This recommendation applies to acute COVID-19 in adults. Some of our recommendations vary according to the severity of COVID-19 illness. Definitions of the categories are based on the World Health Organization (WHO) criteria and can be viewed by clicking the plus (+) signs below.
RECOMMENDATION: We do not recommend Hydroxychloroquine (HCQ) or Chloroquine for treating COVID-19. There is no demonstrable benefit and there is potential toxicity.
DATE OF RECOMMENDATION: 4th February 2022
Definition of mild:
• Symptomatic (any acute COVID-19 related symptoms)
• AND respiratory rate <24/min
• WITHOUT pneumonia or hypoxia
Definition of moderate illness:
• Pneumonia (clinical or radiological) OR hypoxia (SpO2 <94% in adults with no underlying lung disease)
• AND respiratory rate ≤30/min
• AND SpO2 ≥90% on room air
Definition of Severe illness
Pneumonia with ANY ONE of the following:
• severe respiratory distress or respiratory rate >30/min
• SpO2 <90% on room air
• NO invasive or non-invasive respiratory support needed
Definition of critical:
• Requirement for high-level respiratory support: noninvasive ventilation, high-flow oxygen (≥20 litres per minute) or invasive mechanical ventilation
• OR acute respiratory distress syndrome (PaO2/FiO2 ratio of <300)
• OR sepsis
• OR shock
HCQ and Chloroquine have been shown to have antiviral activity in vitro against various RNA viruses like Rabies virus, Chikungunya, Influenza A and B, etc and hence they were considered potential candidates for treating COVID-191. Mechanisms of action are quite varied including interference with ACE-2 receptor glycosylation preventing SARS-CoV-2 binding to target cells, interference with SARS-CoV-2 attempts to acidify the lysosomes, inhibition of cathepsins, alteration of molecular crosstalk between SARS-CoV-2 and target cells and virion assembly and binding.
Some early research shared widely in social and conventional media fueled the belief these drugs were effective. However, these studies were small, non-randomised and/or of low quality2, and some were later retracted due to likely fraudulent research3. Widespread use of HCQ/CQ during the pandemic led to an acute shortage of the drug for conditions such as rheumatological diseases.
There was high quality evidence to suggest that critical outcomes such as all-cause mortality and negative PCR at day 14 were not different between individuals receiving HCQ/CQ vs. Standard of care/placebo. Other important outcomes of interest such as progression to mechanical ventilation and time to clinical improvement (low quality of evidence) were also not different between HCQ/CQ and comparison groups (placebo or standard of care). Furthermore, there was increased incidence of adverse events and QT interval prolongation on electrocardiography with HCQ/CQ use compared to no HCQ/CQ (low quality of evidence). Although, there are no major cost implications, in view of lack of evidence of benefit and evidence of harm, we strongly recommend against the use of HCQ/CQ in COVID-19 of any severity.
