This recommendation applies to acute COVID-19 in adults. Some of our recommendations vary according to the severity of COVID-19 illness. Definitions of the categories are based on the World Health Organization (WHO) criteria and can be viewed by clicking the plus (+) signs below.
RECOMMENDATION:
Due to evidence of selective benefit in patients with no detectable COVID-19 antibodies, we do not recommend use of Casirivimab and Imdevimab (REGEN- COV™) for moderate, severe or critical COVID-19 infection in patients with detectable COVID-19 antibodies. However, where timely antibody testing is available (prior to treatment), and the required dose of 8g is available and the high cost does not preclude its use, Casirivimab and Imdevimab (REGEN- COV™) may be used by some clinicians in a narrow subset of patients: hospitalized COVID-19 patients requiring oxygen support, early in the illness (< 7 days of symptoms), with no detectable COVID-19 antibodies (Conditional recommendation).
DATE OF RECOMMENDATION: 16/08/2021
Definition of moderate illness
- Pneumonia (clinical or radiological) OR hypoxia (SpO2 <94% in adults with no underlying lung disease)
- AND respiratory rate ≤30/min
- AND SpO2 ≥90% on room air
Definition of severe illness:
Pneumonia with ANY ONE of the following:
- respiratory rate >30/min
- severe respiratory distress
- SpO2 <90% on room air
- NO invasive or non-invasive respiratory support needed
Definition of critical illness:
- Requirement for high-level respiratory support: noninvasive ventilation, high-flow oxygen (≥20 litres per minute) or invasive mechanical ventilation
- OR acute respiratory distress syndrome (PaO2/FiO2 ratio of <300)
- OR sepsis
- OR shock
A conditional recommendation is one for which the desirable effects probably outweigh the undesirable effects (weak recommendation FOR an intervention) or undesirable effects probably outweigh the desirable effects (weak recommendation AGAINST an intervention) but appreciable uncertainty exists. This implies that not all will be best served by this recommendation and decisions can be made by the patient and caregiver based on patient values, resources and setting with a clear understanding of the ensuing harms and benefits.
Definition of moderate illness
- Pneumonia (clinical or radiological) OR hypoxia (SpO2 <94% in adults with no underlying lung disease)
- AND respiratory rate ≤30/min
- AND SpO2 ≥90% on room air
Definition of severe illness:
Pneumonia with ANY ONE of the following:
- respiratory rate >30/min
- severe respiratory distress
- SpO2 <90% on room air
- NO invasive or non-invasive respiratory support needed
Definition of critical illness:
- Requirement for high-level respiratory support: noninvasive ventilation, high-flow oxygen (≥20 litres per minute) or invasive mechanical ventilation
• OR acute respiratory distress syndrome (PaO2/FiO2 ratio of <300)
• OR sepsis
• OR shock
Rapid COVID-19 Antibody tests
Antibody tests can indicate that an individual has been exposed to the SARS CoV2 virus in the past and has mounted an immune response against it. Hence a positive result in an antibody test indicates that this this individual has been exposed to SARS COV2 virus in the past and has mounted an immune response against it. A negative antibody test indicates that the individual has not mounted an immune response against SARS CoV2 virus. This drug is only recommended in those with a negative antibody test (seronegative). Only antibody tests validated by ICMR should be used and interpreted in accordance with ICMR guidelines dates 12 February 2021 (12) https://www.icmr.gov.in/pdf/covid/kits/Antibody_based_tests_12022021.pdf
The clinical efficacy of monoclonal antibodies (mAbs) in COVID-19 is believed to be related to direct binding of the virus and neutralization of the infected host cell. Alternatively they may bind to viral antigens expressed on the surface of infected cells and stimulate antibody dependent phagocytosis and cytotoxicity via the Fc portion of the mAbs(1). The clinical benefit of mAbs is likely to be in early disease where the individual is unable to mount a robust pathogen specific immune response. This has been demonstrated in use of mAbs in outpatients with COVID-19 (2). However, it has been noted that some individuals may be antibody negative or have lost antibodies in those who present with severe disease. Therefore, it is possible that in individuals who are antibody negative there may be some evidence of clinical benefit even in those who are hospitalized with severe disease. The Recovery trial (3) studied the monoclonal antibody cocktail Casirivimab-Imdevimab at a dose of 4gm + 4gm in those hospitalized with moderate to severe COVID-19 illness requiring oxygen support. In the seronegative patients critical outcomes including mortality, progression to non-invasive or invasive mechanical ventilation or discharged alive from hospital all favored the antibody cocktail. This modest benefit seemed more pronounced when used in <80 years of age, when used <7 days from symptom onset and those in early disease (on low flow oxygen).Considering that, seronegative status is what determines this modest benefit, the high cost and generalizability may preclude its use in the majority of the population. However in a closely selected group of patients who have not mounted an immune response against COVID-19 this high dose antibody cocktail REGEN-COV™ may be beneficial.
