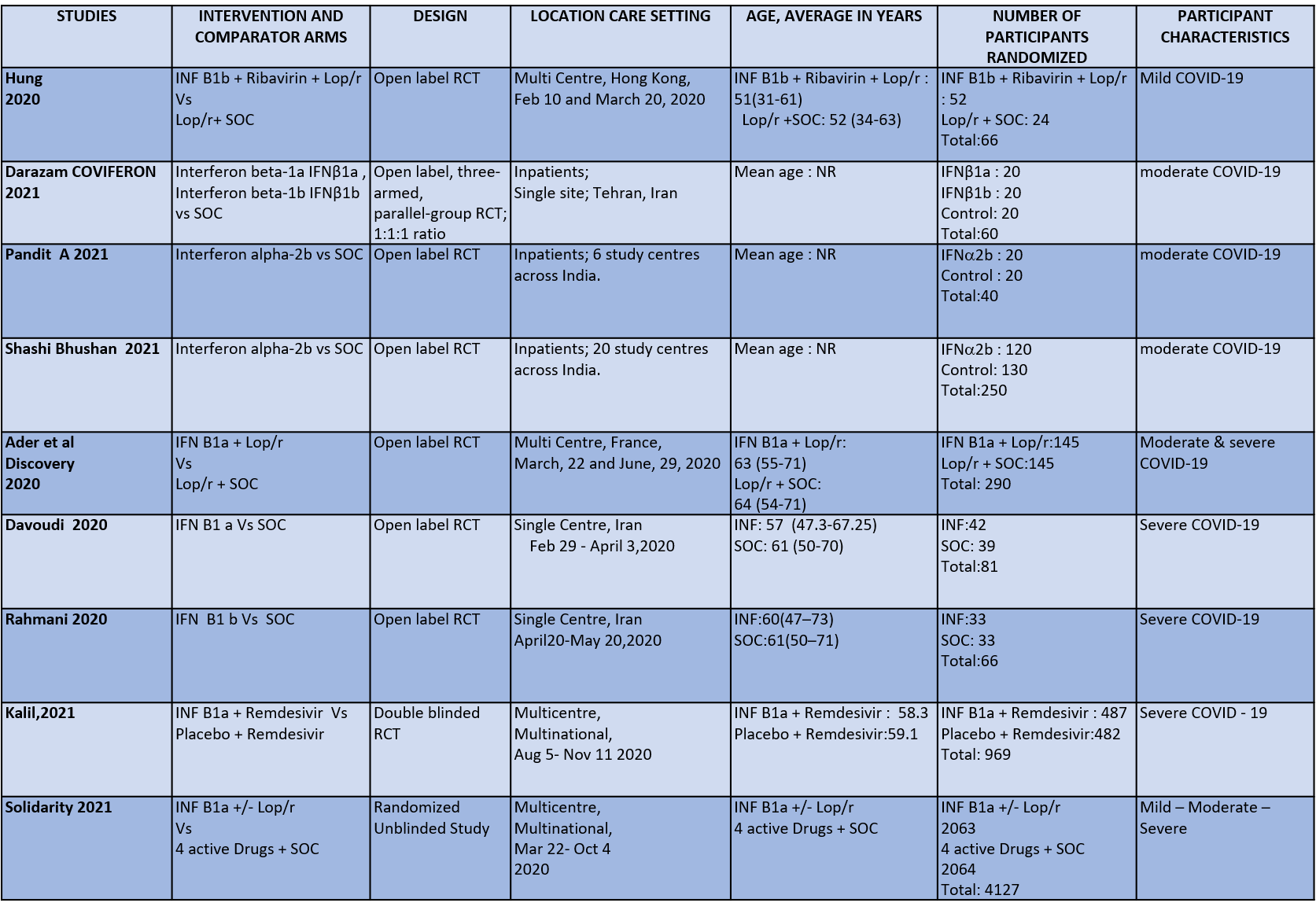
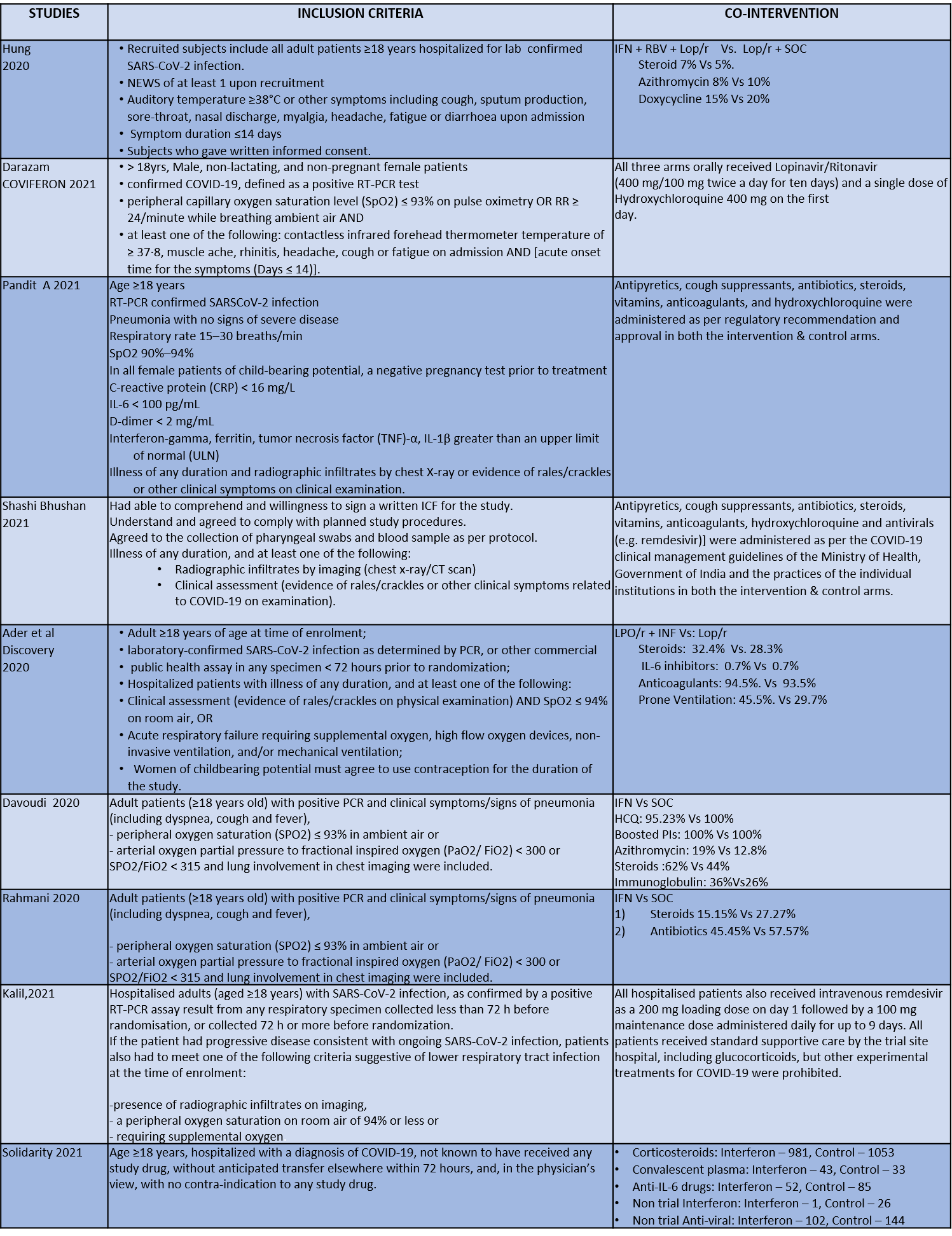
We found 9 RCTs that matched the PICO question as pre-defined by the expert working group. The trials included a total of 5949 participants, all of whom were adults. Two trials were reported from India8-9, three from Iran9-12, two from Russia15,16, and one from France13, all of which were of varying disease severity ranging from moderate to severe. Also, the trials had participants who were enrolled to varying interventions i.e. some studies compared interferon with standard of care or placebo whereas others with active comparators. All trials were done on hospitalized patients with confirmed COVID-19 infection.
Each trial and its results are described below; characteristics of the trials are summarized in the “Summary of characteristics of included trials” table. All the trials had varying risk of bias across all domains. Risk of bias for each domain per trial is displayed alongside the forest plots below.
The following comparisons were investigated in the trials (we compared outcomes for arms randomized to Interferon vs. standard of care or active comparators).
● Five trials8-12 compared Interferon with standard of care(440 participants)
● Four trials13-16 compared Interferon with an active comparator (e.g.: Remdesivir, Ribavirin, Lopinavir/Ritonavir, Tocilizumab)