Date of latest search: 30th July 2021
Date of completion & presentation to Expert Working Group: 18th November 2021
Date of planned review: 18th May 2022
Evidence synthesis team: Umang Agrawal, Siddhant Shetty, Anupa Thampy, Anjely S, Richard Kirubakaran, Bhagteshwar Singh, Priscilla Rupali
Explanations
a. Downgraded by one level (indirectness). Arabi et al contributes to 33% of the weight of outcome (only severe patients included).
b. Downgraded by one level (serious imprecision) [11% benefit and 53% harm increases uncertainty]
c. Downgraded by one level: Risk of Bias (serious)
d. Downgraded by one level due to: Wide confidence interval, only a single study used to assess this outcome
e. Downgraded by one level due to serious risk of attrition and reporting bias.
f. Downgraded by one level due to high heterogeneity: I2= 81%
g. Downgraded by one level: for serious imprecision for the given outcome (wide CI)
h. Downgraded by one level: Risk of bias unclear to high in most of the studies
i. Downgraded by one level: Wide confidence interval and optimal information size (OIS) criteria not met
j. Downgraded by one level: One study showing higher incidence in the standard of carearm has high risk of attrition and reporting bias
k. Downgraded by one level due to high heterogeneity:I2 = 59%
l. Downgraded by 2 levels: CI very wide
SARS‐CoV‐2 is a novel coronavirus that has caused a pandemic since December 2019. Over 350 million people have been diagnosed with COVID‐19, and as of 25th January 2022, over 5.6 million people have died.4 Severe disease is characterized by hypoxia and progressive acute respiratory distress syndrome appears to be the main driver for mortality, although patients can experience a syndrome with clinical and laboratory features of severe systemic inflammation, termed a “cytokine storm”. At the other end of the spectrum, asymptomatic infection is not uncommon; national Italian data described this in approximately 10% of all people with a confirmed COVID‐19 diagnosis, though more recent estimates have been higher5. More recently, wide‐ranging longer‐term morbidity has been described in the absence of a severe initial illness.6
This review aims to provide a summary of the available evidence from randomized controlled trials (RCT) of Hydroxychloroquine/Chloroquine for treatment of COVID-19, for any duration, which could guide clinicians and researchers regarding the appropriate use of this drug in the future.
We searched PubMed and Epistemonikos, for studies of any publication status and in any language. We also reviewed reference lists of systematic reviews and included studies. We performed all searches up to 31st July 2021.