Date of latest search: 03rd July 2021
Date of completion & presentation to Expert Working Group: 06th July 2021
Date of planned review: 06th January 2022
Evidence synthesis team: Hanna Alexander, Naveena George, Bhagteshwar Singh, Priscilla Rupali and Richard Kirubakaran
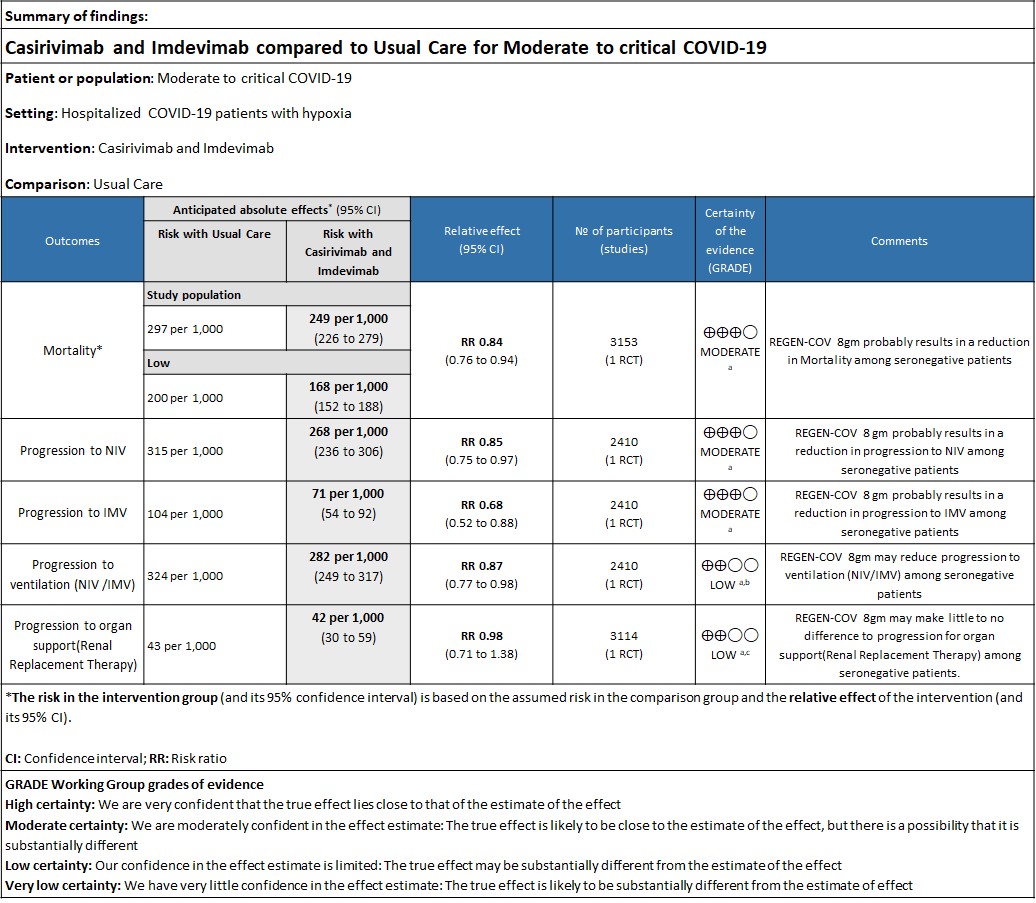
Please note that this summary of findings table presents results for seronegative patients only, i.e. those with no detectable antibodies to SARS CoV2.
Explanations
a. Downgraded by one level for serious indirectness due to baseline Risk is different in our setting with is 20%.
b. Downgraded by one level for serious imprecision as RR is 0.87(95% CI 0.77 to 0.98)
c. Downgraded by one level for serious imprecision as RR is 0.98 (95% CI 0.71 to 1.38)
REGN-COV™ is a cocktail made up of two noncompeting, neutralizing human IgG1 antibodies that target the receptor binding domain of the SARS-CoV-2 spike protein, thereby preventing viral entry into human cells through the angiotensin-converting enzyme 2 (ACE2) receptor(4). The combination is manufactured by Regeneron pharmaceuticals. The drug was approved by the FDA for emergency use on 21 November 2020 in patients ≥12 years of age, weighing at least 40 kilograms with a positive SARS-CoV-2 PCR test and at high risk for progression to severe COVID-19.
The authorized dosages are 1,200mg (600mg+600mg) or 2400mg (1200mg+1200mg) of Casirivimab and Imdevimab administered together as a single intravenous (IV) infusion over 60min, within 10 days of symptom onset.
The dose used in this study was 8g (4gm + 4gm), however the optimal dosing for each category of severity has not been established as yet.The CDSCO in India, on 5 May 2021, granted an EUA to Roche (Genentech) and Regeneron for use of REGEN-COV™ in the country. The announcement came in light of the second wave of the COVID-19 pandemic in India(5). This review aims to provide a summary of the available evidence for use of REGEN-COV™ for treatment of COVID-19 in severe category of patients and thus guide clinicians and researchers regarding the appropriate use of this drug.
We searched PubMed, Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language. We also reviewed reference lists of systematic reviews and included studies. We performed all searches up to 03 July 2021.
We searched the above databases and found 11 records. After removing duplicates and excluding that did not match our PICO question, one randomized controlled trial was selected and included for the analysis.
We extracted data for the following outcomes, pre-defined by the Expert Working Group:
- Critical (primary for this review):
- All-cause (in hospital) Mortality at day 28
- Time to clinical recovery
- Progression to severe disease- requiring O2,
- Progression to non-invasive ventilation, mechanical ventilation or other respiratory support
- Time to clinical improvement (WHO Ordinal scale-decrease in 2 scales)
- Discharge from hospital
- Important (secondary):
- Length of hospital stay
- Time to viral clearance (negative PCR)
- Organ support (non-respiratory)
- Length of ICU stay
- Adverse events:
- All
- Serious
- Infusion-related adverse events (if reported, record rate of infusion used)
Two reviewers independently assessed eligibility of search results using the online Rayyan tool. Data extraction was performed by one reviewer, and checked by another, using a piloted data extraction tool on Microsoft Word. Risk of bias (RoB) was assessed using the Cochrane RoB v2.0 tool, with review by a second reviewer and compared with covidnma database. If there was a difference in more than one domain it was assessed by a third independent author.We planned to use risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs) using Review Manager (RevMan) v5.4. We used GRADE methodology to make summary of findings tables on GRADEPro GDT.