Our expert working group classified mortality, time to clinical improvement as primary outcomes and duration of hospitalization, progression to oxygen therapy/ventilation or progression to ICU and adverse events as secondary outcomes.
Primary Outcomes
As presented in the ‘Summary of findings’ table, there is low or very low certainty evidence regarding the effect of Interferon on mortality, time to clinical improvement (not requiring oxygen or hospitalised care), duration of hospital stay, progression to ICU care and any adverse events. The evidence is of moderate quality for outcomes of progression to oxygen or ventilation (non-invasive or invasive), all adverse events, drug-related adverse events and serious adverse events.
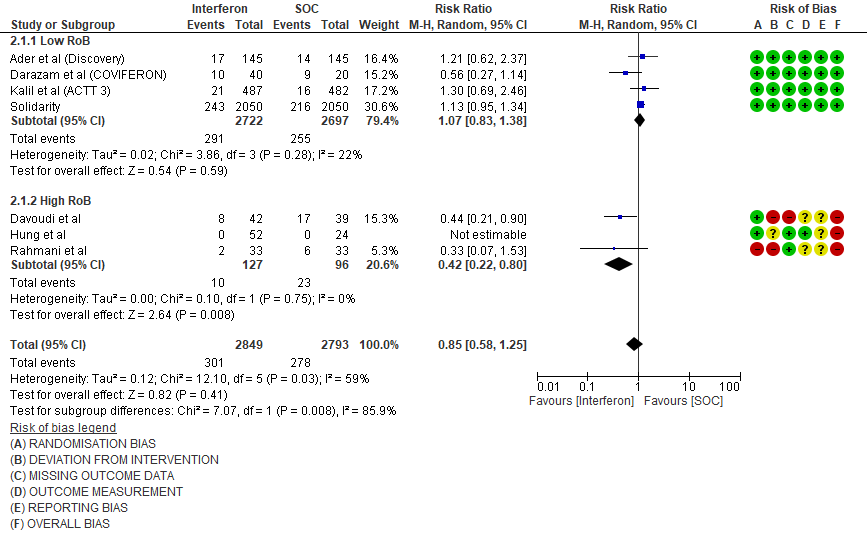
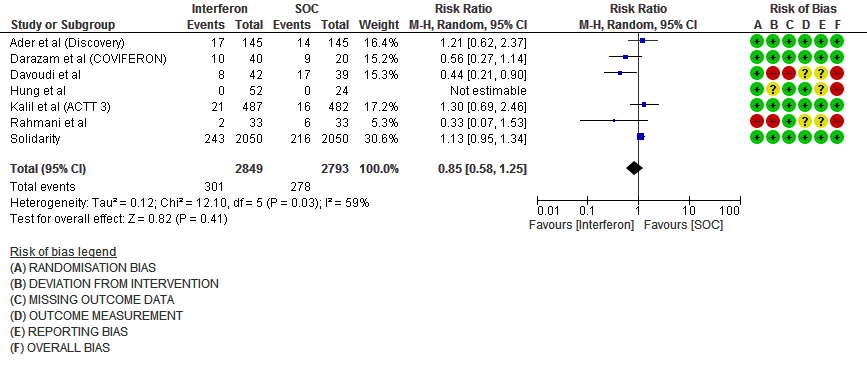
a) All-cause mortality: Very low certainty of evidence in 5642 patients in seven RCTs10-16 found a very uncertain effect of Interferon on all-cause mortality (RR 0.85; 95% CI 0.58 to 1.25). There were no significant differences observed even when trials were stratified by severity, risk of bias or comparators.
b) Time to Clinical improvement [mean days](time to achieve >2 point reduction in the WHO ordinal score):Low certainty of evidence in 121 patients intwo RCTs8,11suggests that Interferon may result in no difference in mean time to clinical improvement(MD 14 days higher; 1.12 lower to 1.39 higher days).