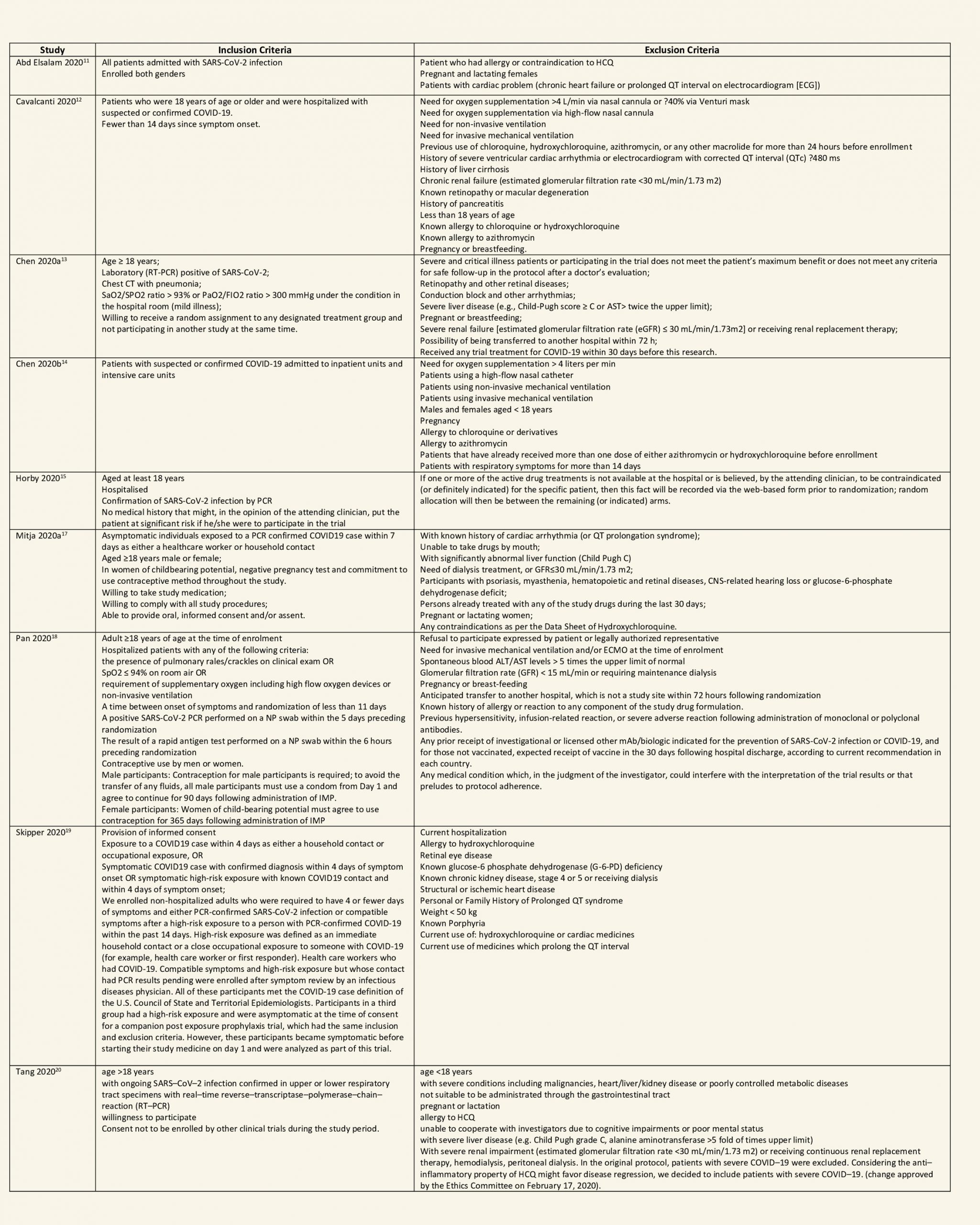
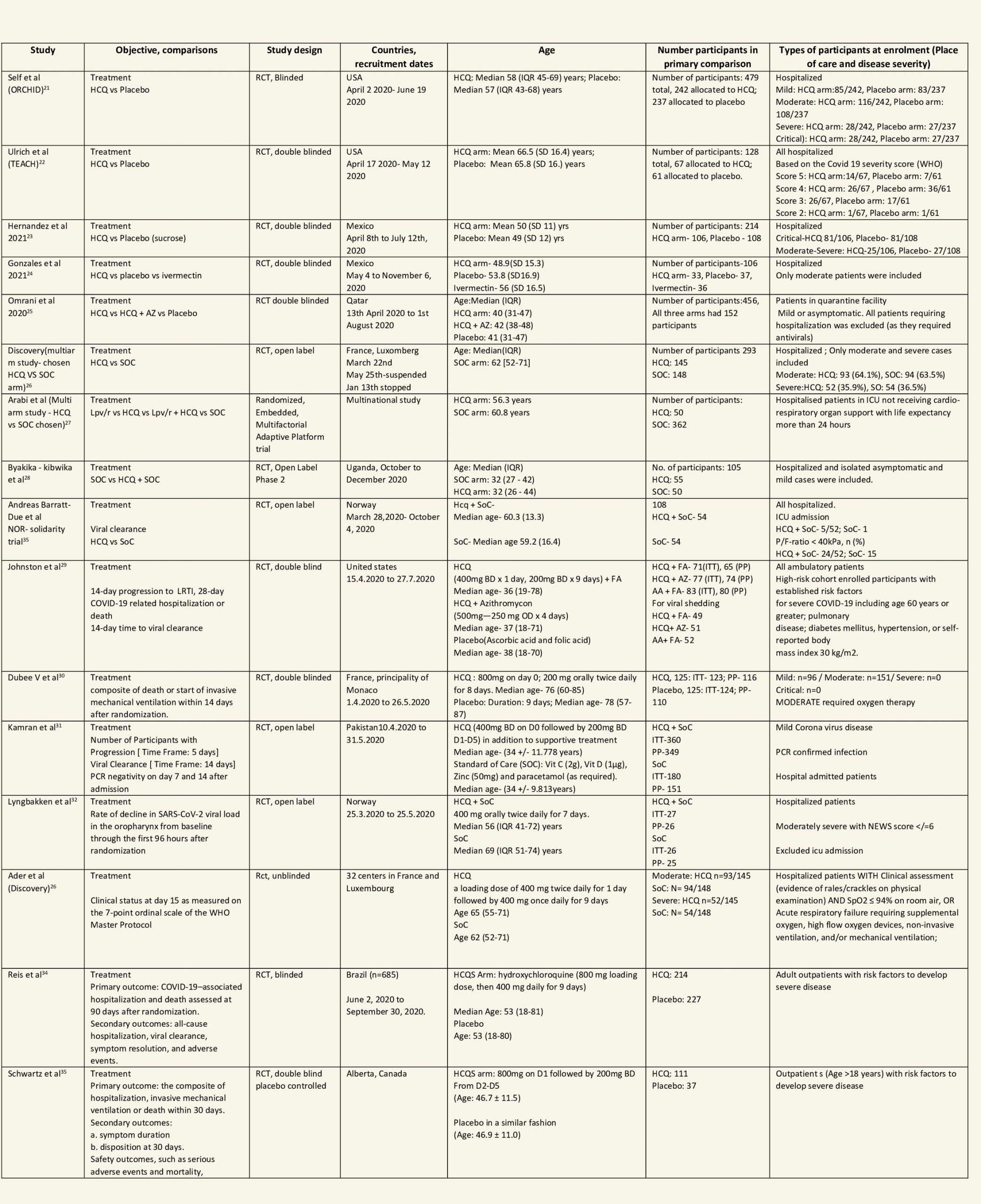
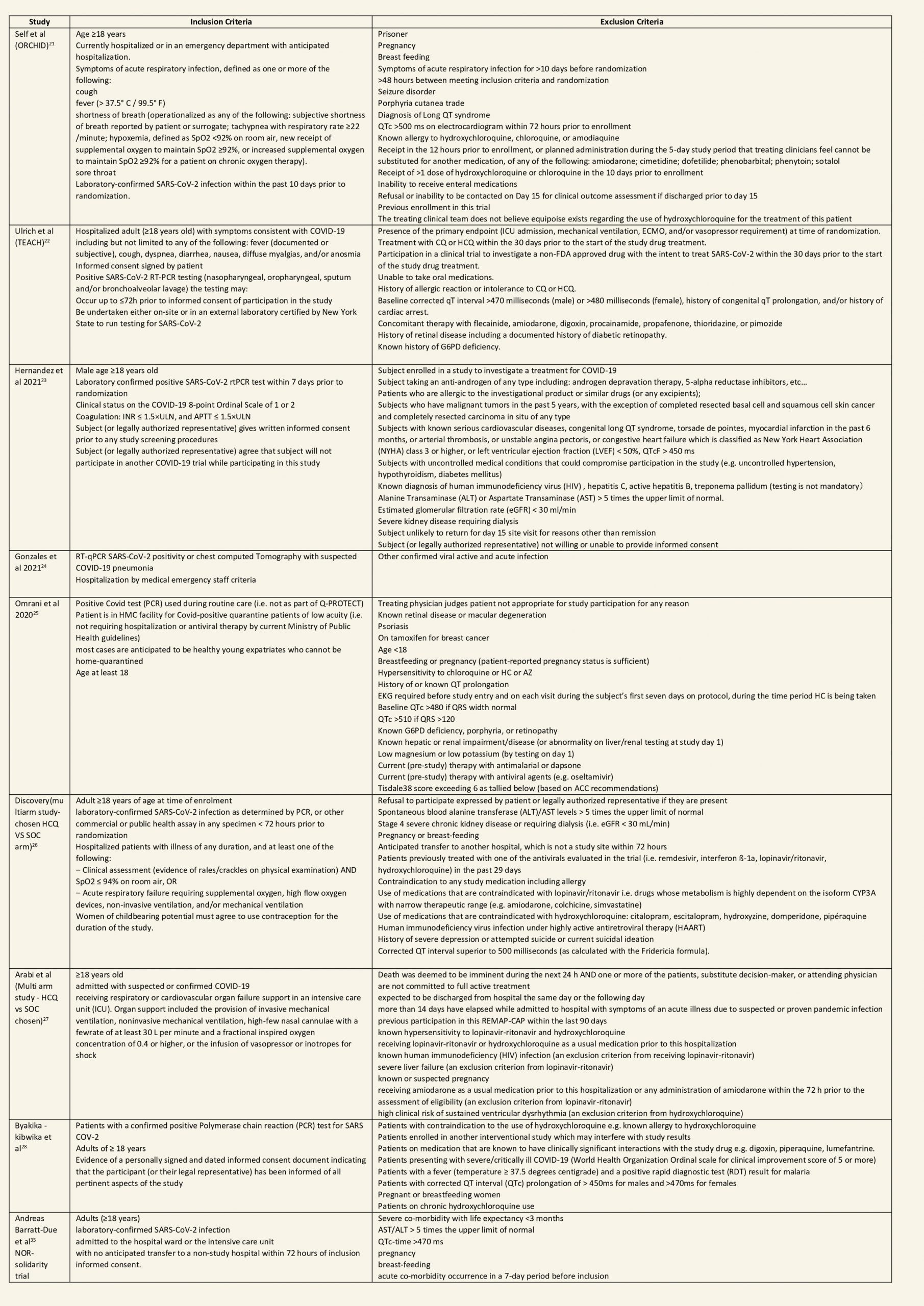
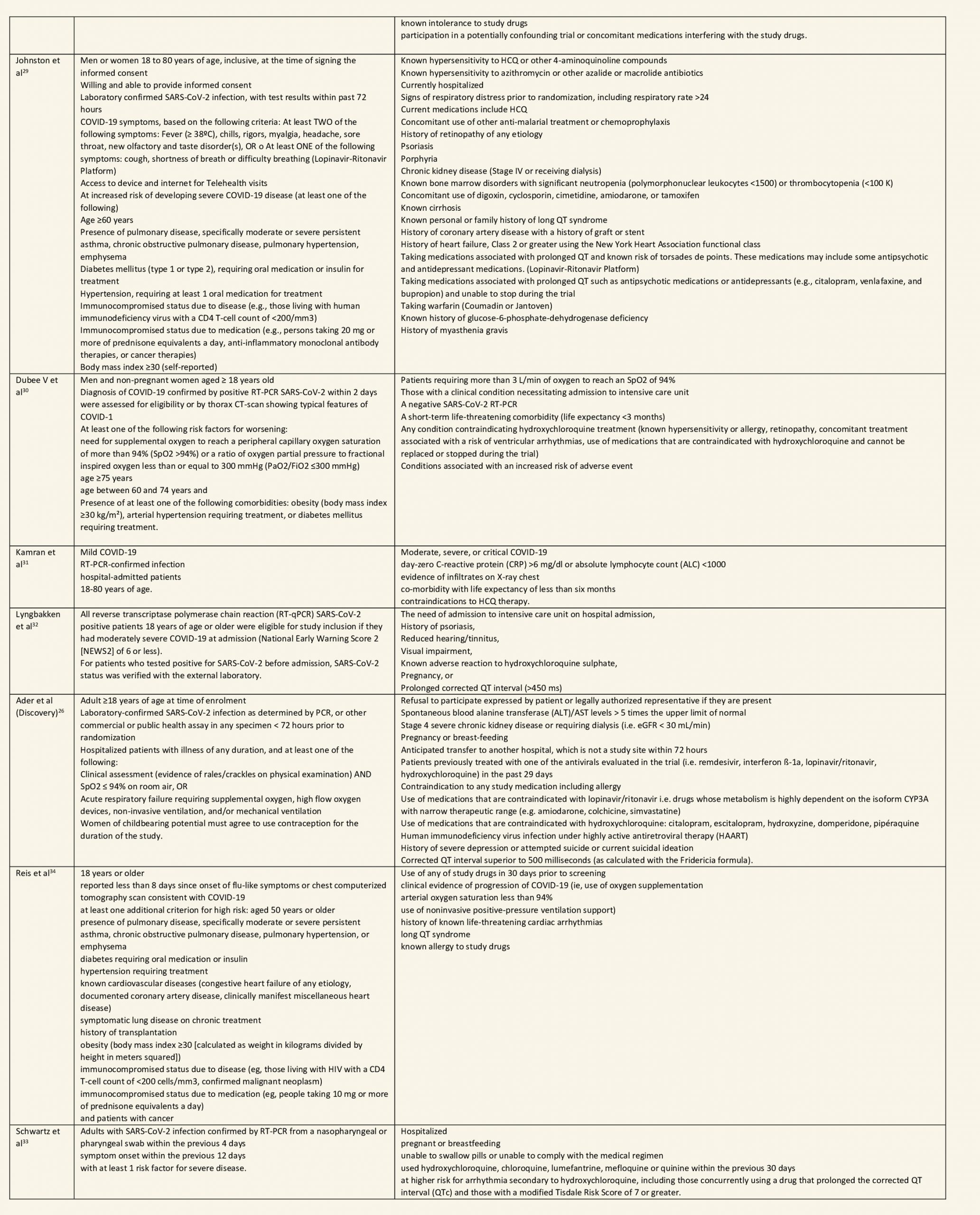
After searching the above databases and removing duplicates, we found 257 records for systematic reviews. After excluding non-RCTs, non-peer reviewed studies and systematic reviews which did not match our PICO question, four systematic reviews were selected. 3 of these were excluded since the AMSTAR scoring for these was found to be of critically low quality. One Cochrane review7 was included for the analysis. The AMSTAR score for the systematic review was found to be of moderate quality. The systematic review selected had 14 RCTs. 4 of these were excluded as they did not match the PICO question. We also went ahead and searched new RCTs to update the Cochrane review. 16 new RCTs were found which matched our PICO. A total of 26 RCTs were included for meta-analysis.
Data extraction was performed in context with the following PICO:
Population:
Patients who are symptomatic with confirmed or suspected COVID-19:
-
-
- Inpatients: Critical, Severe, Non-severe
- Outpatients
-
Intervention: HCQ or Chloroquine any dose, duration
Control: Standard of care/placebo
Outcomes:
Critical
- All- Cause mortality
- Progression to Mechanical ventilation
- Progression to non-invasive ventilation
- Participants with serious adverse events.
Important:
- Negative Respiratory PCR at D14 from randomization
- Time to clinical improvement
- Participants with any adverse events
- Participants with prolonged QTc on ECG
Two reviewers independently assessed eligibility of search results using the online Rayyan tool. Data extraction was performed by one reviewer, and checked by another, using a piloted data extraction tool on Microsoft Word. Risk of Bias (RoB) assessment for the 10 studies in the Cochrane review were included directly from the Cochrane Review7 . RoB for the 16 new RCTs was assessed using the Cochrane RoB v1.0 tool. For all outcomes, one review author performed the assessment, and cross-checked it against the assessment by another review author (who used consensus approach). The whole RoB assessment was scrutinised by the whole author team for this review. We planned to use risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). We planned to meta-analyze if appropriate, i.e., if outcomes were measured and reported in a similar way for similar populations in each trial, using random-effects models with the assumption of substantial clinical heterogeneity (the appropriateness of which would be checked by the Methodology Committee), and the I2 test to calculate residual statistical heterogeneity, using Review Manager (RevMan)v5.4. We used GRADE methodology to make summary of findings tables on GRADEPro GDT.
