This recommendation applies to acute COVID-19 in adults. Some of our recommendations vary according to the severity of COVID-19 illness. Definitions of the categories are based on the World Health Organization (WHO) criteria and can be viewed by clicking the plus (+) signs below.
RECOMMENDATION:
We do not recommend use of Colchicine in inpatients with non-severe, severe or critical illness as there is moderate quality evidence to suggest that the critical outcomes are unchanged. No recommendation is given for mild infection in out-patient settings as further evidence is required to inform practice.
DATE OF RECOMMENDATION: 03rd September 2021
Mild infection
- Symptomatic (any acute COVID-19 related symptoms
- AND respiratory rate <24/min
- WITHOUT pneumonia or hypoxia
Mild or moderate illness without hypoxia
- Pneumonia (clinical or radiological)
- AND respiratory rate ≤30/min
- AND SpO2 ≥94% on room air
Moderate illness with hypoxia
NO features of severe or critical illness (see below).
- Hypoxia (SpO2 <94% on room air)
- AND respiratory rate ≤30/min
- AND SpO2 ≥90% on room air
Severe infection
Pneumonia with ANY ONE of the following:
- respiratory rate >30/min
- severe respiratory distress
- SpO2 <90% on room air
- NO invasive or non-invasive respiratory support needed
Critical infection
- Requirement for high-level respiratory support: non-invasive ventilation, high-flow oxygen (≥20 litres per minute) or invasive mechanical ventilation
- OR acute respiratory distress syndrome (PaO2/FiO2 ratio of <300)
- OR sepsis
- OR shock
Colchicine has been used as an anti-inflammatory drug in the past and in COVID-19, it has been thought to reduce vascular endothelial inflammation by targeting the “inflammasome”. There is extensive experience with the use of Colchicine in diseases like Gout and the mechanism of action here is believed to be due to impairment of neutrophil function, prevention of macrophage activation and degranulation of mast cells and thus prevention of inflammation. The evidence from RCTs indicates that in hospitalized patients with COVID-19 with non-severe or severe to critical illness, colchicine use was not associated with reduction in hospital stay, progression to invasive mechanical ventilation or mortality suggesting that there was no benefit to using Colchicine for this group of patients. In the single randomized control study conducted in outpatients with COVID-19, there was a slight reduction to hospital admission in those given Colchicine, however the other critical outcomes like mortality or progression to invasive mechanical ventilation were not convincingly reduced. The confidence intervals were wide and event rates were low in both the placebo and colchicine groups and hence since it was a single trial the evidence was deemed insufficient to come to a robust conclusion. In