Secondary Outcomes
a) Clinical Improvement at Day 15: Very Low certainty evidence in 281 patients from two RCT8,9, found that the evidence is very uncertain about the effect of interferon on clinical improvement at day 15 (RR 1.13; 95% CI 0.78 to 1.65).
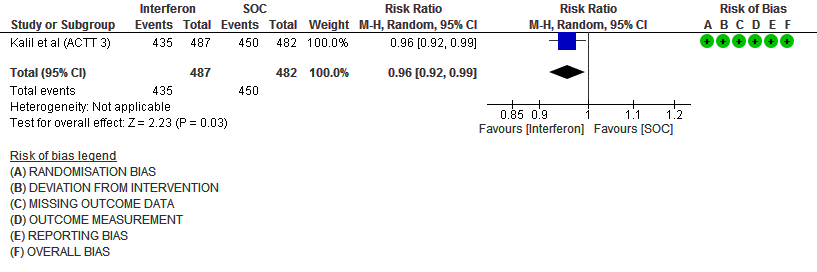
b) Clinical recovery at Day 28 (time to achieve WHO score 1,2,3 or not requiring hospitalization for oxygen or medical care): Moderate certainty evidence in 969 patients from one RCT15, found that Interferon made little to no difference in clinical recovery at day 28 (RR 0.96; 95% CI 0.92 to 0.99).
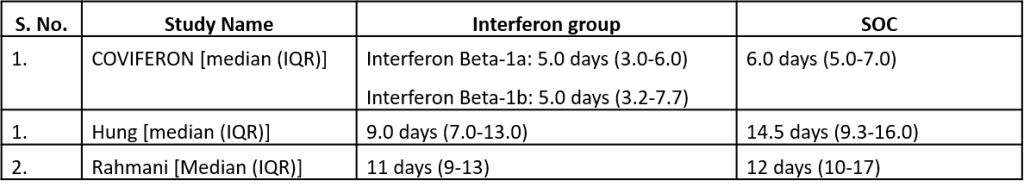
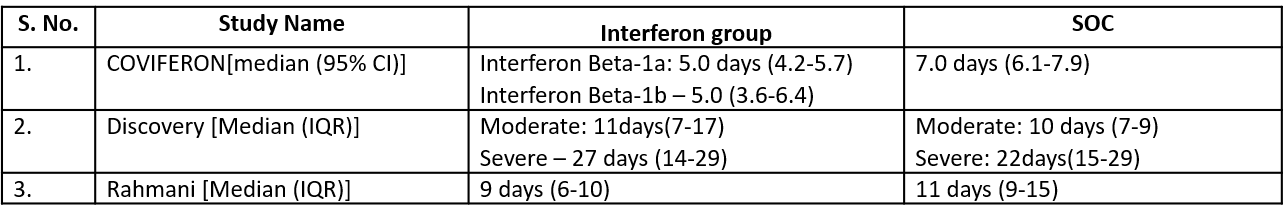
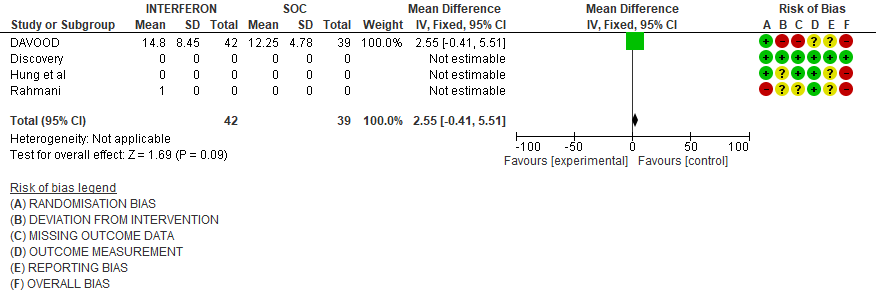
c). Duration of hospital stay(days): Very low certainty of evidence in 81 patients from one RCT11suggested that the effect of interferon on the mean duration of hospitalization was
higher in patients receiving Interferon MD 2.55 days higher (0.41 days lower to 5.51 higher).
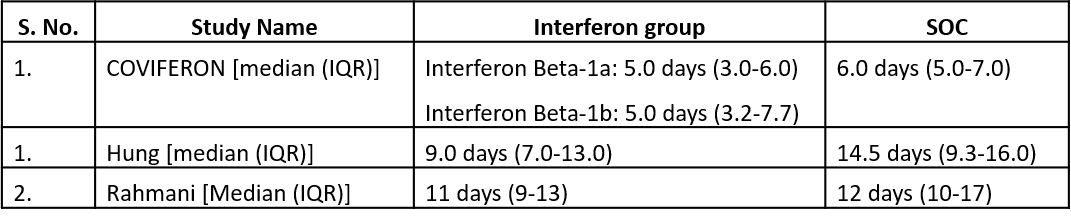
Three studies10,12,14 reported duration of hospital stay in median (IQR).
