This recommendation applies to acute COVID-19 in adults. Some of our recommendations vary according to the severity of COVID-19 illness. Definitions of the categories are based on the World Health Organization (WHO) criteria and can be viewed by clicking the plus (+) signs below.
RECOMMENDATION: The group recommends against the use of Azithromycin for COVID-19 infection in all categories of severity (strong recommendation). There was high certainty evidence to demonstrate that Azithromycin did not impact mortality or progression of illness in the treatment of COVID-19 infection.
DATE OF RECOMMENDATION: 27th September 2021
Definition of mild:
• Symptomatic (any acute COVID-19 related symptoms)
• AND respiratory rate <24/min
• WITHOUT pneumonia or hypoxia
Definition of moderate illness:
• Pneumonia (clinical or radiological) OR hypoxia (SpO2 <94% in adults with no underlying lung disease)
• AND respiratory rate ≤30/min
• AND SpO2 ≥90% on room air
Definition of Severe illness
Pneumonia with ANY ONE of the following:
• severe respiratory distress or respiratory rate >30/min
• SpO2 <90% on room air
• NO invasive or non-invasive respiratory support needed
Definition of critical:
• Requirement for high-level respiratory support: noninvasive ventilation, high-flow oxygen (≥20 litres per minute) or invasive mechanical ventilation
• OR acute respiratory distress syndrome (PaO2/FiO2 ratio of <300)
• OR sepsis
• OR shock
Azithromycin is a macrolide antibiotic which has shown activity against SARS-CoV-2 in vitro1. It is also known for its immunomodulatory properties, of potential utility during the inflammatory phase of COVID-192. Azithromycin has been widely used in the care of patients with COVID-19 in India. The home care treatment kits provided by many non governmental organizations for patients advised home isolation for COVID-19 also contain Azithromycin3. Hence it is important to consider the question of whether its use for COVID-19 is justifiable.
After detailed review of available evidence, we do not recommend the use of azithromycin for any category of patients with COVID-19 infection for the following reasons:
- There was high certainty of evidence to suggest that critical outcomes like all-cause mortality and progression to mechanical ventilation were not different with or without azithromycin.
- Further, all other outcomes of interest including duration of hospitalisation (moderate certainty of evidence); time to clinical improvement, progression to oxygen requirement and viral clearance (low certainty of evidence), were not different in the azithromycin and comparison groups (usual standard of care).
- In the hospitalised non-severe group, the proportion of patients with viral clearance at day 6 was found to be higher with azithromycin, but this observation was from a single small study with very low certainty of evidence, and did not translate into clinically significant outcomes.
- The widespread use of a broad-spectrum antibiotic like azithromycin in a pandemic situation is likely to contribute to antibiotic resistance, an outcome which is not measured in any of the trials. Although there are no major cost implications of the use of azithromycin and undesirable side-effects were few in this analysis, it has been associated with an increase in cardiovascular deaths in other studies. We strongly recommend against its use in all categories of COVID-19 as there is currently no evidence to suggest any significant desirable effects.
Date of latest search: 3rd July 2021
Date of completion & presentation to the Expert Working Group: 12th August 2021
Date of planned review: 12th February 2022
Evidence Synthesis Team: Jisha Sara John, Gina Chandy, Richard Kirubakaran and Priscilla Rupali
Explanations
a. Downgraded by one level for serious imprecision as the confidence interval is very wide
b. Downgraded by 2 levels because of High Risk of Bias
c. Downgraded by 2 levels for very serious imprecision because of wide CI
d. Downgraded by 1 level for serious imprecision as the mean difference of less than a day is not clinically significant
e. Downgraded by 1 level for serious imprecision as optimal information size criteria were not met, very few events documented
f. Downgraded by 1 level because of some concerns and high Risk of Bias in studies.
g. Downgraded by 1 level for serious inconsistency as the I2 was greater than 50%
h. Downgraded by 1 level for serious indirectness as all categories of severity are included
i. downgraded by 1 level as outcomes at 6 and 14 days were included
Subgroup Analysis
Explanations
a. Downgraded by 2 level due to wide 95% CI, and with low number of events
b. Downgraded by 1 level due to some concerns in ROB
c. Downgraded by1 level due to wide 95% CI
d. Downgraded by 2 levels for very serious imprecision, as the CI were wide and event rates were very low; optimal information size (OIS) was inadequate
e. Downgraded by 2 levels for very serious ROB.
f. Downgraded by 1 level for serious imprecision, as the event rate was low and OIS was inadequate.
g. The ECG measurement at a homecare setting was not reliable.