addition, in all the subgroups studies, Colchicine was found to cause significant adverse events and diarrhea as compared to the control groups again leading to a fair bit of uncertainty regarding implementation of this drug. Further trials are essential to be confident of effects produced by Colchicine and frame a recommendation for this sub group of patients. Overall, there seems to be no benefit of Colchicine over standard of care in hospitalized severe or non-severe COVID-19 illness, and the data available in outpatients with COVID-19 so far is inconclusive.
Date of latest search: 15th July 2021.
Date of completion & presentation to Expert Working Group: 22nd July 2021.
Date of planned review: 15th January 2022
Evidence synthesis team: Padmanaban Venkatesan, Divya Deodhar, Richard Kirubakaran and Priscilla Rupali.
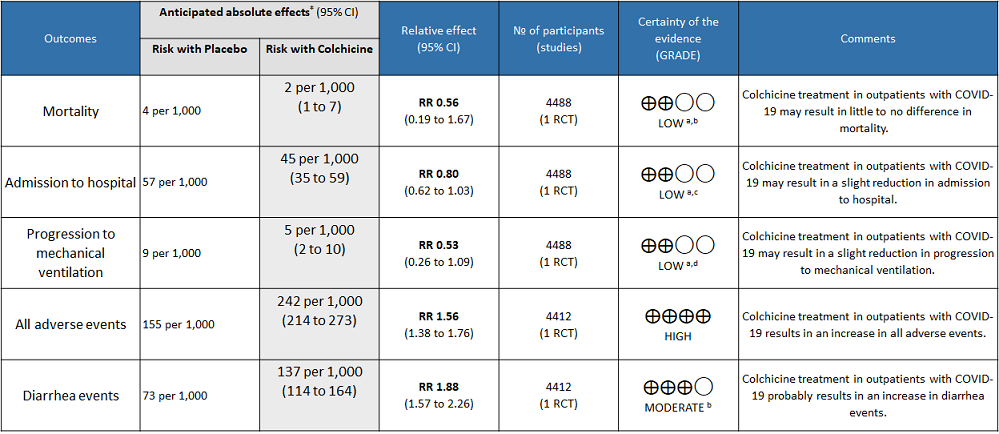
Colchicine compared to Placebo for outpatients with COVID-19
Explanations
a. Downgraded by one level for serious imprecision as the confidence interval crosses 1
b. Downgraded by one level for serious imprecision because of small number of events
c. Downgraded by one level for very small absolute difference(1.2% points) which doesn't cross the clinical decision making threshold
d. Downgraded by one level for serious imprecision because of small number of events
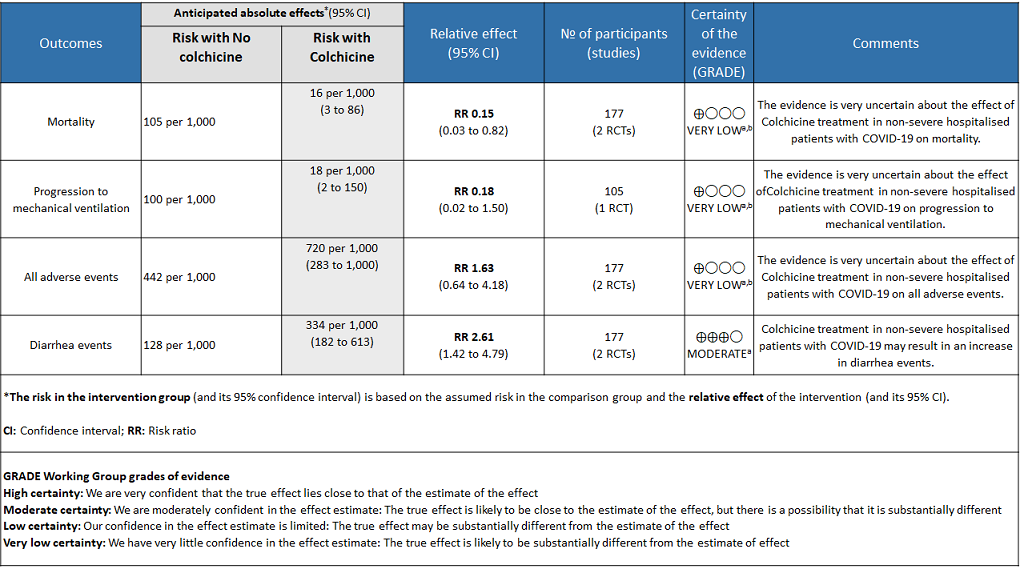
Colchicine compared to no Colchicine for hospitalised non-severe COVID-19 patients
Explanations
a. Downgraded by one level for risk of bias
b. Downgraded by two levels for very serious imprecision as the sample size is small and the confidence interval is large
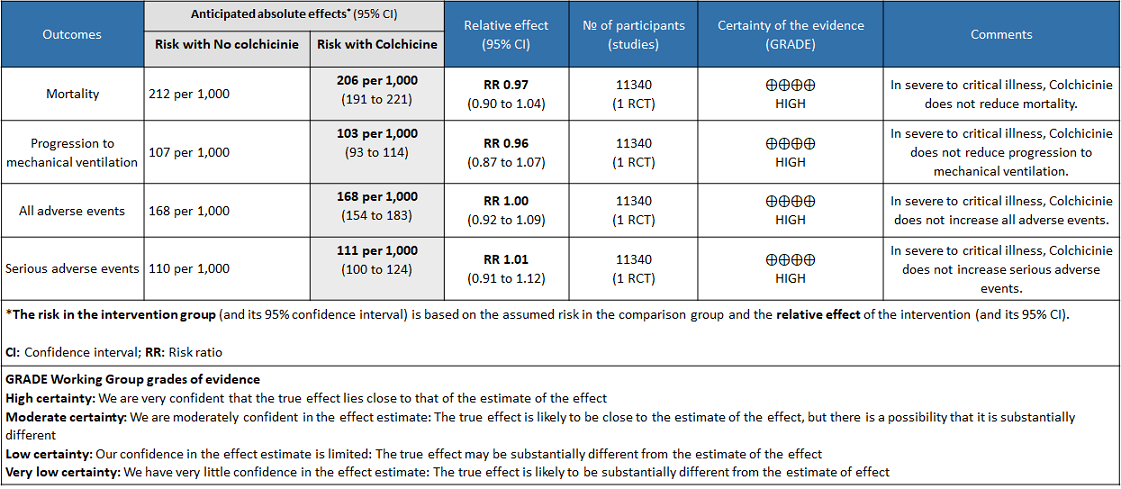
Colchicine compared to No colchicine for hospitalised patients with severe COVID-19
As of July 25, 2021, COVID-19 infection has affected more than 3 crore people and killed more than 4 lakh people in India as per official records1. As the health care systems in India continue to struggle, there is a dire need for cheaper and effective drugs to prevent hospitalization and reduce mortality due to COVID-19. Since inflammation is known to play a major role in pathogenesis of COVID-19, anti-inflammatory drugs are used to treat COVID-192,3. In particular, activation of the NLRP3 inflammasome of the innate immune system is found to increase the inflammatory cytokines IL-1β, IL-6 and TNF which cause tissue damage in COVID-194. It reduces inflammation by multiple mechanisms including inhibiting NLRP3 inflammasome and so was proposed as a potential drug to treat COVID-195. There is extensive experience with the use of Colchicine as an anti-inflammatory drug in Gout. However, adverse effects including multiple debilitating gastrointestinal side effects especially diarrhea have precluded the wide usage of this drug in gout and other rheumatic diseases.