Three studies10,12,14 reported duration of hospital stay in median (IQR)
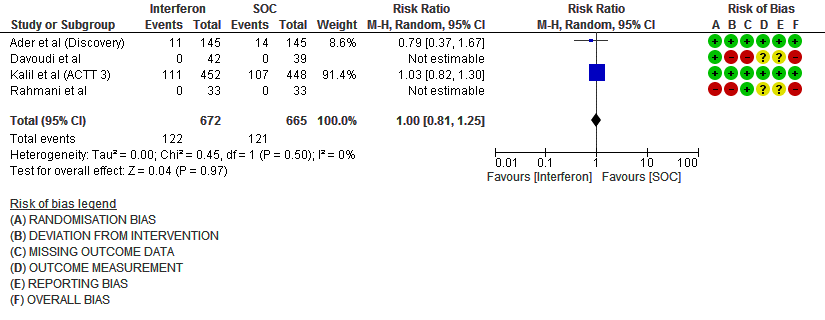
d) Progression to oxygen therapy/NIV at Day 28: Moderate certainty evidence in 1337 patients from 4 RCTs10,11,13,15, found that Interferon probably does not reduce the need for oxygen therapy/NIV at day 28(RR 1.00; 95% CI 0.81 to 1.25).
e. Progression to Invasive mechanical ventilation at Day 28: Moderate certainty evidence in 5300 patients from six RCTs10-13,15-16, found that Interferon probably does not reduce progression to mechanical ventilation at day 28 (RR 1.02; 95% CI 0.87 to 1.20).
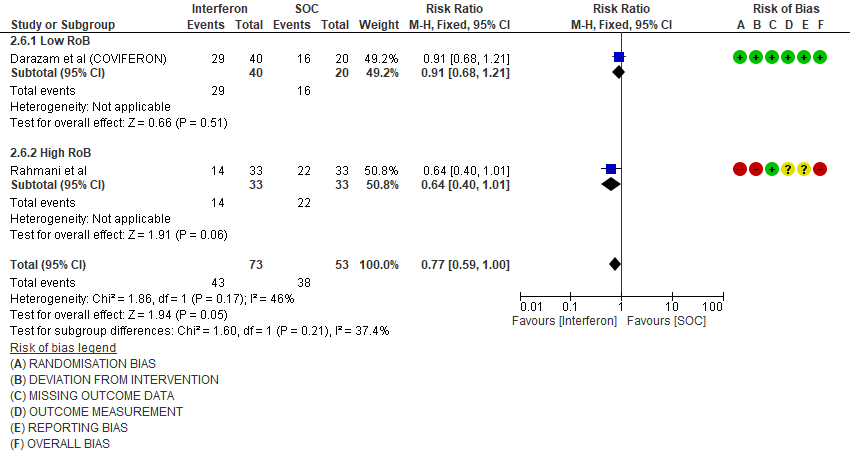
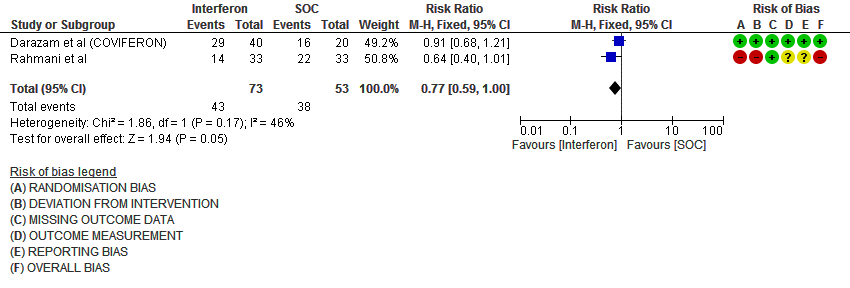
f. Progression to intensive care: Low certainty evidence in 126 patients from two RCTs10,12, found that Interferon may reduce admission into ICU (RR 0.77; 95% CI 0.59 to 1.00).
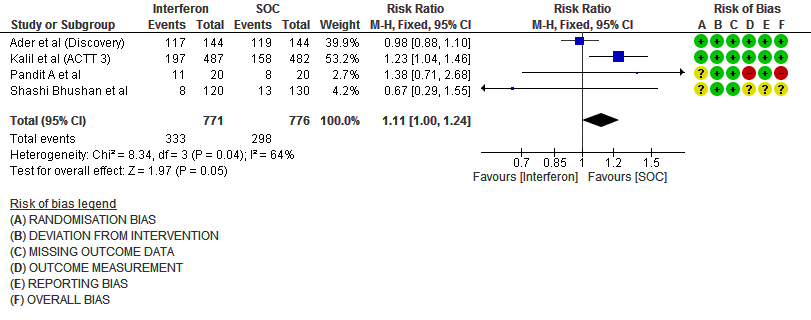
g. Any Adverse events: Low certainty evidence in 1547 patients from four RCTs8-9,13,15, found that Interferon may slightly increase adverse events(RR 1.11; 95% CI 1.00 to 1.24).
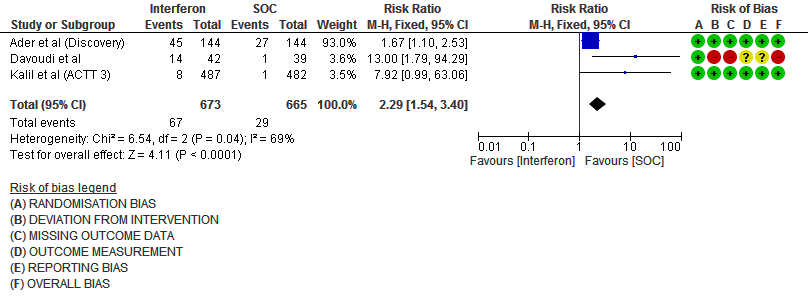
h. Drug-related Adverse events: Moderate certainty evidence in 1338 patients from three RCTs11,13,15, found that Interferon probably increases drug-related adverse events when compared to standard of care or active comparator(RR 2.29; 95% CI 1.54 to 3.40).