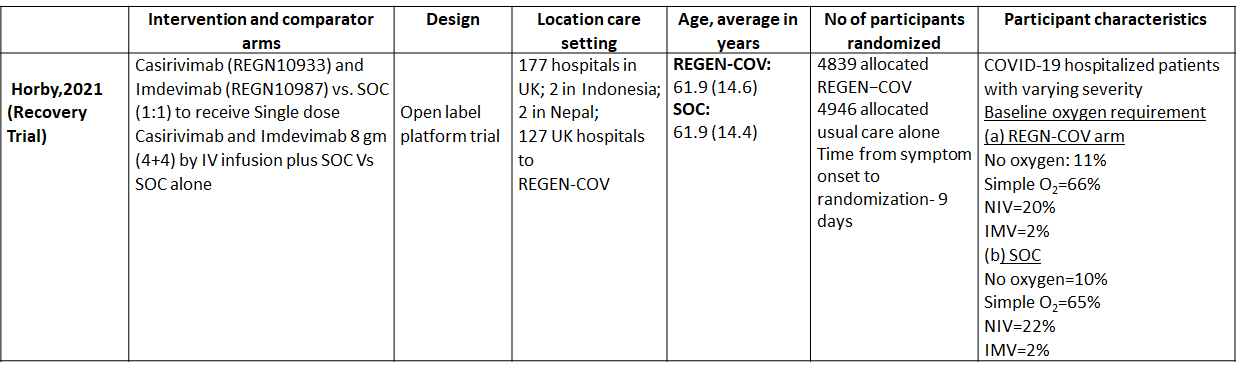
We found one RCT that matched the PICO question as pre-defined by the expert working group. The trial included a total of 9785 participants, all of whom were adult hospitalized patients with varying severity of diseases. This study was done in 177 hospitals in UK; 2 in Indonesia; 2 in Nepal; 127 UK hospitals.
The results are described below; characteristics of the trials are summarised in the Summary of characteristics of included trials table.
The following comparisons were investigated in the trial (we compared outcomes for arms randomized to Casirivimab and Imdevimab combination (REGEN-COV™) vs usual care.
Outcomes
As presented in the ‘Summary of findings’ tables, the evidence is of moderate certainty about the effect of Casirivimab and Imdevimab combination (REGEN-COV™) 8 gm as a single dose intravenous infusion on mortality, progression to Invasive mechanical ventilation, progression to non-invasive mechanical ventilation and progression to organ replacement therapy (Renal replacement therapy) in all patients (seronegative or seropositive). Then a separate analysis for all outcomes was done in all the seronegative patients as well. The intervention was delivered at a median of 9(6-12) days in both the groups.
a. Mortality:
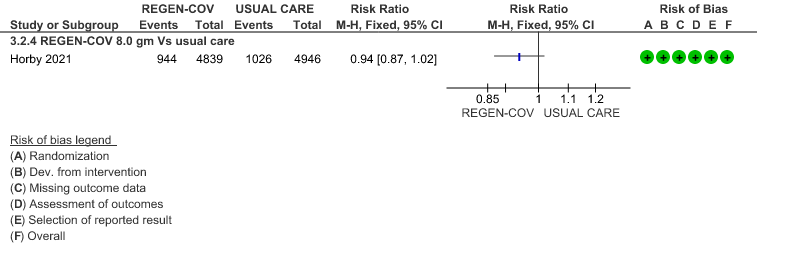
All patients: Mortality in all (seropositive and seronegative) revealed that REGEN-COV™ resulted in a mild reduction in mortality with a RR=0.94 (95% CI 0.87-1.02) suggesting that this mild benefit may be due to the effect on REGEN-COV™ on seronegative patients rather than in all patients.
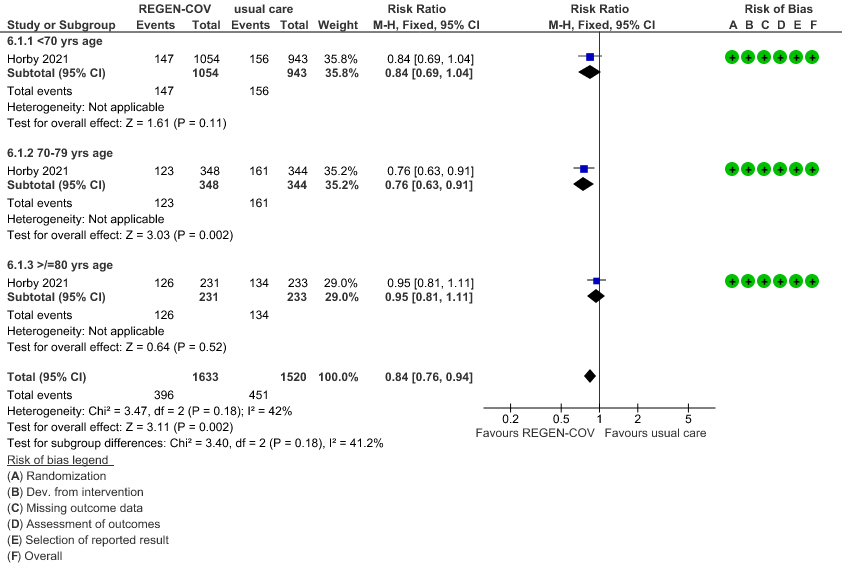
Seronegative: Moderate certainty evidence from 1 trial in 3153 patients, revealed that REGEN-COV™ 8gm probably reduces mortality in seronegative patients; RR 0.84; (95% CI 0.76 to 0.94). The mortality reduction was 20% with a NNT of 19 to prevent 1 death.
The group also felt that the projected mortality in the control group was very high and felt that the baseline risk in the Indian population was around 20% rather than what was projected in the trial as 29% and hence the NNT was baseline risk was modified was 25.
b. Progression to non-invasive ventilation (NIV):
All patients: Progression to non-invasive ventilation was mildly reduced by REGEN-COV™ with a RR=0.95(95% CI 0.87-1.04) suggesting that this mild benefit may be due to the effect on REGEN-COV™ on seronegative patients rather than in all patients.
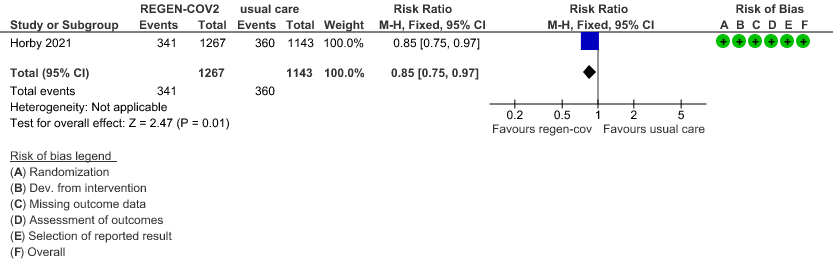
Seronegative: Moderate certainty of evidence in 1 trial with 2,410 patients revealed that REGEN-COV™ 8 gm reduces the progression to non-invasive mechanical ventilation in seronegative patients with (RR 0.85; 95 percent CI 0.75 to 0.97).
c. Progression to invasive mechanical ventilation (IMV):
All patients: Progression to invasive mechanical ventilation was mildly reduced by REGEN- COV™ with a RR=0.86 (95% CI 0.71-1.04) suggesting that this mild benefit may be due to the effect on REGN-COV™ on seronegative patients rather than in all patients.