A scoping search for use of Colchicine in COVID-19 was done. The criteria for selection of this systematic review were based on the following:
Included and only meta-analysed data from randomized controlled trials
Had a recent latest search date (within the last three months)
Included all available outcomes
Matched the pre-defined PICO question closely
Scored a high or moderate score on the AMSTAR 2 quality assessment tool
To identify all available systematic reviews pertaining to our PICO question, a systematic search of PubMed, Cochrane and the Epistemonikos databases was conducted. The search strategy was designed and validated by the Group’s information specialist. The search results were screened independently by two reviewers against the pre-defined criteria set out above, using Rayyan. 17 articles were found, and 8 articles were identified as systematic reviews on use of Colchicine in COVID-19. But none of the 8 articles exclusively included and only meta-analysed data from randomized controlled trials. So, we decided to conduct our own meta-analysis of randomised controlled trials of Colchicine use in COVID-19.
Population: Outpatients, Non-severe hospitalised patients, severe hospitalised patients.
Following were the outcomes we wanted to extract data for:
1. Primary outcome:
a. Time to clinical recovery
2. Secondary outcome:
a. Inpatients:
-
-
- Need for mechanical ventilation
- All-cause mortality
- Length of stay in hospital
- Admission to critical care
-
b. Outpatients:
-
-
- Admission to hospital
- Time to symptom resolution
-
3. Adverse events:
-
-
- All
- Serious
- Neutropaenia
- Renal impairment
- GI intolerance
-
Cochrane RoB2 tool was used to assess the risk of bias in the randomised control trials. For all outcomes, one review author performed the assessment, and cross-checked it against the assessment by another review author who compared it with the COVID NMA platform. If there was a difference in more than one domain, it was assessed by a third independent author.
We performed our own meta-analysis using Review Manager (RevMan) 5.4 using a fixed‐effects model for each of the critical and important outcomes. We used risk ratios (RR) for dichotomous outcomes with 95% confidence intervals (CIs). We used the I2 statistic to measure residual heterogeneity. We used GRADE methodology to assess the certainty in the evidence and documented this in a ‘Summary of findings’ table using GradeProGDT.
We found 5 randomised controlled trials that met our search criteria6–10.
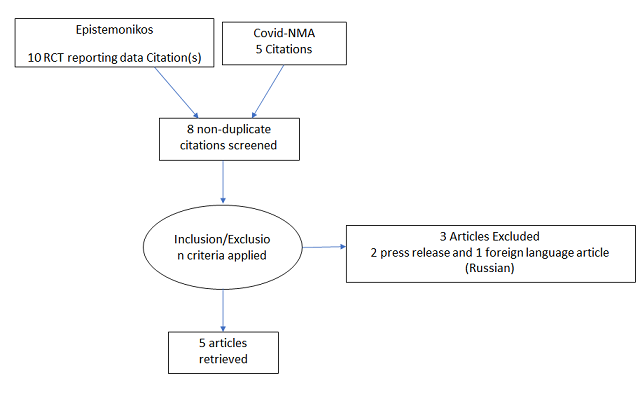
The five RCTs differed in their patient population and so we divided our analysis into three groups:
1. Colchicine compared to Placebo for outpatients with COVID-19 (Tardiff et.al., 2021)
2. Colchicine compared to No colchicine for hospitalised non-severe COVID-19 patients (Defteros et.al., 2020, Lopes et.al., 2021, Salhezdah et.al., 2020)
3. Colchicine compared to No colchicine for hospitalised patients with severe COVID-19 (Horby et.al., 2021)
Colchicine compared to Placebo for outpatients with COVID-19:
- All-cause mortality: Low certainty evidence from 1 RCT6 that included 4488 outpatients with COVID-19 disease indicated that Colchicine makes little to no difference in mortality in outpatients with COVID-19 infection RR=0.56(95% CI; 0.19-1.67).
- Admission to hospital: Low certainty evidence from 1 RCT6 that included 4488 outpatients with COVID-19 disease indicates that colchicine reduces hospital admission by 20% (RR = 0.80;95% CI 0.62-1.03) compared to no colchicine.The number needed to treat (NNT) to reduce one additional admission to hospital is 83.33 outpatients with COVID-19 infection.
- Progression to mechanical ventilation: Low certainty evidence from 1 RCT6 that included 4488 outpatients with COVID-19 disease indicates that colchicine may have reduced progression to mechanical ventilation by 47% (RR=0.53;95% CI 0.26-1.09) compared to no colchicine. The number needed to treat (NNT) to reduce one additional progression to mechanical ventilation is 250 outpatients with COVID-19.
- All adverse events: High certainty evidence from 1 RCT6 that included 4488 outpatients with COVID-19 disease indicates that colchicine increases adverse events by 56% (RR=1.56; 95%CI 1.38-1.76 ) compared to placebo. The number needed to harm (NNH) for one additional adverse event is 11.5 outpatients with COVID-19 infection given Colchicine.
- Diarrhea events: High certainty evidence from 1 RCT6 that included 4488 outpatients with COVID-19 disease indicates that colchicine increases diarrhea events by 88% (RR=1.88; 95%CI 1.57-2.26) compared to placebo. We anticipate that there would be 64 more (from 41 more to 91 more) diarrhea events per 1000 people if colchicine is administered for outpatients with COVID-19. The number needed to harm (NNH) resulting in one additional diarrhea event for 6 outpatients with COVID-19 given Colchicine.