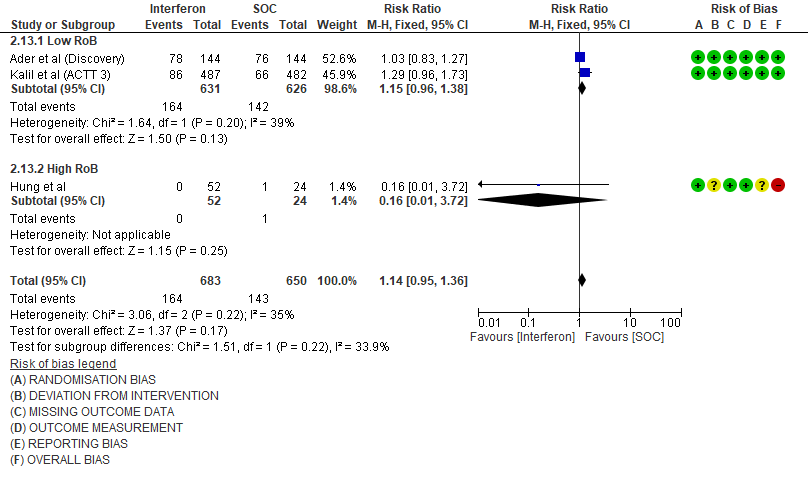
i. Serious Adverse events: Moderate certainty evidence in 1333 patients from three RCTs13-15, found that Interferon probably increases serious adverse events when compared to standard of care or active comparator (RR 1.14; 95% CI 0.95 to 1.36).
Disaggregated data could not be obtained for co-morbidities, inflammatory markers or different age groups. Subgroups of pregnancy, lactation, renal failure, liver failure were excluded from most trials. No data were available separately for immunocompromised individuals in any of the trials.
Forest plots:
Each outcome has been pooled and evaluated separately as a sub group with studies which had a low risk of bias and high risk of bias.
1.Mortality
2. Admission into intensive care (ICU)
3. Progression to Oxygen Therapy
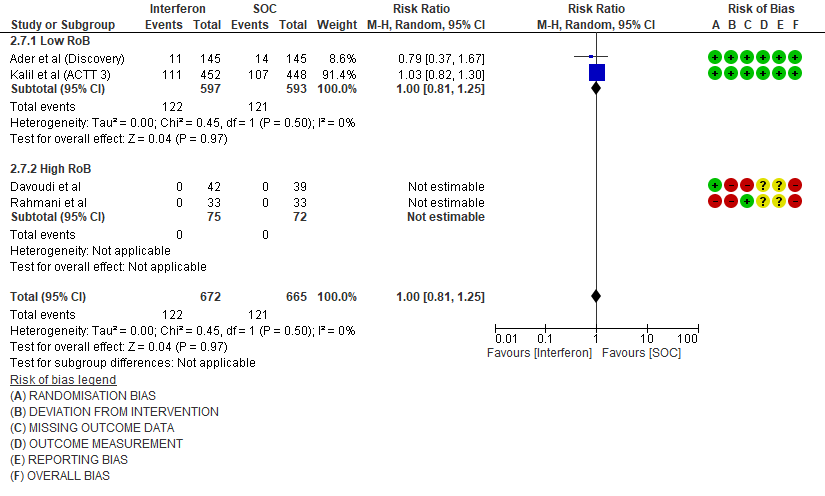
4. Progression to NIV/IMV
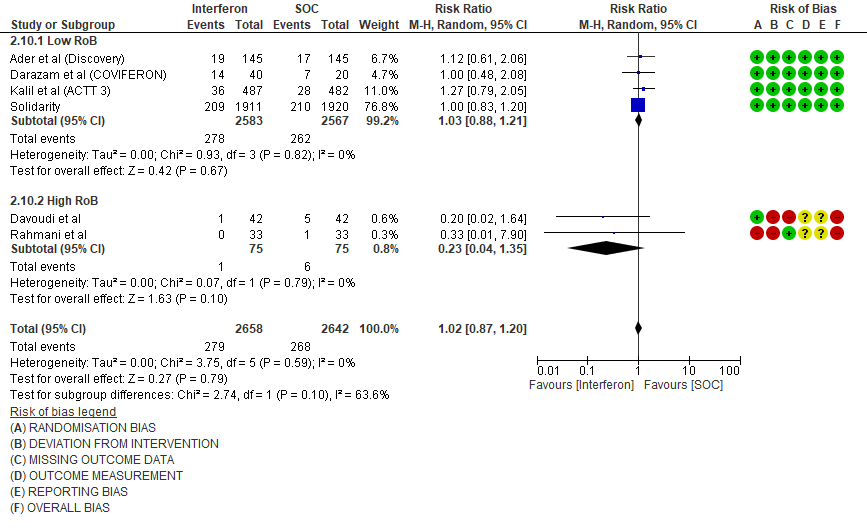
5. Adverse events
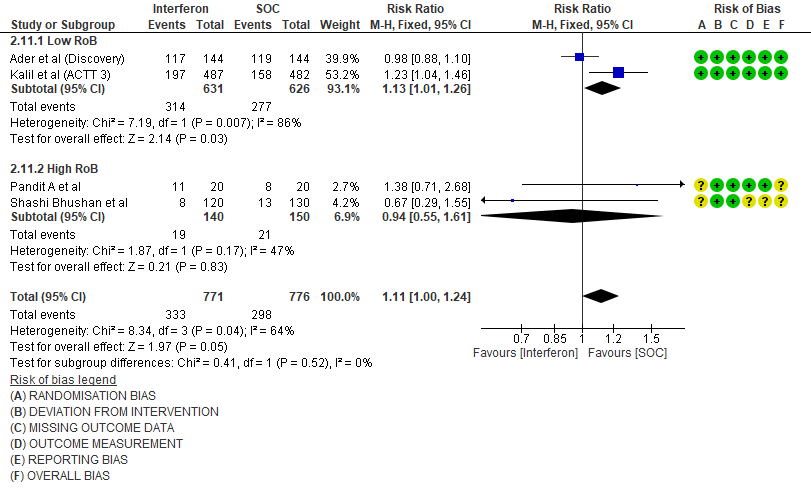
6. Drug related adverse events
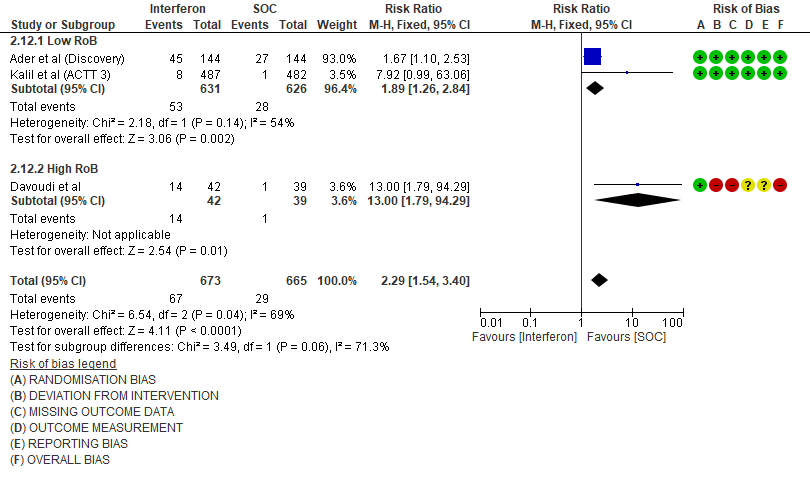
7. Serious Adverse events