Seronegative: Moderate certainty of evidence in 1 trial with 2412 patients (RR 0.68; 95 percent CI 0.52 to 0.88), revealed that REGEN-COV™ 8 gm reduces the progression to invasive mechanical ventilationin seronegative individuals.
d. Progression to invasive mechanical ventilation or non-invasive ventilation:
All patients: Progression to respiratory support (non-invasive ventilation or invasive mechanical ventilation) was mildly reduced by REGEN-COV™ with a RR=0.95(95% CI 0.87-1.04) suggesting that this mild benefit may be due to the effect on REGEN-COV™ on seronegative patients rather than in all patients.
Seronegative: Moderate certainty evidence from 1 trial with 6,646 patients revealed that REGEN-COV™ 8 gm probably results in a reduction in progression to invasive and non-invasive mechanical ventilation in seronegative patients (RR 0.87; 95 percent CI 0.77 to 0.98).
e. Progression to organ replacement therapy (Renal replacement therapy):
All patients: Progression to organ replacement therapy was not reduced by REGEN-COV™ with a RR=1.03 (95% CI 0.85-1.25).
Seronegative: Moderate certainty of evidence in 1 trial with 9670 patients found that REGEN-COV™ 8 gm may make little to no difference in reducing the progression to organ replacement therapy (Renal replacement therapy) in seronegative patients(RR 0.98; 95% CI 0.71 to 1.38).
f. Length of hospital stay: The median length of stay in seronegative individuals given REGEN-COV™ was a median 13 (7->28) days vs 17 (7->28) days given usual care.
g. Hospital discharge by day 28: About 1046/1633 seronegative individuals given REGEN-COV™ vs878/1520 given usual care were discharged alive at day 28.
Subgroup analysis
This trial included pregnant, lactating mothers, adolescents 12-18 years, those with co-morbidities >1 and as also those on chronic oxygen therapy. However, disaggregated data could not be obtained for these categories. Though, immunocompromised and those with renal or liver failure were not excluded from this trial, data was not available separately for this group.
The other subgroup analyses that was requested included high vs low dose, past infection, interaction and efficacy with variants, vaccination status, however these outcome variables were not available in the journal article or supplementary data.
Baseline antibody status was available in both the groups and we did a separate analysis for the outcomes of interest.
a. Mortality: In the seronegative group 396/1633 vs 411/2696 in the seropositive died by 28 days; RR=1.56 (1.37-1.76)
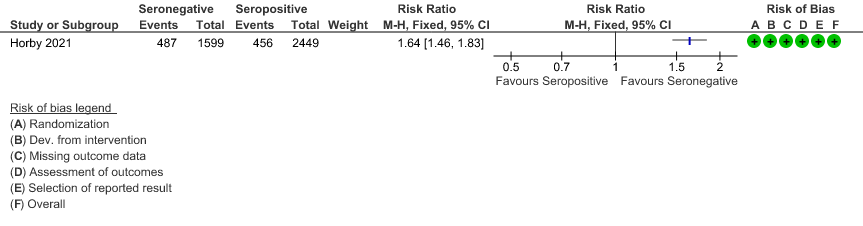
b. Progression to invasive mechanical ventilation or death: In the seronegative group 487/1599 vs 456 out of 2449 in the seropositive group progressed to invasive mechanical ventilation or death; RR=1.64(1.46-1.83).
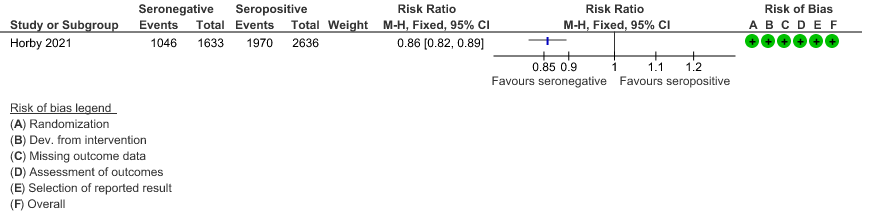
c. Discharged alive from hospital: In the seronegative group 1046/1633 vs 1970/2636 in the seropositive group were discharged alive from hospital; RR=0.86(0.82-0.89).