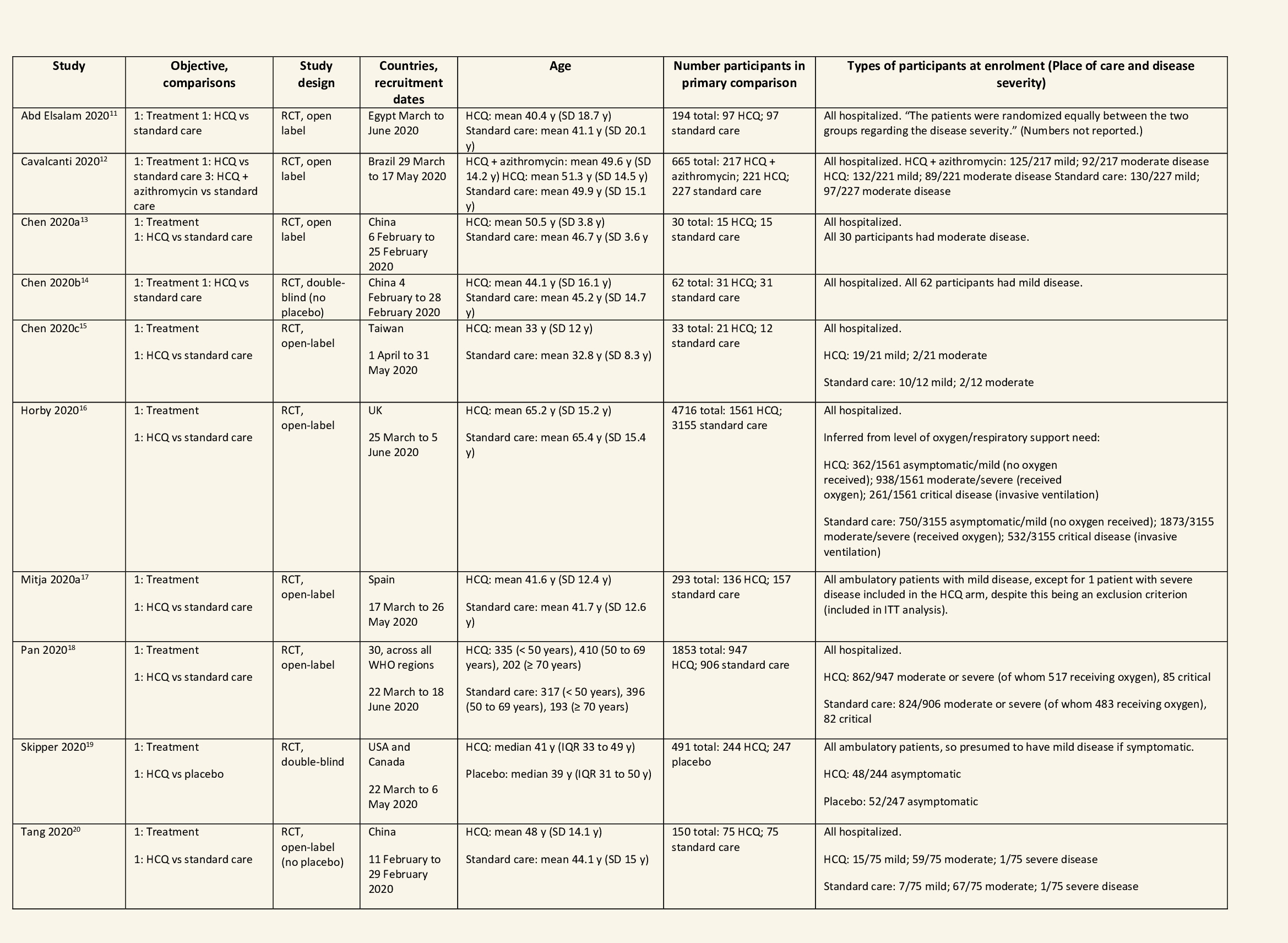
We found 26 RCTs (10 from the Cochrane review and 16 new RCTs) that matched the PICO question. The severity of disease varied from mild to critical. A few important details of these trials are provided below:
- Trials using placebo: 9/26
- Trials recruiting hospitalized participants: 20/26
- Severity of included participants:
-
- Mild disease only: 7/26
- Moderate disease only: 3/26
- Only severe disease: 1/26
- The remaining trials included participants with illness of mixed severity
-
Each trial and its results are described below; characteristics of the trials are summarized in the Summary of characteristics of included trials table. All the trials have varying risk of bias across all domains. Risk of bias for each domain per trial is displayed alongside the forest plots below.
Hydroxychloroquine/Chloroquine compared to Standard of Care
Characteristics of all the trials have been summarized in the Summary of characteristics of included trials table. All the trials have varying risk of bias across all domains. Our expert working group classified all-cause mortality, progression to mechanical ventilation, progression to non-invasive ventilation and participants with serious adverse events as critical outcomes, while outcomes like negative respiratory PCR at Day 14 from randomization, time to clinical improvement, participants with any adverse events and participants with prolonged QTc on ECG were classified as important outcomes.
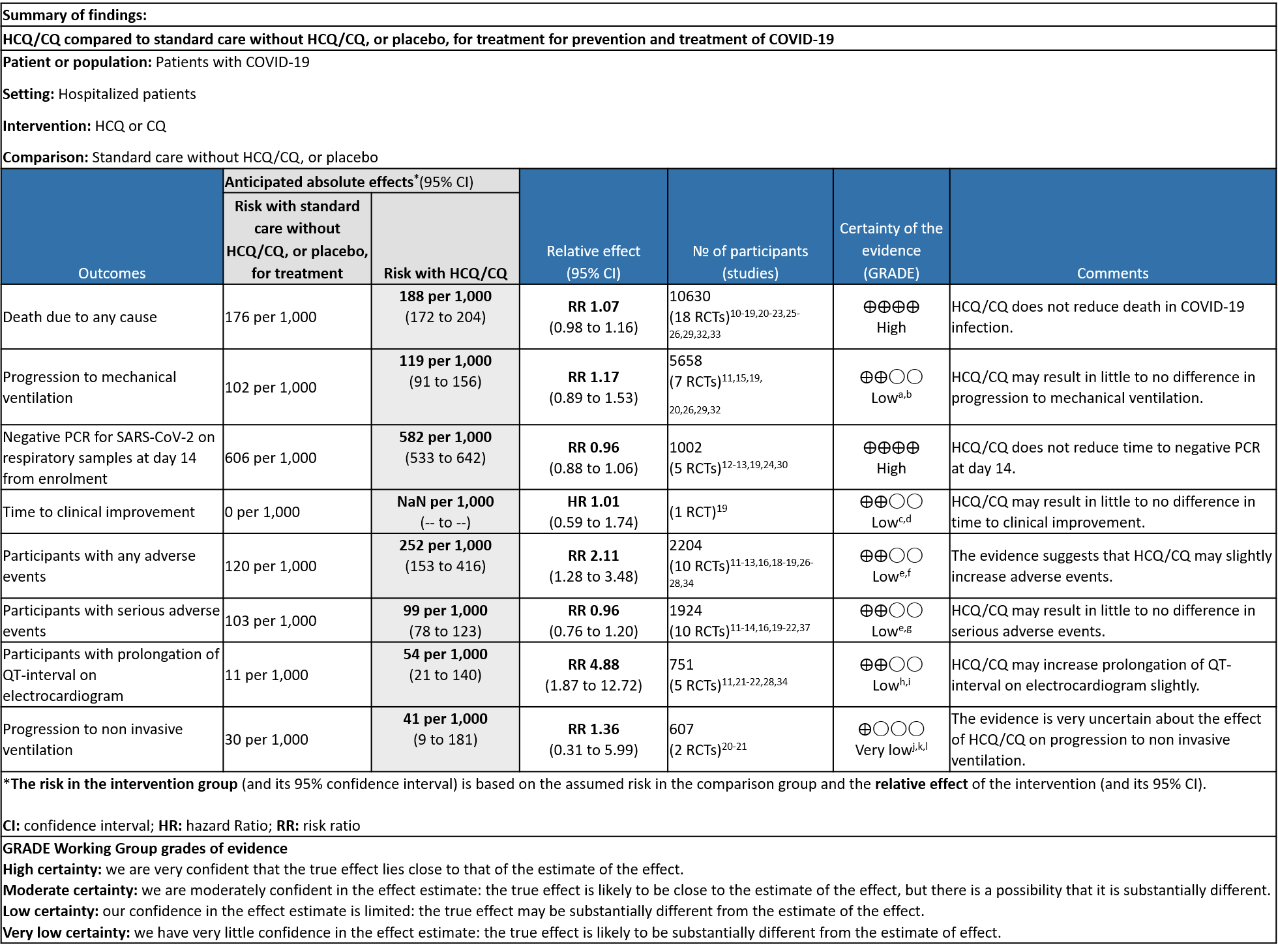
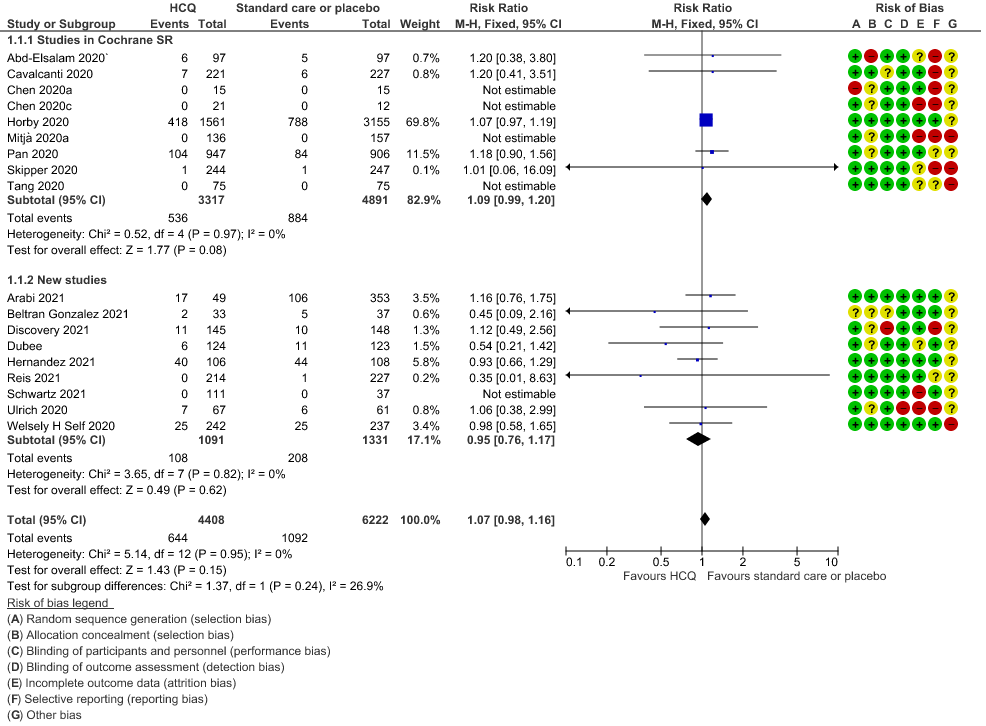
a. All-cause mortality: High certainty of evidence in 10,630 participants from 18 RCTs11-24,26-27,30,33-34 showed that hydroxychloroquine/chloroquine does not reduce mortality in COVID-19 infection RR 1.07 (0.98 to 1.16).