1.Mortality
2. Time to Clinical improvement [mean days](time to achieve >2 point reduction in the WHO ordinal score)
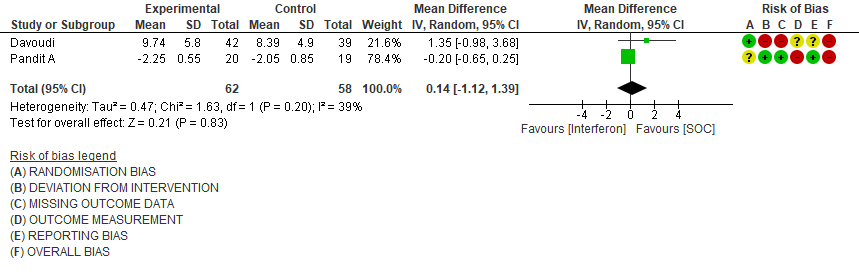
3. Clinical Improvement at Day 15
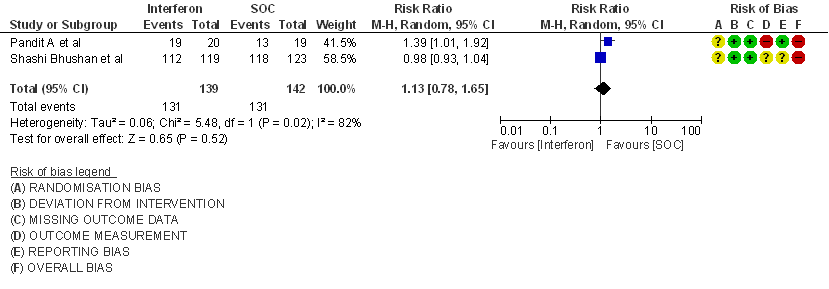
4. Clinical recovery at Day 28 (time to achieve WHO score 1,2,3 or not requiring hospitalization for oxygen or medical care)
5. Duration of Hospital stay (days)
6. Progression to intensive care (ICU)
7. Progression to oxygen therapy/NIV at Day 28
8. Progression to Invasive mechanical ventilation at Day 28
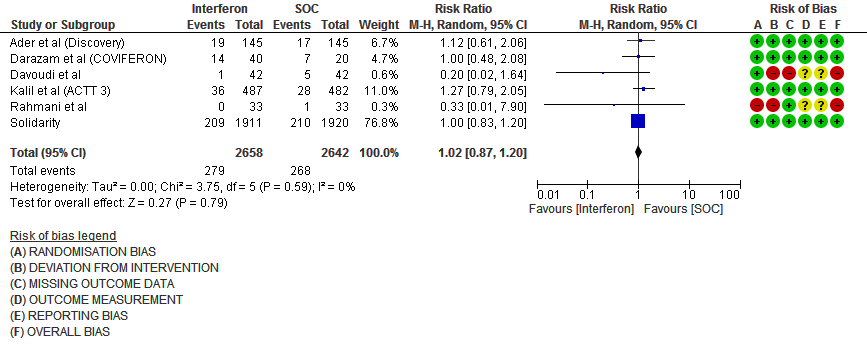
9. Adverse events (no. of patients)
Number of events
10. Drug related adverse events (no. of patients)
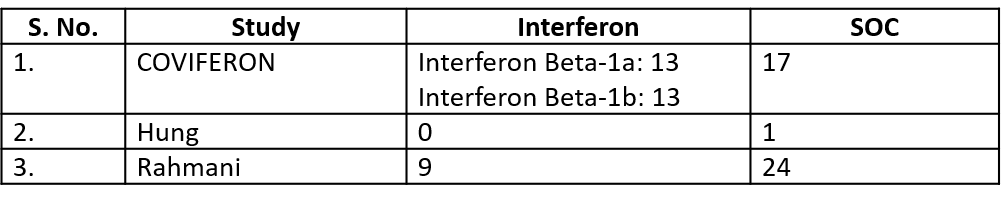
11. Serious Adverse events (no. of events)
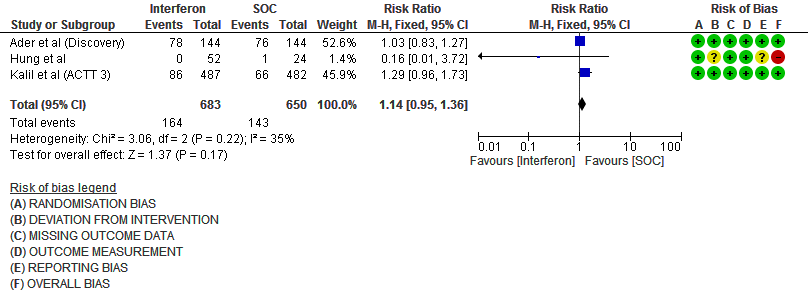
Events
The Anti-Viral Expert Working Group met on 10th February 2022 to consider Interferon as a treatment for COVID-19. Conflict of interest declarations were reviewed by the Steering Committee; none were found to be relevant to Interferons.