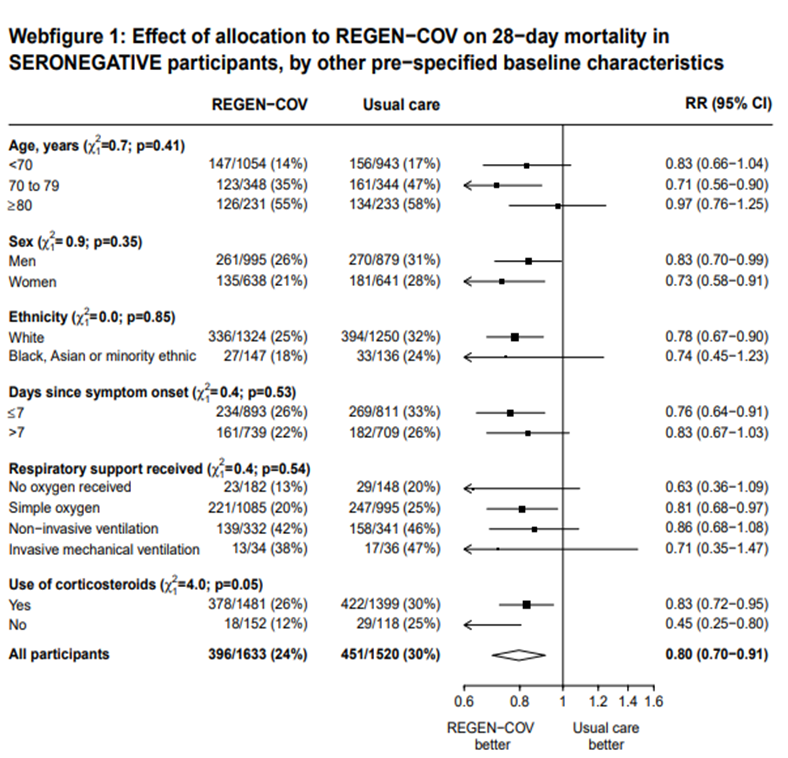
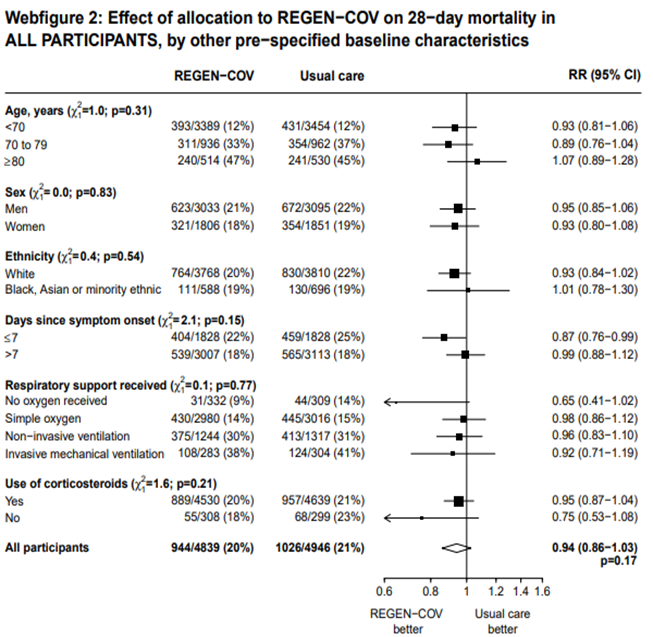
In another pre-specified analysis according to baseline characteristics in seronegative patients, mortality was stratified by
a. Age and the risk of dying in those 80 years of age, RR=0.97(95% CI 0.76-1.25).
b. In those who had symptoms 7 days; RR = 0.83 (95% CI 0.67-1.03)
c. Respiratory support: In those with no oxygen at baseline RR=0.63 (0.36-1.09); on simple oxygen at baseline RR=0.81 (95% CI 0.68-0.97); NIV RR=0.86 (95% CI 0.68-1.08); IMV RR=0.71 (95% CI 0.35-1.47)
1.
(a) Mortality in all (seronegative and seropositive patients)
(b) Mortality in seronegative patients stratified by age
2.
(a) Progression to non-invasive ventilation in all (seronegative and seropositive):
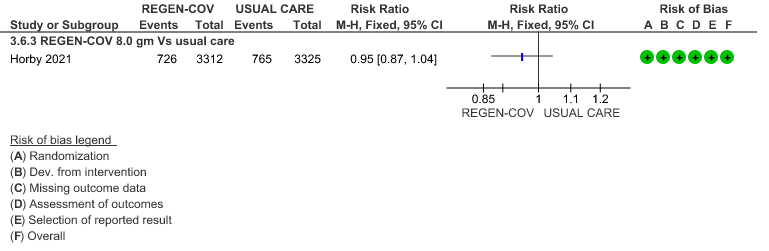
(b) Progression to NIV (seronegative)
3.
(a) Progression to invasive mechanical ventilation (IMV) in all (seronegative and seropositive):
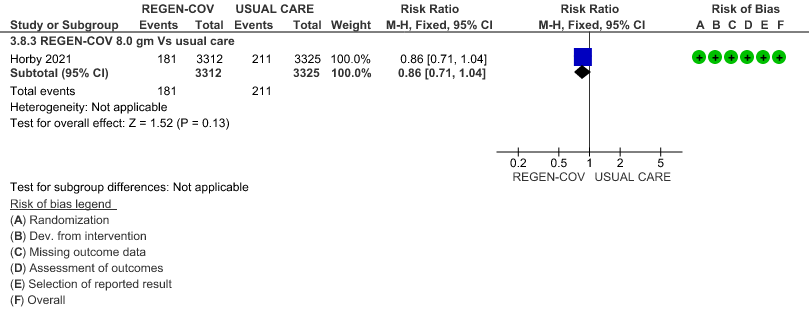
(b) Progressive to IMV in seronegative
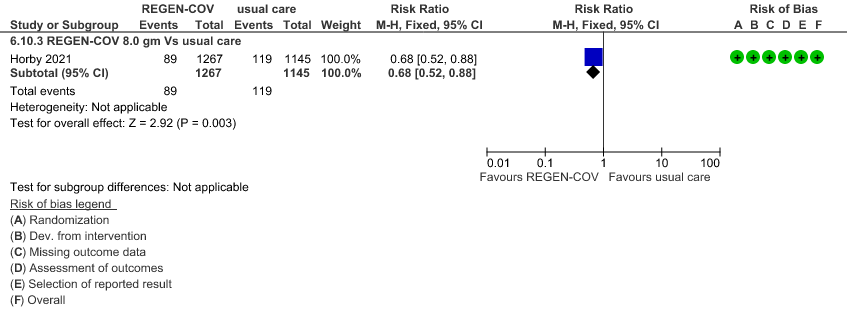
4.
(a) Progression to ventilatory support (IMV or NIV) in all(seronegative and seropositive)
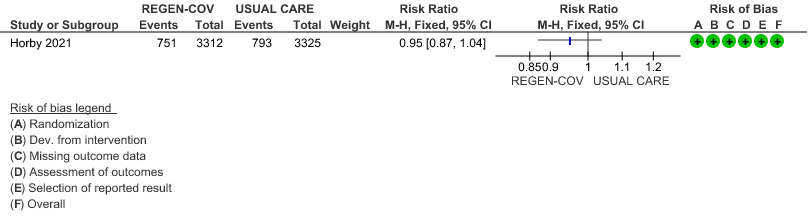
(b) Progression to ventilatory support (IMV or NIV) in seronegative
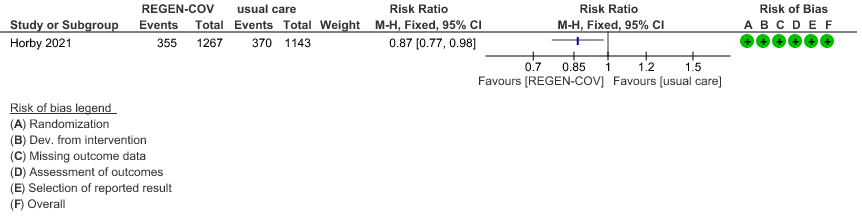
5.