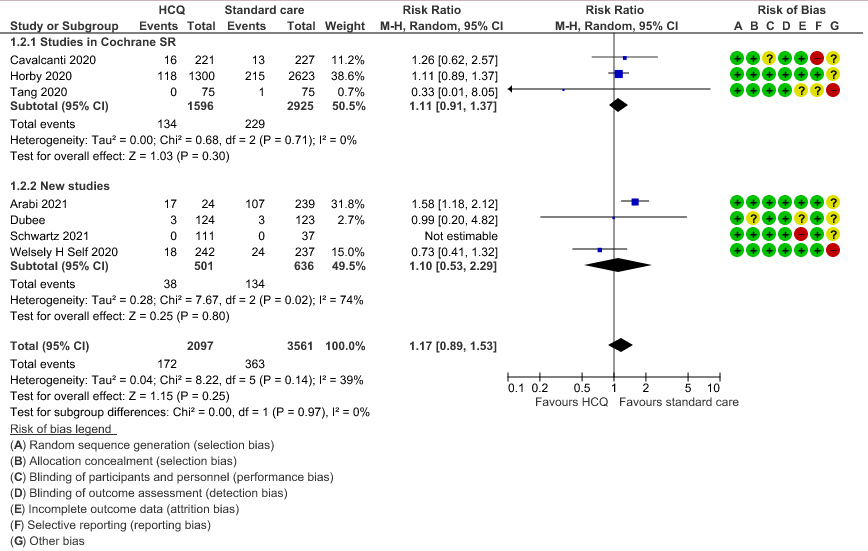
b. Progression to mechanical ventilation: Low certainty of evidence in 5,658 participants from 7 RCTsRCTs12,16,20-21,27,30,33 showed that hydroxychloroquine/chloroquine may result in no difference or even a slight increase in progression to mechanical ventilation in COVID-19 infection RR 1.17 (0.89 to 1.53).
c. Progression to non-invasive ventilation: Very low certainty of evidence in 607 patients from 5 RCTs21-22 showed that the evidence was very uncertain regarding the effect of hydroxychloroquine/chloroquine on progression to non-invasive ventilation RR 1.36 (0.31 to 5.99).
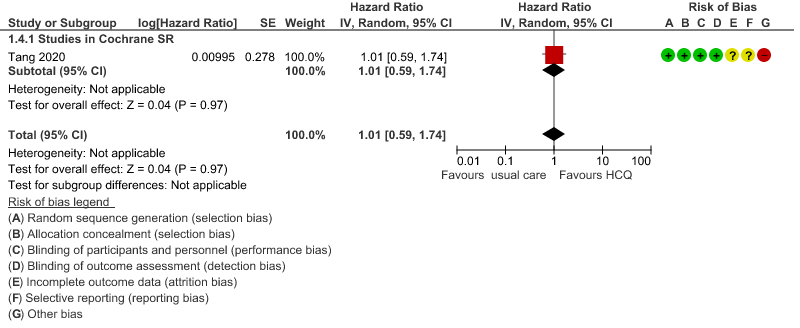
d. Time to clinical improvement: Low certainty of evidence from 1 RCT20 showed that hydroxychloroquine/chloroquine may result in no difference in time to clinical improvement in COVID-19; HR 1.01 (0.59 to 1.74).
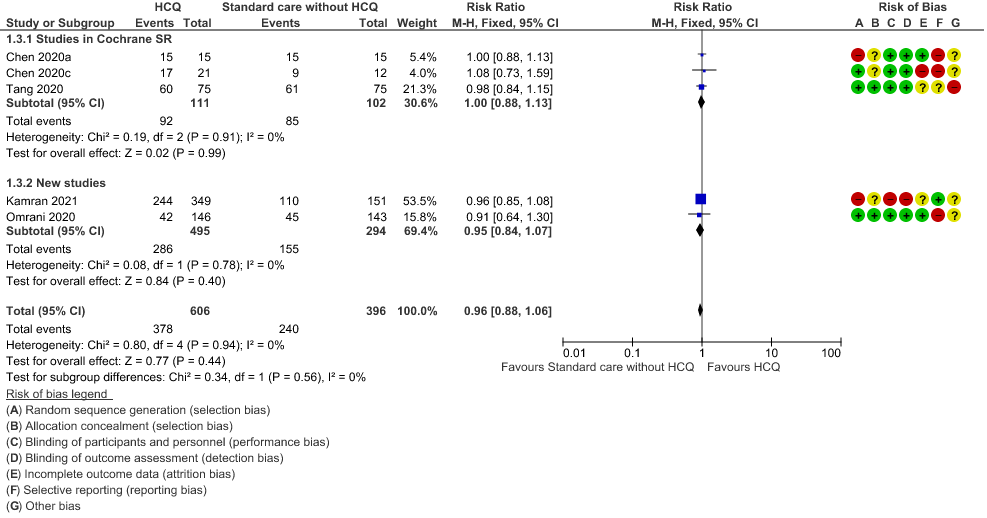
e. Negative PCR at Day 14: High certainty of evidence in 1002 patients from 5 RCTs13-14,20,25,31 showed that hydroxychloroquine/chloroquine does not reduce time to negative PCR for SARS-CoV-2 at day 14; RR 0.96 (0.88 to 1.96).
f. Any adverse events: Low certainty of evidence in 2204 patients from 10 RCTs12-14,17,19-20,27-29,35 showed that hydroxychloroquine/chloroquine may increase adverse events RR 2.11 (1.28 to 3.48).
g. Serious adverse events: Low certainty of evidence in 1924 patients from 10 RCTs12-15,17,20-23,38 showed that hydroxychloroquine/chloroquine may result in no difference in serious adverse events in COVID-19 infection RR 0.96 (0.76 to 1.20).
h. Prolonged QTc: Low certainty of evidence in 751 patients from 5 RCTs12,22-23,29,35 showed that hydroxychloroquine/chloroquine may increase prolongation of QT interval on electrocardiogram RR 4.88 (1.87 to 2.72).