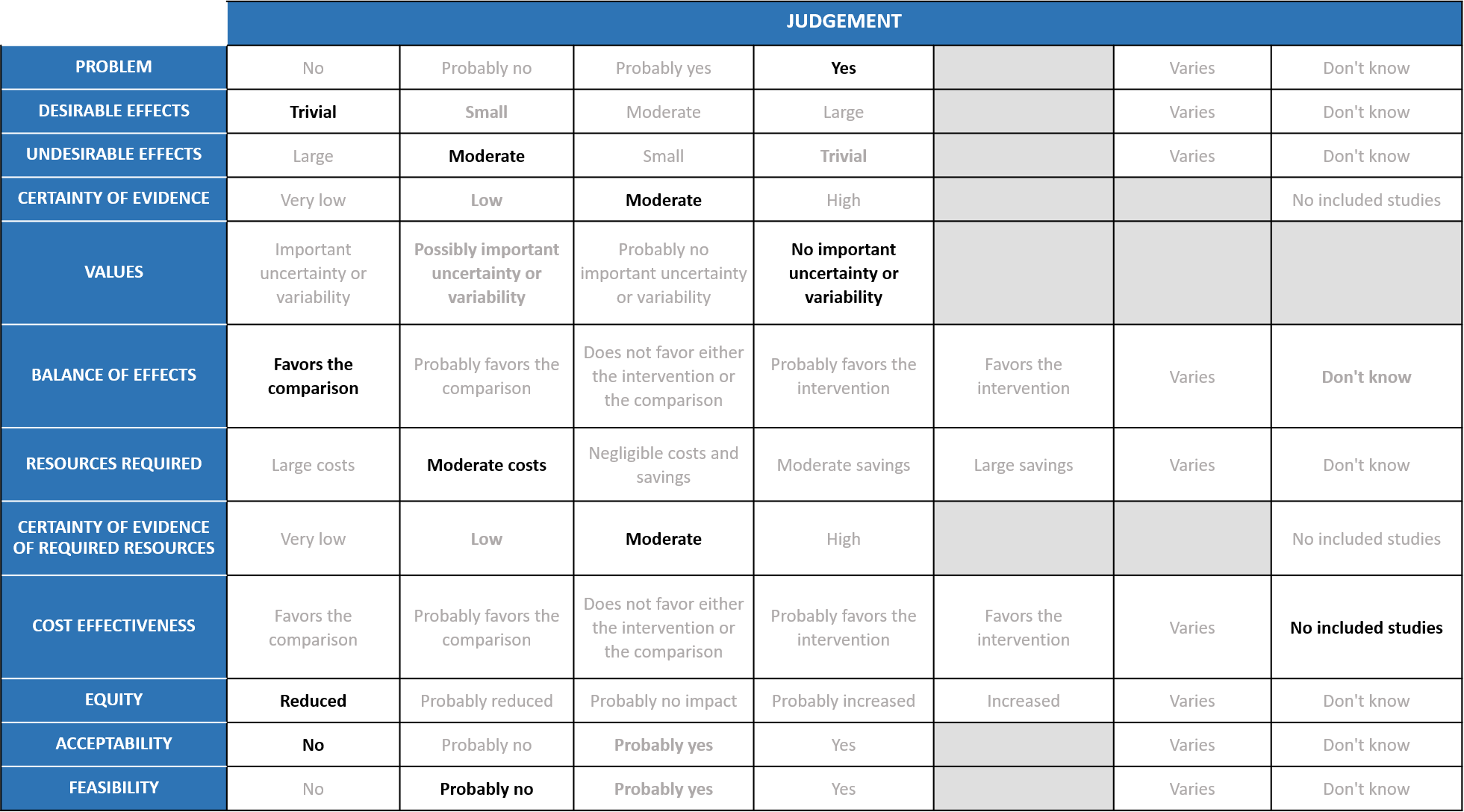
A summary and then more detailed explanations of the Expert Working Group's judgements follow.
Problem
The COVID-19 pandemic in India with more than 41 million cases and over 0.48 million deaths has significantly impacted and stressed the health structure of the country. With a shortage of intensive care unit beds, oxygen and trained personnel the country is facing a major health crisis.
COVID-19 is a priority and there are no effective antiviral treatments and hence any treatment that prevents death or progression to oxygen or ventilation would be beneficial.
Desirable effects
The group agreed that the evidence suggests that Interferondoesnot have a clinically significant effect ontime to clinical improvement, clinical improvement at day 15, duration of hospital, all-cause mortality, clinical recovery at day 28, need for oxygen therapy/ventilation and progression to mechanical ventilation but does reduce ICU admission. Overall, Interferon has hardly any efficacy, so the group discussed and concluded that the desirable effects were trivial (less than trivial).
Undesirable effects
Based on the pooled data the group suggested that the evidence was moderate that Interferons increased all adverse events, drug related adverse events and serious adverse events.
Certainty of evidence
Using GRADE methodology, the evidence synthesis team rated certainty of evidenceas very low for all-cause mortality, clinical improvement at day 15, duration of hospital stay; low for time to clinical improvement, ICU admission; Moderate for need for oxygen/ventilation, progression to mechanical ventilation and clinical recovery at day 28. And for undesirable effects, the certainty of evidence was found to be low for any adverse events and moderate for drug related adverse events and serious adverse events.The group agreed with these judgements and rated the overall certainty of evidence as moderate.
Values
The outcomes studied in this review were all-cause mortality, time to clinical improvement, clinical recovery at day 28, duration of hospital stay, progression to oxygen therapy/NIV, progression to invasive mechanical ventilation, progression to ICU, any adverse events, drug related adverse events and serious adverse events. The group felt that there was no important uncertainty or variability in what outcomes were chosen in the trial. The outcomes were appropriate measured optimally.
Balance of effects
The expert working group felt that the balance of effects favours the comparison. The beneficial effects are less than trivial with moderate adverse effects and hence the group favored the comparison.
Resources required
This is a subcutaneous injection and is usually given in hospital. The cost of PEG INF – Alpha 2b in India is Rs. 18,200, cost of Interferon Alpha 2b available in India is from Rs. 380.95 to Rs. 647.74 per vial. Hence the group concluded moderate cost. The group includes clinicians in different types of Indian hospitals who have a good idea of drug and hospitalization costs.