Colchicine compared to No colchicine for hospitalised non-severe COVID-19 patients:
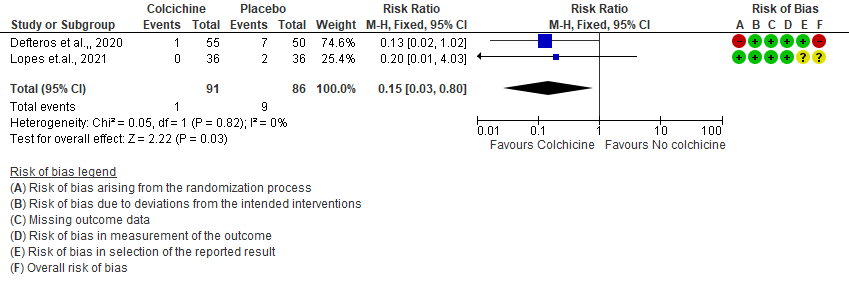
- All-cause mortality: Very low certainty evidence from 2 RCTs7,8 that included 177 hospitalized patients with non-severe COVID-19 disease indicates that the evidence is very uncertain about the effect of Colchicine treatment in hospitalized non-severe COVID-19 patients on mortality [RR= 0.15; 95%CI 0.03-0.82].
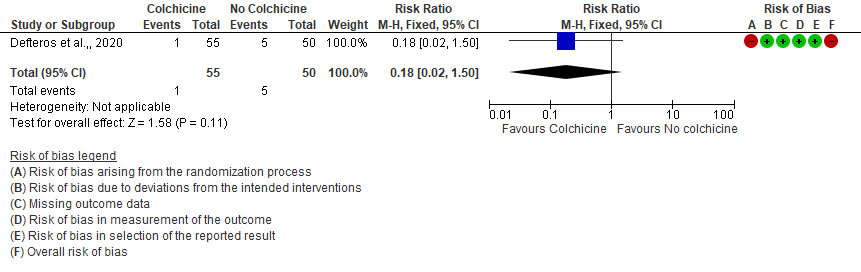
- Progression to mechanical ventilation: Very low certainty evidence from 1 RCT7 that included 105 hospitalised patients with non-severe COVID-19 disease indicates that the evidence is very uncertain about the effect of Colchicine treatment in hospitalized non-severe COVID-19 patients on progression to mechanical ventilation [RR=0.18;95% CI 0.02-1.50].
- All adverse events: Very low certainty evidence from 2 RCTs7,8 that included 177 hospitalized patients with non-severe COVID-19 disease indicates that the evidence is very uncertain about the effect of Colchicine treatment in hospitalized non-severe COVID-19 patients on all adverse events [RR= 1.63; 95% CI 0.64-4.18].
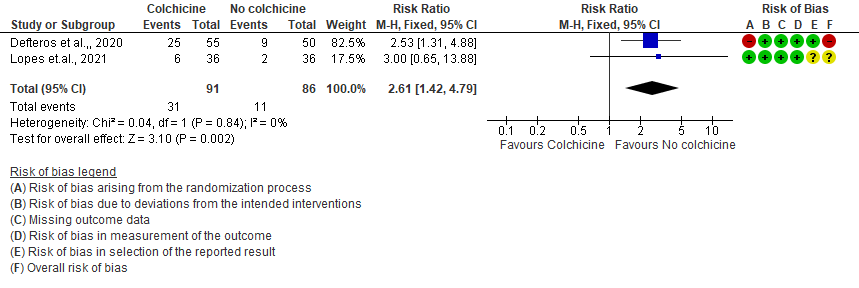
- Diarrhea events: Moderate certainty evidence from 2 RCTs7,8 that included 177 hospitalized patients with non-severe COVID-19 disease indicates that colchicine may increase in diarrhea events by 161% [RR = 2.61; 95% CI 1.42-4.79] compared to placebo. The number needed to harm (NNH) to result in one additional diarrhea event is 5.7 hospitalized non-severe COVID-19 patients.
Colchicine compared to No colchicine for hospitalised patients with severe COVID-19:
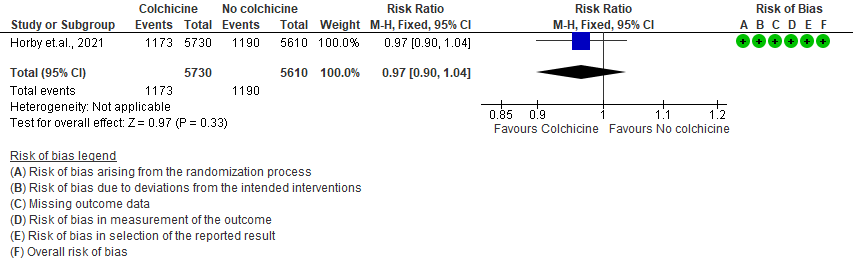
- All-cause mortality: Moderate certainty evidence from 1 RCT10 that included 11340 people with severe COVID-19 disease indicates that colchicine probably does not reduce mortality [RR = 0.97; 95% CI 0.90-1.04].