10 Studies from Singh et al7
16 new RCTS
HCQ/CQ compared to standard care without HCQ/CQ, or placebo
1. Mortality
2. Progression to Mechanical Ventilation
3. Negative PCR at day 14
4. Time to clinical improvement
5. Adverse events
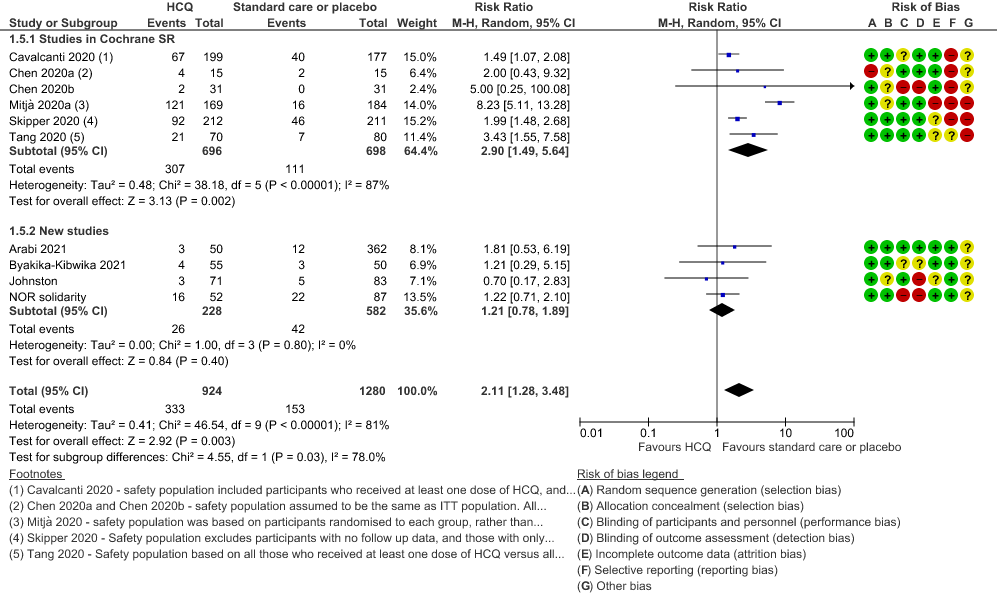
6. Serious adverse events
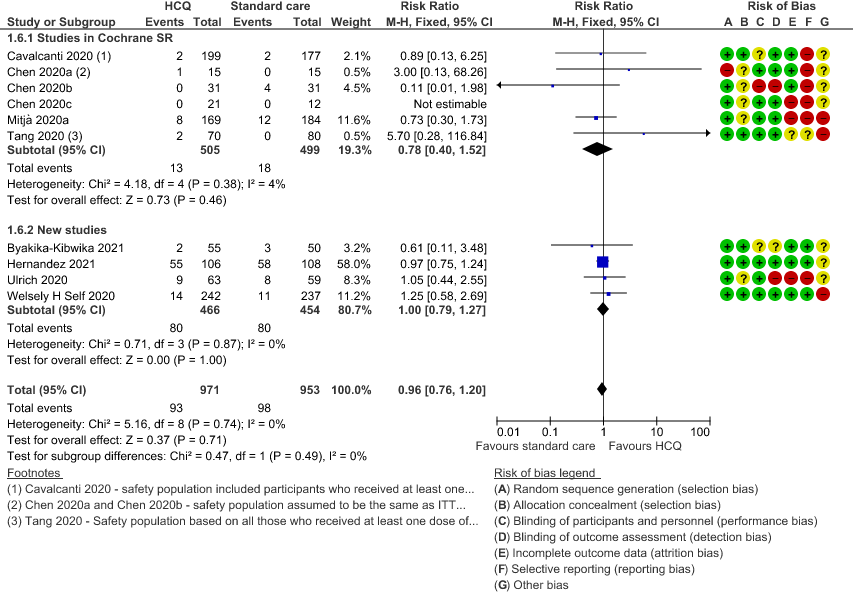
7. Prolonged QTc interval
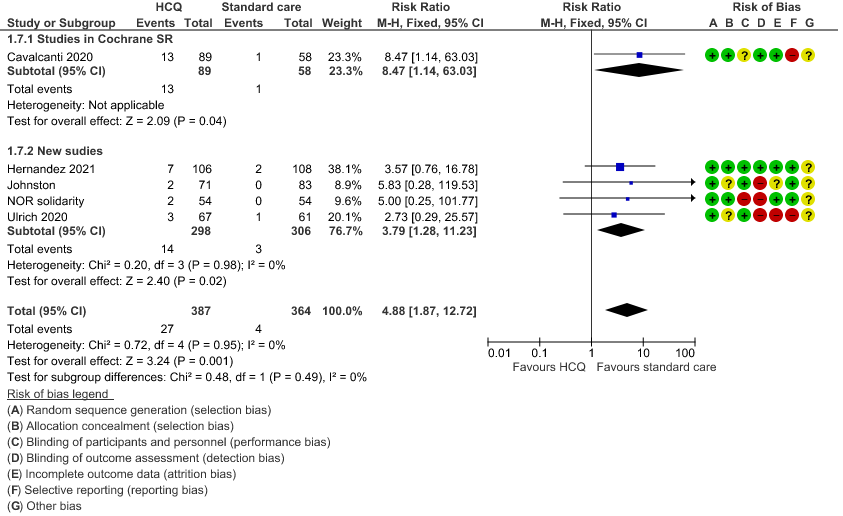
8. Progression to non-invasive mechanical ventilation
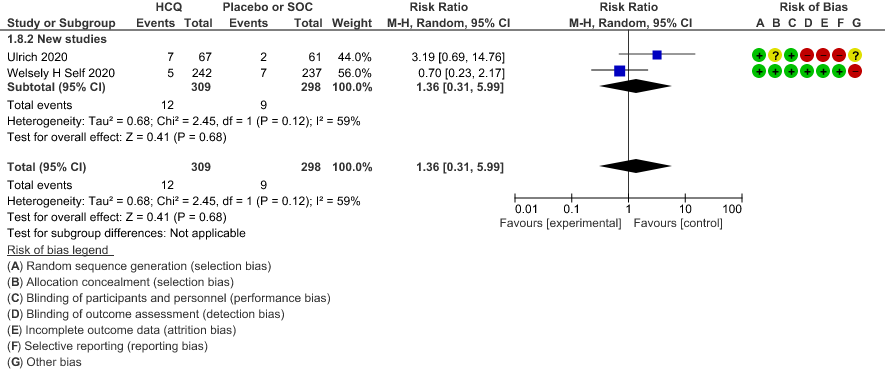
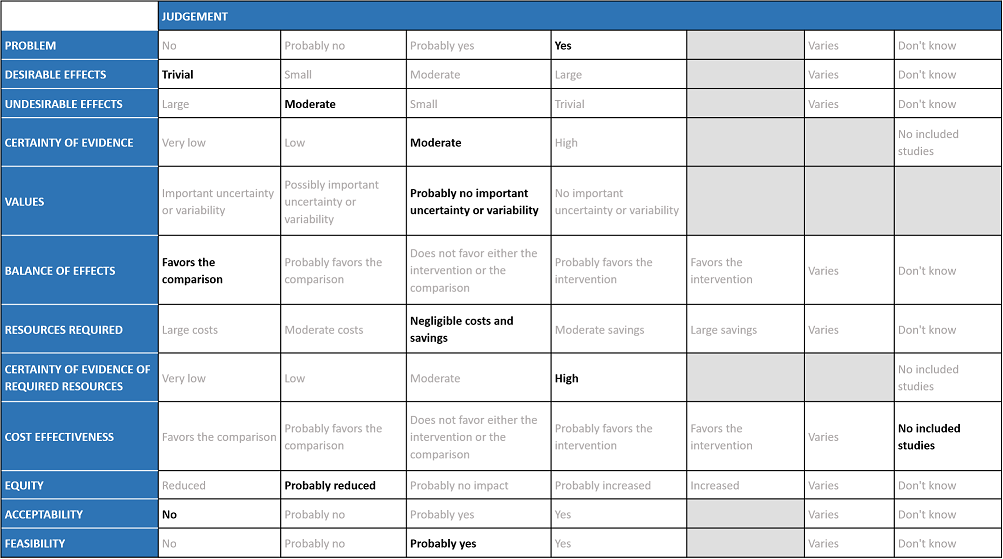
Evidence to decision
The Antiviral Expert Working Group met on 18th November 2021 to consider Hydroxychloroquine/Chloroquine as a treatment for COVID-19. Conflict of interest declarations were reviewed by the Steering Committee; none were found to be relevant to Hydroxychloroquine/Chloroquine.