Certainty of evidence of required resources
Interferon beta is not available in India so the costs may vary in India. However we have been using interferon alpha for many years which experts believe has comparable antiviral effects and in fact may have better antiviral activity as compared to beta interferon. The group was aware of the costs and hence felt that there was moderate certainty of evidence of required resources to implement this intervention.
Cost effectiveness
The group discussed that there are no studies looking at this. And also the costs are high with questionable efficacy and moderate harm.Hence the group favored no included studies.
Equity
The costs are high and would be out of reach of the common man. In addition, these would need to be administered in hospital by trained and skilled staff as this would need to be given subcutaneously. Hence this would definitely lower equity.
Acceptability
The group felt that this would not be an acceptable intervention to any stakeholder as it is costly, injected subcutaneously and has no efficacy but probable harms.
Feasibility
This is probably not a feasible intervention because the cost of the drug is high and difficult to implement subcutaneous administration widely.
Interferons are widely available and have been used for many years in various viral infections. However, they require subcutaneous administration, are relatively expensive, and require monitoring for adverse effects, all of which might make implementation challenging if interferon is later found to be effective and safe for COVID-19 treatment.
Evidence about the utility and effect of Interferon in COVID-19 on reduction in disease progression and clinical improvement was uncertain. Hence in view of lack of benefit but appreciable harm, we recommend against the usage of Interferon as a therapy across all the subgroups of COVID-19 patients.
This strong recommendation against use of interferon will be reviewed as new evidence of efficacy and/or safety emerges. There are a number of ongoing trials listed in international registries. In terms of safety, interferon has been widely used to treat cancers, chronic viral infections (such as hepatitis B and C) and multiple sclerosis and adverse events are known to be common.
There is a severe paucity of evidence with regards to this drug. There is very limited evidence of efficacy, timing and cost effectiveness of Interferons in COVID-19 infection. We need more randomized controlled trials with larger numbers to evaluate the add-on beneficial effects of Interferons to existing antiviral agents. However, this also needs to be balanced against the knowledge that we do have suitable alternative options.
1. Age, comorbidities, prior sensitization, immunocompromised status, dose and time of administration are other aspects that merit further study.
2. In addition, the trials have been done with different types of Interferons alpha and beta. At present due to lower numbers we are unable to tease out the beneficial effects of alpha vs beta. This may need further evaluation.
- Konerman MA, Lok AS. Interferon Treatment for Hepatitis B. Clinics in Liver Disease. 2016 Nov;20(4):645–65.
- Essaidi-Laziosi, M., Geiser, J., Huang, S. et al.Interferon-Dependent and Respiratory Virus-Specific Interference in Dual Infections of Airway Epithelia. Sci Rep 10, 10246 (2020).
- Interferon therapy for COVID-19 and emerging infections: Prospects and concerns | Cleveland Clinic Journal of Medicine
- Ranieri VM, Pettilä V, Karvonen MK, Jalkanen J, Nightingale P, Brealey D, et al. Effect of Intravenous Interferon β-1a on Death and Days Free From Mechanical Ventilation Among Patients With Moderate to Severe Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA. 2020 Feb 25;323(8):725–33.
- Acosta PL, Byrne AB, Hijano DR, Talarico LB. Human Type I Interferon Antiviral Effects in Respiratory and Reemerging Viral Infections. J Immunol Res. 2020;2020:1372494.
- de Weerd NA, Nguyen T. The interferons and their receptors—distribution and regulation. Immunol Cell Biol. 2012;90(5):483–91.
- Baron S, Tyring SK, Fleischmann WR Jr, Coppenhaver DH, Niesel DW, Klimpel GR, et al. The Interferons: Mechanisms of Action and Clinical Applications. JAMA. 1991 Sep 11;266(10):1375–83.
- Pandit A, Bhalani N, Bhushan BLS, Koradia P, Gargiya S, Bhomia V, et al. Efficacy and safety of pegylated interferon alfa-2b in moderate COVID-19: A phase II, randomized, controlled, open-label study. Int J Infect Dis. 2021 Apr;105:516-21.
- Bhushan BLS, Wanve S, Koradia P, Bhomia V, Soni P, Chakraborty S, et al. Efficacy and safety of pegylated interferon-alpha2b in moderate COVID-19: a phase 3, randomized, comparator-controlled, open-label study. Int J Infect Dis. 2021 Oct;111:281-7.
- Rahmani H, Davoudi-Monfared E, Nourian A, Khalili H, Hajizadeh N, Jalalabadi NZ, et al. Interferon beta-1b in treatment of severe COVID-19: A randomized clinical trial. Int Immunopharmacol. 2020 Nov;88:106903.
- Davoudi-Monfared E, Rahmani H, Khalili H, Hajiabdolbaghi M, Salehi M, Abbasian L, et al. A Randomized Clinical Trial of the Efficacy and Safety of Interferon beta-1a in Treatment of Severe COVID-19. Antimicrob Agents Chemother. 2020 Aug 20;64(9).
- Alavi Darazam I, Shokouhi S, Pourhoseingholi MA, Naghibi Irvani SS, Mokhtari M, Shabani M, et al. Role of interferon therapy in severe COVID-19: the COVIFERON randomized controlled trial. Sci Rep. 2021 Apr 13;11(1):8059.
- Ader F, Peiffer-Smadja N, Poissy J, Bouscambert-Duchamp M, Belhadi D, Diallo A, et al. An open-label randomized controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-beta-1a and hydroxychloroquine in hospitalized patients with COVID-19. Clin Microbiol Infect. 2021 Dec;27(12):1826-37.
- Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020 May 30;395(10238):1695-704.
- Kalil AC, Mehta AK, Patterson TF, Erdmann N, Gomez CA, Jain MK, et al. Efficacy of interferon beta-1a plus remdesivir compared with remdesivir alone in hospitalised adults with COVID-19: a double-bind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021 Dec;9(12):1365-76.
- Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, Abdool Karim Q, et al. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med. 2021 Feb 11;384(6):497-511.