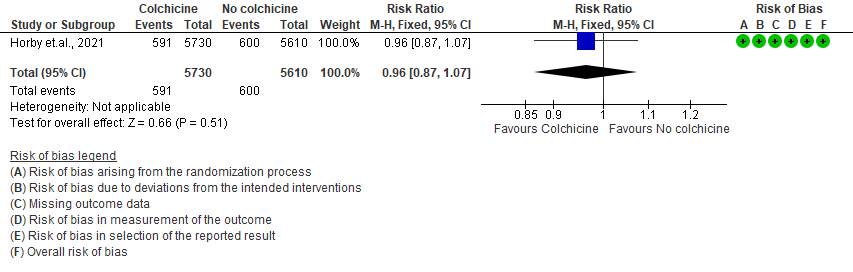
- Progression to mechanical ventilation: Moderate certainty evidence from 1 RCT10 that included 11340 people with severe COVID-19 disease indicates that colchicine probably does not reduce progression to mechanical ventilation [RR=0.96; 95% CI 0.87-1.07].
- All adverse events: Moderate certainty evidence from 1 RCT10 that included 11340 people with severe COVID-19 disease indicates that colchicine probably does not increase/reduce all adverse events [RR=1.00; 95% CI 0.92-1.09].
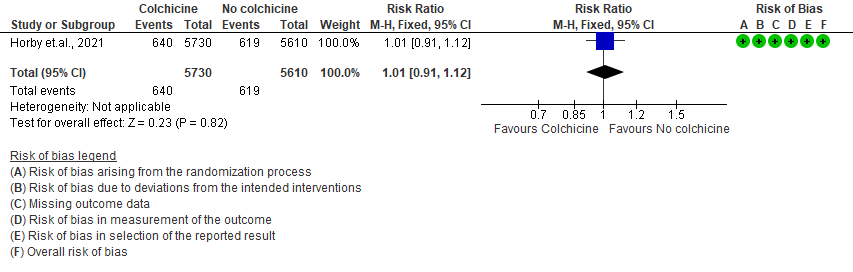
- Serious adverse events: Moderate certainty evidence from 1 RCT10 that included 11340 people with severe COVID-19 disease indicates that colchicine probably does not increase serious adverse events [RR=1.01;95% CI 0.91-1.12].
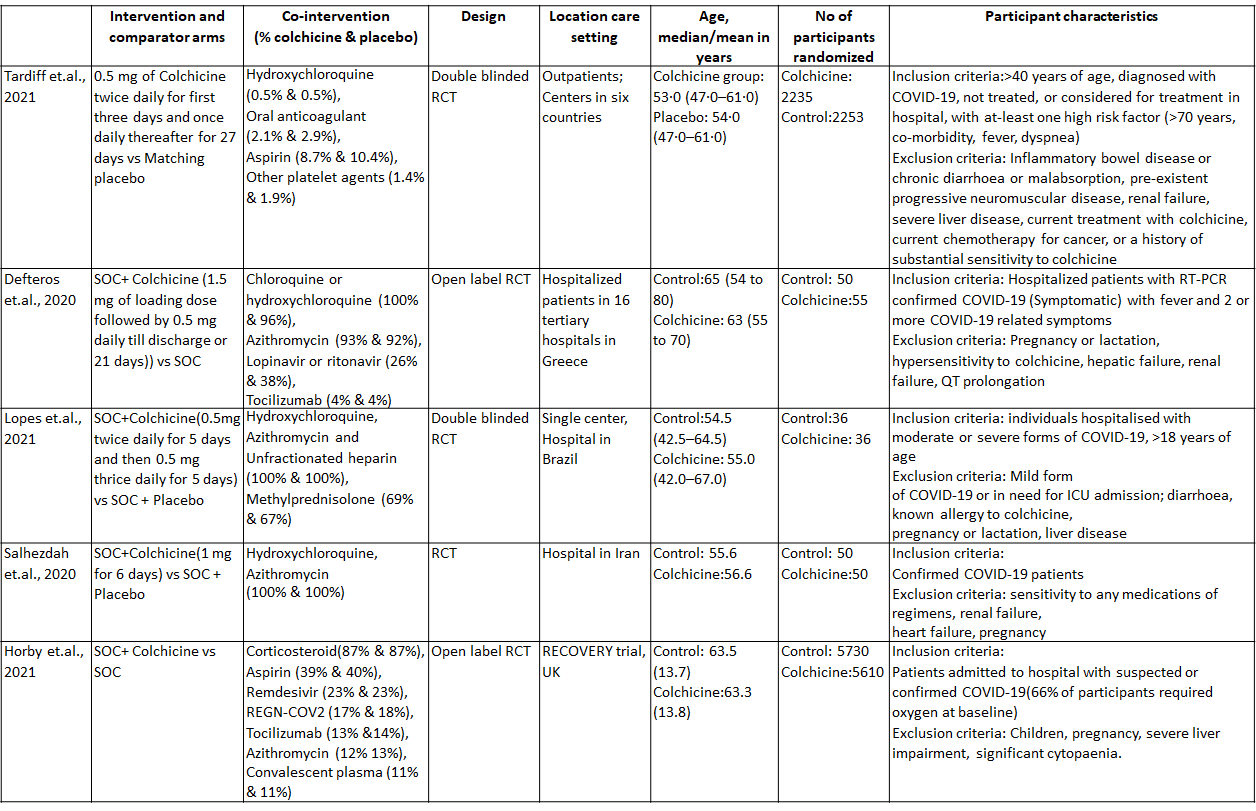
I. Colchicine compared to Placebo for outpatients with COVID-19 (Tardiff et.al., 2021)
1. All-cause mortality
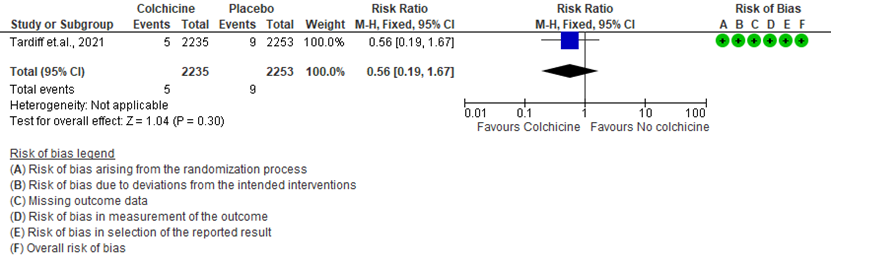
2. Admission to hospital
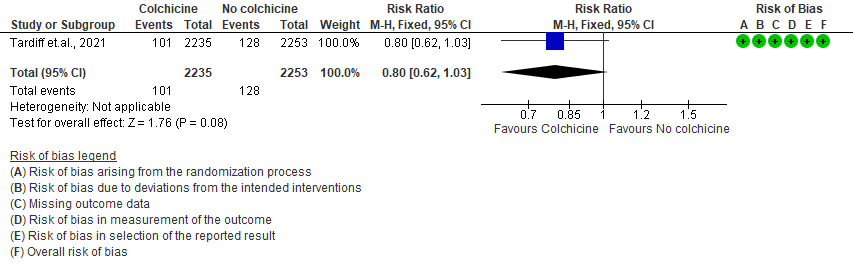
3. Progression to mechanical ventilation
4. All adverse events
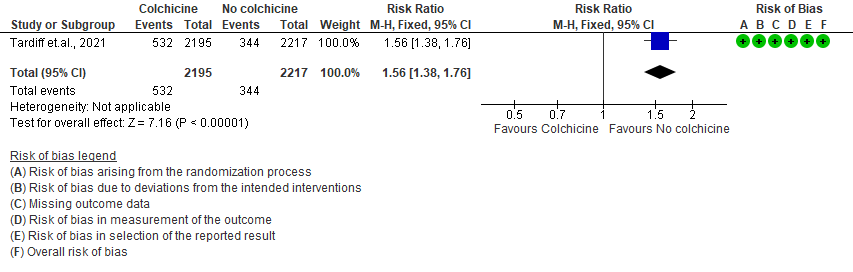
5. Diarrhea events
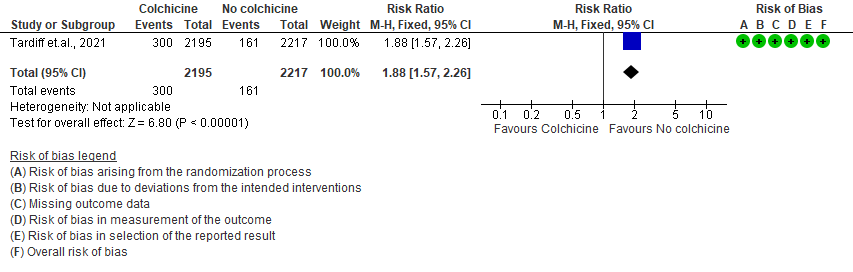
II. Colchicine compared to No colchicine for hospitalised non-severe COVID-19 patients (Defteros et.al., 2020, Lopes et.al., 2021, Salhezdah et.al., 2020)
1. All-cause mortality
2. Progression to mechanical ventilation
3. All adverse events
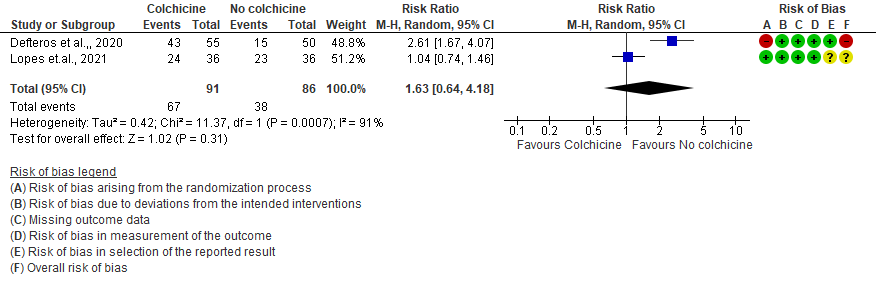
4. Diarrhea
III. Colchicine compared to No colchicine for hospitalized patients with severe COVID-19 (Horby et.al., 2021)
1. All-cause mortality
2. Progression to mechanical ventilation
3. All adverse events
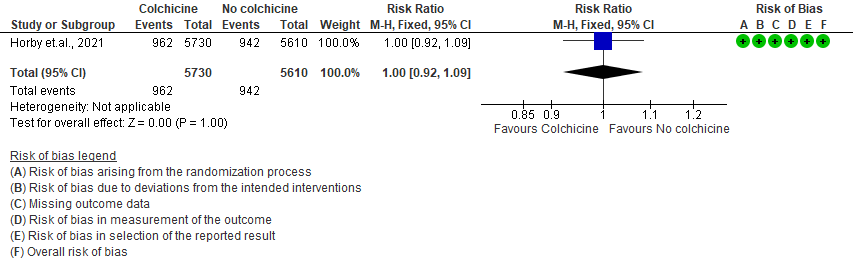
4. Serious adverse events