A summary and then more detailed explanations of the Expert Working Group's (group)judgments follow.
Note that the group decided to apply the recommendation to both Hydroxychloroquine (HCQ) and Chloroquine (CQ), due to their very similar mechanism of action and other properties.
Problem
The COVID-19 pandemic in India with more than 41 million cases and over 0.48 million deaths has significantly impacted and stressed the health structure of the country. The onslaught of the delta variant created a shortage of intensive care unit beds, oxygen and trained personnel with the country facing a major health crisis. The group judged the problem to be of utmost priority.
Desirable effects
The group agreed that there was high quality evidence suggesting that HCQ/CQ did not reduce mortality, or time to negative PCR. There was low to very low-quality evidence suggesting HCQ/CQ did not impact progression to non-invasive mechanical ventilation and mechanical ventilation, or even time to clinical improvement. The group discussed and concluded that the desirable effects were trivial.
Undesirable effects
The pooled data suggested that hydroxychloroquine/chloroquine did not have clinically significant serious adverse events but overall did slightly increase adverse effects and QT prolongation (this is well known adverse effect of HCQ/CQ). However, it was found to be safe when used long term in smaller doses, but several larger trials have used higher doses.
Certainty of evidence
Using GRADE methodology, the evidence synthesis team rated the certainty of evidence high for mortality, negative PCR by day 6; very low for progression to mechanical ventilation and low for adverse events. The overall certainty of evidence was rated as moderate as Horby et al contributed a large number of the total participants16. The group agreed with these judgments and rated the overall certainty of evidence as moderate.
Values
The EWG felt that all the outcomes including those of mortality, negative PCR by day 14, progression to mechanical ventilation and adverse events were expressed variably in the studies. However, there is probably no important uncertainty or variability on how people would value the main outcomes. Some concerns about limited evidence on need for hospitalisation and time to clinical improvement; therefore evidence presented was skewed towards those more important for severely-critically unwell patients.
Balance of effects
The group felt that the balance of effects favors the comparison. The group felt that there is no convincing evidence to prove the benefit of this intervention but possibly some harms. The group also noted that in many studies there was an active comparator drug that could also have adverse effects, however the assumption made in this analysis here, is that this would be equal to no HCQ/CQ which may not be entirely accurate. In addition some studies mentioned patients with suspected COVID-19 were given HCQ/CQ which again could overestimate the side effects and underestimate the efficacy or vice versa.
Resources required
The group felt that there were negligible costs and savings. The erratic supply chain had led to an increased demand for this drug during the initial phase of the first wave of the pandemic despite its uncertain clinical benefits until the drug was removed from the ministry of health guidelines for covid-19 on August 20th 2021. Costs of managing adverse events, or monitoring associated with specific events such as QT prolongation, might not be negligible. The group includes clinicians in different types of Indian hospitals who have a good idea of drug and hospitalization costs.
Certainty of evidence of required resources
No studies reporting this were reviewed by the group but the clinicians in the group were aware of the cost and hence felt that there was high certainty of evidence for required resources to implement this intervention. Drug is not expensive, but costs of managing prolonged QT can be considerable (involves ECG, monitoring etc)
Cost effectiveness
No effectiveness studies were included, so with a no-HCQ/CQ comparison, this could be proposed to favour the comparison, but as there is no evidence for this, we have agreed to not pass judgement on this. Also, we would not treat people with “placebo” so we can’t use that to consider it more cost effective.
Equity
At this point in time this intervention probably reduces equity due to cost and need for hospitalization. There was an observed indirect impact on equity for patients who are using HCQ/CQ for other indications, many of whom are taking it long-term, when demand soared for it for covid-19 treatment.
Acceptability
The group felt that this intervention is likely to have no acceptance because there is no observed effect but only harm.
Feasibility
This is probably a feasible intervention because it is an oral drug and of low cost. However, manufacturing may lag behind demand and also it causes significant discomfort with high-dose acute use. That’s why/how it got sold/used in large quantities.
Hydroxychloroquine/Chloroquine is widely available and is used frequently for the treatment of malaria and some autoimmune conditions. It is an oral drug, and hence easy to administer in the outpatient setting. It is relatively inexpensive, but has moderate risk of adverse effects. It has been used indiscriminately in the treatment of COVID-19 without evidence of efficacy, and may be associated with direct or indirect harms. Its use may lead to a false sense of security and delays in getting appropriate medical care, as well as implementation of other measures which are of proven benefit. The evidence currently does not justify the use of Hydroxychloroquine/Chloroquine.
Our strong recommendation against the use of HCQ/CQ applies to all subgroups of patients with COVID-19. The group considered all trials of HCQ/CQ and found no subgroups where there was benefit in clinically meaningful endpoints whether evaluated by severity or co morbidities. There is limited data available assessing its use in patients with COVID-19 with liver or kidney disease.
The recommendation against the use of Hydroxychloroquine/Chloroquine will be reviewed as and when any new evidence emerges. Although the evidence for discontinuation of the drug because of the undesirable effects reported with widespread use such as cardiac arrhythmias, QT prolongation & CQ retinopathy (bullous maculopathy) have so far been low, this needs careful monitoring as the potential for drug-related harm cannot be ruled out and may emerge in future with the reported dosage used in the treatment of COVID-19.
With moderate to high quality evidence of lack of efficacy of hydroxychloroquine/chloroquine in the treatment of COVID-19, and some evidence of harm, it is undesirable to pursue further research into the use of hydroxychloroquine/chloroquine for the treatment of COVID-19.
- Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19?. Int J Antimicrob Agents. 2020;55(5):105938. doi:10.1016/j.ijantimicag.2020.105938.
- Lee TC, Senecal J, Hsu JM, McDonald EG. Ongoing Citations of a Retracted Study Involving Cardiovascular Disease, Drug Therapy, and Mortality in COVID-19. JAMA Internal Medicine. 2021 Nov 1;181(11):1535–7
- Mehra MR, Ruschitzka F, Patel AN. Retraction—Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. The Lancet. 2020 Jun;395(10240):1820.
- COVID-19 Map [Internet]. Johns Hopkins Coronavirus Resource Center. [cited 2021 Dec 2]. Available from: https://coronavirus.jhu.edu/map.html
- COVID-19 integrated surveillance: key national data [Internet]. [cited 2021 Dec 2]. Available from: https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-integrated-surveillance-data
- Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020 Aug 11;m3026.
- Singh B, Ryan H, Kredo T, Chaplin M, Fletcher T. Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19. Cochrane Infectious Diseases Group, editor. Cochrane Database of Systematic Reviews [Internet]. 2021 Feb 12 [cited 2021 Nov 29];2021(2). Available from: http://doi.wiley.com/10.1002/14651858.CD013587.pub2
- Phase 2 randomized study on chloroquine, hydroxychloroquine or ivermectin in hospitalized patients with severe manifestations of SARS-CoV-2 infection [Internet]. [cited 2021 Nov 29]. Available from: https://www.tandfonline.com/doi/epub/10.1080/20477724.2021.1890887?needAccess=true
- Eze P, Mezue KN, Nduka CU, Obianyo I, Egbuche O. Efficacy and safety of chloroquine and hydroxychloroquine for treatment of COVID-19 patients-a systematic review and meta-analysis of randomized controlled trials. Am J Cardiovasc Dis. 2021 Feb 15;11(1):93–107.