(a) Progression to organ replacement therapy (Renal replacement therapy) in all:
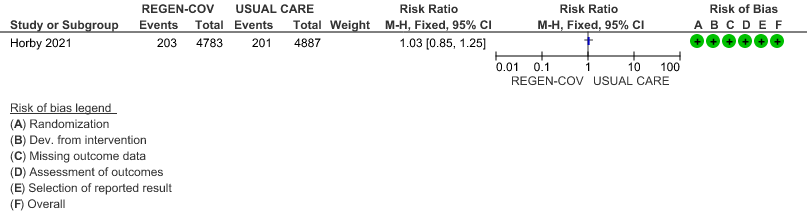
(b) Progression to organ replacement therapy (Renal replacement therapy) in seronegative:
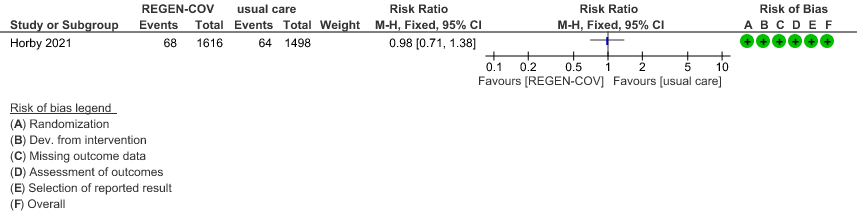
6.
(a) Outcomes of interest in the seropositive vs seronegative individuals:
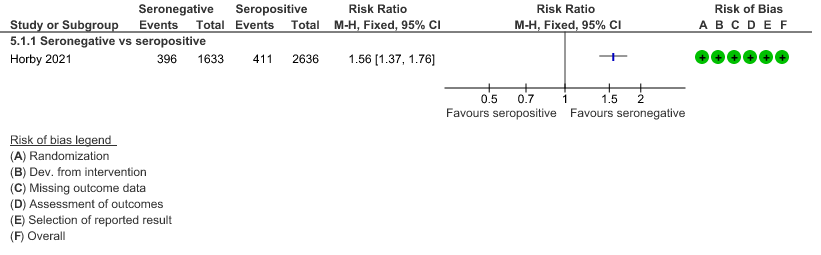
(b) Progression to IMV or death
(c) Discharged alive from hospital
7. Outcomes of interest as per baseline characteristics [web figures of tables taken from Supplementary material Recovery trial for REGEN-COV™ – (Ref 6)]
Horby PW, Mafham M, Peto L, et al. Casirivimab and imdevimab in patients admitted to hospital with covid-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv 2021.06.15.21258542v1[Preprint].2021. https://www.medrxiv.org/content/10.1101/2021.06.15.21258542v1.supplementary-material)
Evidence to decision – REGEN-COV™ 8gm for moderate (hypoxia), severe and critical COVID-19 illness.
Summary of judgements
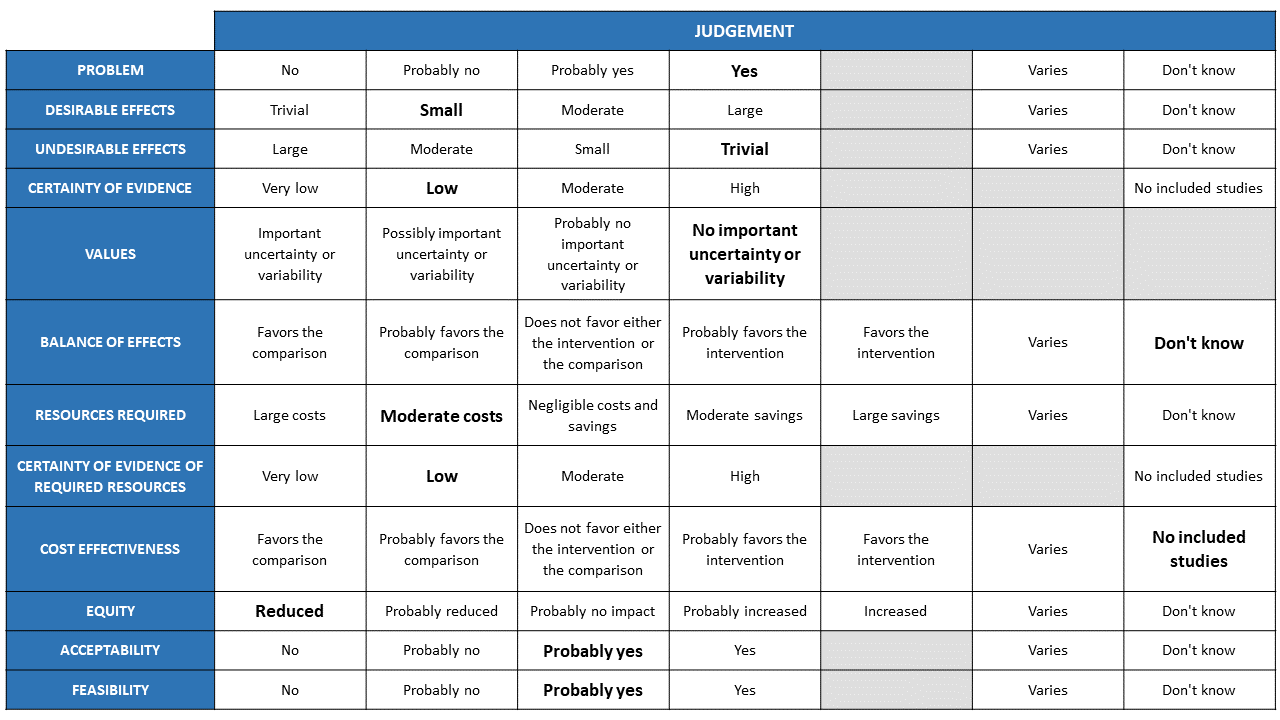
The Antibody Expert Working Group met on 6th July 2021 to consider Casirivimab and Imdevimab combination (REGEN-COV™ ) 8gm as a treatment for COVID-19. Conflict of interest declarations were reviewed by the Steering Committee; none were found to be relevant to Casirivimab and Imdevimab.