The Anti-inflammatory Expert Working Group met on 22nd July 2021 to consider Colchicine as a treatment for COVID-19. Conflict of interest declarations were reviewed by the Steering Committee; none were found to be relevant to Colchicine
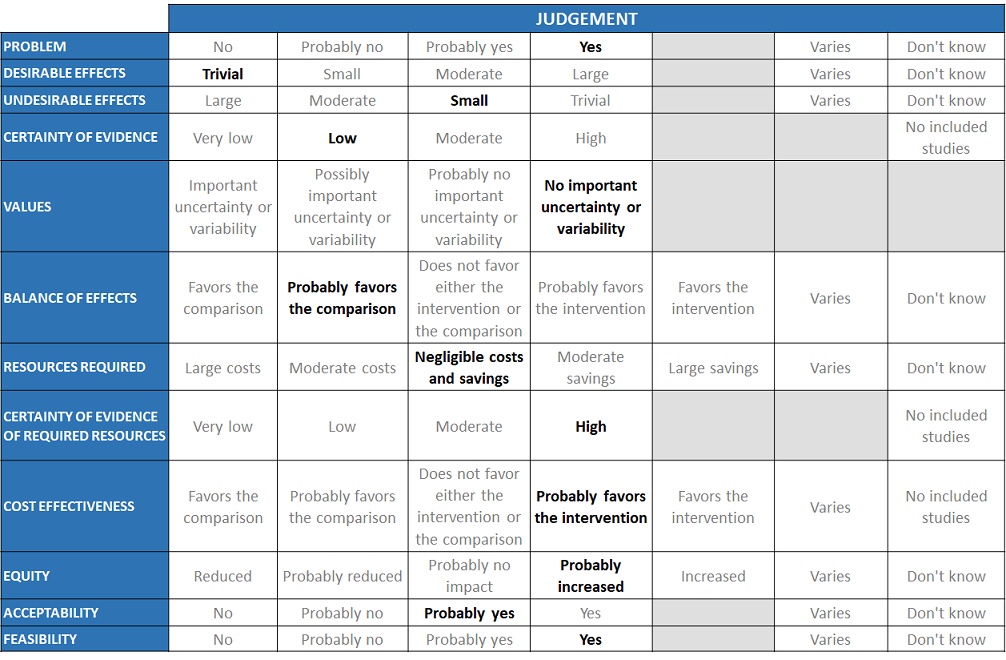
A summary and then more detailed explanations of the Expert Working Group's judgements follow.
Problem
The COVID-19 pandemic in India with more than 30 million cases and over 0.39 million deaths has significantly impacted and stressed the health structure of the country. With a shortage of intensive care unit beds, oxygen and trained personnel the country is facing a major health crisis. This has prompted many irrational and experimental treatment interventions like Colchicine in all severity of patients across the country in patients hospitalized in Covid-19 without a clear indication or evidence in which subgroup of population or disease severity the drug is effective for. The group judged the problem to be of utmost priority.
Desirable effects
The group agreed that the evidence suggests that Colchicine does not have a clinically significant effect on mortality and progression to mechanical ventilation in all the sub groups of severity but may reduce admission to hospital in outpatients. This the group noted was significant but the evidence was from 1 trial with very few events in each of the arms suggesting we could not be confident of the effect of Colchicine for this outcome.
Undesirable effects
The group noted that the certainty of evidence for adverse events and gastrointestinal side effects especially diarrhea was of moderate significance in all subgroups. Comparatively higher number of patients with Colchicine experienced adverse events, especially diarrhea. Hence the group felt that diarrhea which is a well-known adverse effect of Colchicine based on experience with use in gout is an inconvenience to most patients when used for treatment in patients with mild COVID-19 infection.