- Kashour Z, Riaz M, Garbati MA, AlDosary O, Tlayjeh H, Gerberi D, et al. Efficacy of chloroquine or hydroxychloroquine in COVID-19 patients: a systematic review and meta-analysis. Journal of Antimicrobial Chemotherapy. 2021 Jan 1;76(1):30–42.
- Abd-Elsalam S, Esmail ES, Khalaf M, Abdo EF, Medhat MA, Ghafar MSAE, et al. Hydroxychloroquine in the Treatment of COVID-19: A Multicenter Randomized Controlled Study. The American Journal of Tropical Medicine and Hygiene. 2020 Oct 7;103(4):1635–9.
- Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med. 2020 Nov 19;383(21):2041–52.
- Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial [Internet]. 2020 Apr [cited 2021 Nov 29] p. 2020.03.22.20040758. Available from: https://www.medrxiv.org/content/10.1101/2020.03.22.20040758v3
- Chen L, Zhang Z-Y, Fu J-G, Feng Z-P, Zhang S-Z, Han Q-Y, et al. Efficacy and safety of chloroquine or hydroxychloroquine in moderate type of COVID-19: a prospective open-label randomized controlled study [Internet]. 2020 Jun [cited 2021 Nov 29] p. 2020.06.19.20136093. Available from: https://www.medrxiv.org/content/10.1101/2020.06.19.20136093v1
- Chen J, Liu D, Liu L, Liu P, Xu Q, Xia L, et al. [A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19]. Zhejiang Da XueXue Bao Yi Xue Ban. 2020 May 25;49(2):215–9.
- The RECOVERY Collaborative Group. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020 Nov 19;383(21):2030–40.
- Mitjà O, Corbacho-Monné M, Ubals M, Tebé C, Peñafiel J, Tobias A, et al. Hydroxychloroquine for Early Treatment of Adults With Mild Coronavirus Disease 2019: A Randomized, Controlled Trial. Clinical Infectious Diseases. 2020 Jul 16;ciaa1009.
- Maisonnasse P, Guedj J, Contreras V, Behillil S, Solas C, Marlin R, et al. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020 Sep 24;585(7826):584–7.
- Skipper CP, Pastick KA, Engen NW, Bangdiwala AS, Abassi M, Lofgren SM, et al. Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19: A Randomized Trial. Annals of Internal Medicine. 2020 Oct 20;173(8):623–31.
- Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020 May 14;m1849.
- Self WH, Semler MW, Leither LM, Casey JD, Angus DC, Brower RG, et al. Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients With COVID-19: A Randomized Clinical Trial. JAMA. 2020 Dec 1;324(21):2165–76.
- Ulrich RJ, Troxel AB, Carmody E, Eapen J, Bäcker M, DeHovitz JA, et al. Treating COVID-19 With Hydroxychloroquine (TEACH): A Multicenter, Double-Blind Randomized Controlled Trial in Hospitalized Patients. Open Forum Infectious Diseases. 2020 Oct 1;7(10):ofaa446.
- Hernandez-Cardenas C, Thirion-Romero I, Rivera-Martinez NE, Meza-Meneses P, Remigio-Luna A, Perez-Padilla R, et al. Hydroxychloroquine for the Treatment of Severe Respiratory Infection by Covid-19: A Randomized Controlled Trial [Internet]. 2021 Feb [cited 2021 Nov 30] p. 2021.02.01.21250371. Available from: https://www.medrxiv.org/content/10.1101/2021.02.01.21250371v1
- Gonzalez JLB, Gámez MG, Enciso EAM, Maldonado RJE, Palacios DH, Campos SD, et al. Efficacy and safety of Ivermectin and Hydroxychloroquine in patients with severe COVID-19. A randomized controlled trial [Internet]. 2021 Feb [cited 2021 Nov 30] p. 2021.02.18.21252037. Available from: https://www.medrxiv.org/content/10.1101/2021.02.18.21252037v1
- Omrani AS, Pathan SA, Thomas SA, Harris TRE, Coyle PV, Thomas CE, et al. Randomized double-blinded placebo-controlled trial of hydroxychloroquine with or without azithromycin for virologic cure of non-severe Covid-19. EClinicalMedicine. 2020 Dec;29–30:100645.
- Ader F, Peiffer-Smadja N, Poissy J, Bouscambert-Duchamp M, Belhadi D, Diallo A, et al. An open-label randomized controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-β-1a and hydroxychloroquine in hospitalized patients with COVID-19. Clinical Microbiology and Infection. 2021 Dec 1;27(12):1826–37.
- Arabi YM, Gordon AC, Derde LPG, Nichol AD, Murthy S, Beidh FA, et al. Lopinavir-ritonavir and hydroxychloroquine for critically ill patients with COVID-19: REMAP-CAP randomized controlled trial. Intensive Care Med. 2021 Aug;47(8):867–86.
- Byakika-Kibwika P, Lamorde M, Mayanja-Kizza H, Merry C, Colebunders B, Van geertruyden J-P. Update on the efficacy, effectiveness and safety of artemether–lumefantrine combination therapy for treatment of uncomplicated malaria. Ther Clin Risk Manag. 2010;6:11–20.
- Johnston C, Brown ER, Stewart J, Karita HCS, Kissinger PJ, Dwyer J, et al. Hydroxychloroquine with or without azithromycin for treatment of early SARS-CoV-2 infection among high-risk outpatient adults: A randomized clinical trial. EClinicalMedicine. 2021 Mar;33:100773.
- Dubée V, Roy P-M, Vielle B, Parot-Schinkel E, Blanchet O, Darsonval A, et al. Hydroxychloroquine in mild-to-moderate coronavirus disease 2019: a placebo-controlled double blind trial. Clinical Microbiology and Infection. 2021 Aug 1;27(8):1124–30.
- Kamran SM, Moeed HA, Mirza Z-H, Naseem A, Azam R, Ullah N, et al. Clearing the Fog: Is Hydroxychloroquine Effective in Reducing Coronavirus Disease-2019 Progression? A Randomized Controlled Trial. Cureus [Internet]. 2021 Mar 30 [cited 2021 Nov 30];13(3). Available from: https://www.cureus.com/articles/54281-clearing-the-fog-is-hydroxychloroquine-effective-in-reducing-coronavirus-disease-2019-progression-a-randomized-controlled-trial
- Lyngbakken MN, Berdal J-E, Eskesen A, Kvale D, Olsen IC, Rueegg CS, et al. A pragmatic randomized controlled trial reports lack of efficacy of hydroxychloroquine on coronavirus disease 2019 viral kinetics. Nat Commun. 2020 Dec;11(1):5284.
- Schwartz I, Boesen ME, Cerchiaro G, Doram C, Edwards BD, Ganesh A, et al. Assessing the efficacy and safety of hydroxychloroquine as outpatient treatment of COVID-19: a randomized controlled trial. cmajo. 2021 Apr;9(2):E693–702.
- Reis G, Moreira Silva EA dos S, Medeiros Silva DC, Thabane L, Singh G, Park JJH, et al. Effect of Early Treatment With Hydroxychloroquine or Lopinavir and Ritonavir on Risk of Hospitalization Among Patients With COVID-19: The TOGETHER Randomized Clinical Trial. JAMA Network Open. 2021 Apr 22;4(4):e216468.
- Barratt-Due A, Olsen IC, Nezvalova-Henriksen K, Kåsine T, Lund-Johansen F, Hoel H, et al. Evaluation of the Effects of Remdesivir and Hydroxychloroquine on Viral Clearance in COVID-19: A Randomized Trial. Ann Intern Med. 2021 Sep;174(9):1261–9.9
Covid Management Guidelines India Group – Anti-Viral Working Group – Hydroxychloroquine/Chloroquine. Covid Guidelines India; Published online on February 04th, 2022; URL: Hydroxychloroquine / Chloroquine – Covid Guidelines India (indiacovidguidelines.org) (accessed <date>)