A summary and then more detailed explanations of the Expert Working Group's judgements follow.
Problem
The COVID-19 pandemic in India, which has resulted in more than 30 million illnesses and 0.39 million deaths, has wreaked havoc on the country's health system. The country is experiencing a significant health crisis due to a lack of intensive care unit beds, oxygen, and skilled people. This has resulted in many irrational and experimental treatment interventions, such as the use of Casirivimab and Imdevimab in combination in all severity of patients across the country in patients hospitalized with Covid-19, with no clear indication or evidence of which subgroup of the population or disease severity the drug is effective for. The group judged the problem to be of utmost priority.
Desirable effects
The group discussed the anticipated desirable effects of REGEN-COV™ 8gm in covid-19. They agreed that the drug will be effective in seronegative patients who are hypoxic (Oxygen Saturation< 93%). The absolute effect needs to consider that the baseline risk in Indian population is low. Having considered this the effect size will be considered for different mortality rates.
Undesirable effects
The group concluded that there was insufficient data to demonstrate that the negative impacts are significant. There were very few events leading to adverse events in the evidence evaluated, and both groups had the same number of incidents.
Certainty of evidence
Using GRADE methodology, the evidence synthesis team rated the certainty of evidence as Moderate for mortality, progression to non-invasive ventilation and invasive mechanical ventilation; low certainty for progression to NIV/IMV and progression to organ support (renal replacement therapy) in seronegative patients. The group methodologist proposed that the evidence be lowered as well, because the baseline risk in our population is minimal. Because mortality, progression to NIV, and IMV are critical outcomes, the panel deemed the overall certainty of evidence is moderate.
Values
The group reviewed the evidence and concluded that there is no significant uncertainty or variability in how the outcomes are valued by the various stakeholders.
Balance of effects
The group favored the intervention as it had moderate certainty evidence of benefit and very few undesirable effects demonstrated.
Resources required
The group believed that the resources needed to implement this intervention are significantly high, as the drug is expensive and is delivered intravenously and considerably high resources are needed to securely give the drug in a healthcare facility protecting the healthcare workers and other patients.
Certainty of evidence of required resources
The group believed that no studies reporting this had been evaluated by the group, but because the physicians in the group were aware of the expense, they considered there were confident of the resources required to implement this intervention.
Cost effectiveness
Although no published research was utilized to make this decision, the expert working group stated that due to the drug's high cost and resources involved to administer the same, this intervention may be utilized differently by clinicians, administrators, as well as patients from different socioeconomic groups as well as health care settings. Hence the group felt that the cost effectiveness may vary.
Equity
The group concluded that using the intervention would definitely reduce equity. This is a costly intervention overall and it is likely to create inequalities between those who can afford it and those who cannot, as well as between private and public health care sectors.
Acceptability
The group predicted that different stakeholders' reactions to the intervention would differ. It is contingent on who will bear the financial burden. Hence the group felt that acceptability would vary among patients, clinicians and administrators.
Feasibility
The group felt that the practicality of implementing this intervention varies according to cost, health-care setting, and availability of resources.
The recommendations being made are primarily from a single randomized trial done in the United Kingdom. Casirivimab and Imdevimab antibody cocktail from the review of evidence seems to be cost effective and useful only in seronegative individuals. It is believed to reduce mortality, progression to non-invasive and invasive mechanical ventilation when used within 7 days of symptom onset or relatively early in illness along with usual care including steroids. The reported drug related reactions were very low and no clear cut drug interactions have been reported in the trials thus far. However, it would be prudent to be prepared to handle anaphylaxis and other allergic reactions. Casirivimab and Imdevimab in the recommended dose of 8g in the hypoxic moderate to critical group is not available in India, however clinicians are beginning to use 3 vials of 2.4g which is being marketed in India based on CDSCO approval.
Since the actual costs and resources required to administer this drug are huge, therefore its widespread use in the Indian setting will not be practical or feasible. In addition, an antibody test which is validated and recommended by the Indian Council of Medical Research (7,8,12)is advised with a swift turnaround time to enable appropriate administration of this drug, which can be a challenge in most settings. However, in certain situations where costs are less of a consideration it may be of some benefit in selected sub-groups who are unable to mount a swift and adequate immune response of their own against COVID-19. So while it may work in those with no detectable antibodies against COVID-19 whether we can extrapolate to a benefit in immunosuppressed individuals remains to be seen.
There has been concern that this drug may not be effective in infections caused by variants, however this has been dispelled as data indicates that this drug is effective even against prevailing variant strains causing moderate to critical COVID-19 infection (9). In India the second wave of COVID-19 illness has been attributed to the delta variant which has now spread to different parts of the world causing another wave of infections(10).
In addition, various studies have revealed that the seropositivity rate in various communities in India vary from 30-60% (ref) and hence judicious administration where it is likely to be most likely to be beneficial is advised (11). Though the optimal use of this drug may be further modified as evidence emerges, hospitals need to make clear explicit guidelines as to when this drug should be administered taking into account the onset of symptoms, severity of illness, antibody status considering the cost implications.
Disaggregated subgroup data was not available for pregnancy, co morbidities and immunosuppression and hence at this point it is not possible to comment whether there are specific benefits or harms in this subgroup with the use of this antibody cocktail. Though it would have interesting to note variables like prior vaccination, infecting strain and prior infection and studied their effect on efficacy this study did not do so. However, there were some pre-specified subgroup analysis which yielded pertinent information.
a. Mortality was compared across all age strata -<70 years, 70-79 years and >80 years. Though the point estimate was in the direction of efficacy across all age groups, it was statistically significant and most marked in the 70-79 age group. It was least impressive in the elderly age group of >80 years of age
b. When the overall group was compared to just the seronegative group there was a clear superiority of benefit noted with Casirivimab-Imdevimab in the subgroups < 80 years of age, administered within 7 days of onset and those on just simple oxygen.