Certainty of evidence
Using GRADE methodology, the evidence synthesis team rated certainty of evidence was found to be low for desirable effects and moderate for adverse events (diarrhea) for outpatients, whereas in hospitalized non-severe it was very low for critical outcomes of mortality, progression to invasive mechanical ventilation but it was moderate for diarrhea. In severe to critical hospitalized patients, evidence was judged to be moderate for all critical events including adverse events. The group agreed with these judgements and rated the overall certainty of evidence as moderate.
Values
The EWG felt that all the outcomes including those of admission to hospital, mortality and progression to mechanical ventilation were expressed variably in the studies. However, there is no important uncertainty or variability on how people would value the main outcomes.
Balance of effects
The group discussed at length regarding implementation of Colchicine. There was a slight effect in reducing admission to hospital but otherwise other critical outcomes were not impacted significantly by Colchicine for a categoric routine implementation in any of subgroups. Even in the out-patients, though there was a reduction in admission to hospital there were significantly higher side effects noted in this subgroup of patients with diarrhea being particularly significant. This was also in keeping with the group’s experiences with the use of Colchicine in the past for other indications. Hence since the marginal benefit if at all was noted in the outpatient group with mild COVID-19 infection which is the sub group with the lowest mortality and morbidity anyway and hence the group felt that the balance of effects probably favors the comparison, rather than the intervention.