While, the recovery trial results indicate that using 4+4 gm of the cocktail in seronegative patients with COVID-19 was significantly associated with reduction in mortality and time to hospital discharge the baseline risk of mortality in the placebo group was much higher (>30%) than what is seen in Indian patient’s ( 15-20%). Hence the absolute benefits will be lower and number needed to treat will be higher. Hence caution needs to be exercised as such an expensive intervention will need to be justified. Though data is emerging that this monoclonal antibody cocktail works against the presently circulating variants (B.1.117, B.1.351, B.1.617 etc) it is uncertain that it would continue to maintain its activity against newly emerging variants. In addition, it seems to prudent to use this drug only when the individual is seronegative on a validated antibody test (12) which will be increasingly rare as the pandemic goes on as individuals will either get infected or will be vaccinated. It is possible that with a waning neutralizing antibody response as has been noted with the currently available vaccines in India COVISHIELD and COVAXIN, and possibly a natural infection as well, this drug may assume a role again in specific high-risk category of patients who are at high risk of progression and unable to mount a suitable immune response. Since it is mandatory to have the serostatus of participants before administering the cocktail, centers using this approach would have to have a validated antibody (anti-spike IgG) testing available round the clock (12). It will also become clearer as evidence emerges as to the optimal timing as to when this drug may be delivered in the natural evolution of COVID-19 infection in a patient requiring oxygen.
However, it is a safe drug as has been observed in trials thus far with very rare infusion reactions and no drug interactions or modification required with renal or hepatic impairment.
Monitoring the continued use of this drug is imperative as it will provide valuable information regarding efficacy and utility of the drug with rising seroprevalence and emerging variants. It will also be important to determine whether the immunocompromised, elderly, high risk group of adults, pregnant women and children in the severe to critical category may have a greater benefit with this drug if they have a poor immune response to vaccination or natural infection. We will continue to monitor the relevance of this therapeutic intervention as new evidence emerges for updating this recommendation.
Several interventions like steroids and IL-6 inhibitors have been shown to decrease mortality in moderate, severe and critical categories of COVID-19 infections. Casirivimab Imdevimab is efficacious in early illness and likely functions as an antiviral drug. Hence research priorities would include
1. Optimal timing of Casirivimab-Imdevimab administration in those with systemic inflammation in severe to critical COVID-19 illness
2. Identification of the high risk population that is likely to benefit with early administration of Casirivimab Imdevimab.
3. Analyse if Casirivimab Imdevimab combination is cost effective in severe to critical COVID-19 infection.
4. Casirivimab Imdevimab efficacy in new emerging variants causing severe to critical COVID-19 infection.
- Winkler ES, Gilchuk P, Yu J, et al. Human neutralizing antibodies against SARS-484 CoV-2 require intact Fc effector functions for optimal therapeutic protection. Cell 2021; 485 184(7): 1804-20 e16.
- Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a Neutralizing 511 Antibody Cocktail, in Outpatients with Covid-19. N Engl J Med 2021;384:238-51.DOI: 10.1056/NEJMoa2035002.
- Horby PW, Mafham M, Peto L, et al. Casirivimab and imdevimab in patients admitted to hospital with covid-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv 2021.06.15.21258542v1 [Preprint]. 2021. www.medrxiv.org/content/10.1101/2021.06.15.21258542v1.
- Commissioner O of the. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 [Internet]. FDA. FDA; 2020 [cited 2021 Jun 29]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19
- Roche receives Emergency Use Authorisation in India for its investigational Antibody Cocktail (Casirivimab and Imdevimab) used in the treatment of Covid-19 | Cipla [Internet]. [cited 2021 Jun 29]. Available from: https://www.cipla.com/press-releases-statements/roche-receives-EUA-India-investigational-antibody-cocktail-casirivimab-Imdevimab-covid.
- Horby PW, Mafham M, Peto L, et al. Casirivimab and Imdevimab in patients admitted to hospital with covid-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv 2021.06.15.21258542v1 [Preprint]. 2021. https://www.medrxiv.org/content/10.1101/2021.06.15.21258542v1.supplementary-material
- The National SARS-CoV-2 Serology Assay Evaluation Group*. Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis 2020; 20: 1390–1400 Published Online September 23, 2020 https://doi.org/10.1016/ S1473-3099(20)30634-4.
- National Task force for COVID-19. Advisory on Strategy for COVID-19 Testing in India (Version VI, dated 4th September 2020). Indian Council of Medical Research. Department of Health Research - Ministry of Health and Family Welfare, Government of India.
- Hoffmann M, Hofmann-Winkler H, Krüger N, et al. SARS-CoV-2 variant B.1.617 586 is resistant to Bamlanivimab and evades antibodies induced by infection and 587 vaccination. bioRxiv 2021: 2021.05.04.442663.
- Petra Mlcochova, Steven Kemp, Mahesh Shanker Dhar et al. SARS-CoV-2 B.1.617.2 Delta variant emergence and vaccine breakthrough. bioRxiv preprint doi: https://doi.org/10.1101/2021.05.08.443253; this version posted June 28, 2021 accessed 4 August 2021.
- Owen Dyer. Covid-19: Two thirds in India carry antibodies, while research suggests country’s death toll is 10 times official figure. Cite this as: BMJ 2021;374:n1856 http://dx.doi.org/10.1136/bmj.n1856.
- Indian Council of Medical Research. Ministry of Health and Family Welfare, Government of India. Guidance on rapid antibody test kits for COVID-19. Accessed at URL https://www.icmr.gov.in/pdf/covid/kits/Antibody_based_tests_12022021.pdf on August 11, 2021.
Covid Management Guidelines India Group – Anti-body Working Group. Casirivimab – Imdevimab. Covid Guidelines India; Published online on August 11, 2021; URL : https://indiacovidguidelines.org/casirivimab-imdevimab-moderate-to-severe/?preview=true (accessed ).