Resources required
The group felt that the costs were negligible to small. The group discussed and concluded that the requirement of resource is negligible cost and savings. The group includes clinicians in different types of Indian hospitals who have a good idea of drug and hospitalization costs.
Certainty of evidence of required resources
No studies reporting this were reviewed by the group but the clinicians in the group were aware of the cost and hence felt that there was high certainty of evidence for required resources to implement this intervention.
Cost effectiveness
The group discussed that even though there was no research evidence that evaluated cost effectiveness of Colchicine. It is a cheap drug in an Indian context, it would probably favor the intervention if found useful in outpatients, though with the limited evidence available from 1 trial the group did not favor its widespread implementation at this point. It does favor the comparison in inpatients, as this intervention did not provide any clinical benefit and in fact lead to an increased incidence of adverse events especially diarrhea.
Equity
At this point in time this intervention would probably increase equity as the cost is low, the drug is widely available and clinicians have prior experience in the use of this drug, if found useful.
Acceptability
The group felt that this intervention is likely to have wide acceptance by all the relevant stakeholders (policy-makers, patients and clinicians) as it is an oral drug that is probably quite safe, but taking evidence and cost into account a well-informed clinician would be unlikely to use it.
Feasibility
This is a feasible intervention if found efficacious as it is easy to deliver and available easily over the counter in the country.
Colchicine is not recommended for use in all hospitalized patients including those patients with severe disease as there are no beneficial effects on all-cause mortality and progression to mechanical ventilation. It also results in significant adverse effects especially diarrhea which can be debilitating in hospitalized patients. No recommendation can be made for the use of Colchicine in outpatients because of its uncertain effects in this group of patients even though the drug is cheap and easily accessible. The limited evidence available from 1 trial is unconvincing and does not impact critical outcomes suggesting that further trials are required to unequivocally suggest that this drug reduces hospital admission.
Clinicians may still choose to use Colchicine in outpatients though the effects are trivial with low certainty of evidence in the absence of other medications for this group of patients. Diarrhea has been noted as an undesirable side effect especially in outpatients and non-severe hospitalized patients. Other adverse events were increased in all groups.
The current recommendation is based on the evidence to date and will be updated as new evidence emerges.
Our recommendation against the use of colchicine applies to all sub-groups of COVID-19 patients. Colchicine use is relatively contra-indicated in pregnant and lactating women, children, patients with severe hepatic and renal impairment and those on concomitant strong CYP-3A4 or P-glycoprotein inhibitors. Such patients were not included in the analyzed trials of colchicine.
We evaluated Colchicine in various subgroups of severity. In out-patients with mild COVID-19 infection, Colchicine seemed to reduce admission to hospital but the evidence was of low certainty suggesting we were not confident of its effects. Other critical outcomes were suggested Colchicine made little to no difference in these outcomes. In hospitalized non-severe COVID-19 patients, evidence was of very low certainty for all critical outcomes as the number of patients and events evaluated for these outcomes were extremely small. In hospitalized severe to critical group of patients with COVID-19 we were able to determine with a moderate degree of certainty that Colchicine did not impact critical outcomes and thus would not be recommended in this subgroup of patients.
Colchicine commonly produces gastrointestinal toxicities like abdominal pain, diarrhea and vomiting. It usually produces rhabdomyolysis when given for a long duration >6 months when given along with other medications which can cause muscle injury like statins. Since cardiac muscle may also be affected, when medications like Digoxin are given caution needs to be exercised as it may result in arrhythmias. Some medications like antifungal azoles, protease inhibitors, antibiotics like Clarithromycin and Telithromycin and Nefazodone are also likely to cause an increase in level of Colchicine thus leading to adverse effects. Myopathy is not a concern in short course as would be used in covid 19; diarrhea is more common with doses above 2 mg /day, although again does not cause permanent damage and usually not of serious concern.
The recommendation against the use of Colchicine will be reviewed as and when new evidence emerges.
Colchicine was not found to be beneficial in any of the trials in hospitalized patients in severe or critical categories. There is still equipoise regarding the benefit of colchicine in mild out-patient category as the evidence was of low certainty and reduced admission to hospital. This benefit probably needs to be explored further in other large randomized controlled trials. This is a cheap drug albeit with a few side effects but if found to have a signal towards benefit should be definitely explored.
- Tardif J-C, Bouabdallaoui N, L’Allier PL, et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. The Lancet Respiratory Medicine. 2021;0(0). doi:10.1016/S2213-2600(21)00222-8
- Deftereos et al, Effect of Colchicine vs Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized with Coronavirus Disease 2019The GRECCO-19 Randomized Clinical Trial
- Lopes et.al, Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial.
- FarhadSalehzadeh, FarhadPourfarzi, SobhanAtaei et.al. The Impact of Colchicine on The COVID-19 Patients; A Clinical Trial Study. doi:10.21203/rs.3.rs-69374/v1
- Horby et al. Colchicine in patients admitted to hospital with 3 COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. RECOVERY TRIAL
Covid Management Guidelines India Group – Anti-inflammatory working Group – Colchicine. Covid Guidelines India; Published online on September 03rd , 2021; URL: https://indiacovidguidelines.org/colchicine/ (accessed <date>).