Explanations
a. Downgraded by 1 level, some concerns in two studies
b. Downgraded by 2 levels for very serious imprecision, due to wide 95% CI, OIS not met.
c. Downgraded by 1 level for serious ROB, some concerns in a single study
d. Downgraded by 1 level for serious inconsistency, I2 value is 58%
e. Downgraded by 1 level for serious imprecision, as the effect estimate is 0.70 days is not clinically significant
f. Downgraded by 2 levels for very serious ROB, single study with High ROB
g. Downgraded by 1 level for serious indirectness, as time to clinical improvement is measured as fever resolution.
h. Downgraded by 1 level for serious imprecision, due to wide 95% CI, OIS not met.
Explanations
a. Downgraded by 1 level, for serious imprecision, as CI was wide (0.74 to 1.16)
b. Downgraded by 1 level, for serious imprecision, as CI was wide (0.63 to 1.33)
Azithromycin is a synthetic macrolide which has been used against bacterial, rickettsial and mycobacterial diseases. It also has antiviral and anti-inflammatory actions and hence has been used previously for treatment of SARS-CoV or MERS-CoV. In vitro studies suggest possible effect against SARS-CoV-2 also (1). Azithromycin’s effect on pH of the Golgi network and recycling endosome is postulated to interfere with intracellular SARS-CoV-2 activity and replication(4). The virus has a furin-like cleavage site in the spike protein and as this drug might reduce levels of furin(enzyme), it could interfere with the cell entry of SARS-CoV-2. Azithromycin’s ability to reduce levels of proinflammatory cytokines such as IL-6 could reduce the intensity of the SARS-CoV-2 triggered cytokine storm, along with associated tissue damage(5). It is an easily available drug and has been utilized for Covid 19 rampantly in India(6). The doses used are 250mg or 500mg of the drug per day for duration of 3 to 10 days. This review aims to provide a summary of the available evidence from randomized clinical trials of Azithromycin for treatment of acute COVID-19, so the Expert Working Group can provide a recommendation to guide clinicians and researchers regarding the appropriate use of this drug.
Towards identifying all relevant studies, we conducted a systematic search of PubMed, covid-nma.org and the Epistemonikos databases in July 2021. We also reviewed reference lists of systematic reviews and included studies.
We included randomized controlled trials (RCTs) testing Azithromycin at any dose for any duration, in people with confirmed COVID‐19, and extracted data for the following pre-defined outcomes:
- Critical (primary for this review):
- Progression to:
- Oxygen therapy
- Ventilation: non-invasive or invasive
- Critical or Intensive care (any reason)
- Duration of hospitalization
- Need for hospitalization (out-patients)
- Progression to:
- Important (secondary):
- Mortality (all-cause) – at 28-30 days, or in-hospital
- Time to clinical improvement
- Time to negative PCR for SARS-CoV-2
- Complications of COVID-19:
- thrombotic events
- pulmonary infections/fibrosis
- Long covid/post-acute sequalae COVID
- Secondary infections
- Adverse events: All and serious adverse events
Two reviewers independently assessed eligibility of search results using Rayyan. We identified a total of 78 articles of which 9 were excluded as they were duplications and 55 as they were not the right study design or were for the wrong drug as intervention. The remaining 14 articles comprised of 12 RCT’s and 2 systematic reviews.
Two reviewers extracted data from each included study, and assessed risk of bias using the Cochrane Risk of bias (RoB) v2.0 tool. The entire RoB assessment was scrutinized by the whole team for this review, to reach consensus.
The systematic reviews were assessed by the AMSTAR grading tool and were found to be moderate to high quality evidence. We used RevMan 5.4 to perform meta‐analysis using a random‐effects model for outcomes where pooling of effect estimates was appropriate. We used risk ratios (RR) for dichotomous outcomes with 95% confidence intervals (CIs). We used the I2 statistic to measure residual heterogeneity. We used GRADE methodology to assess the certainty in the evidence, and documented this in a ‘Summary of findings’ table using GradeProGDT.
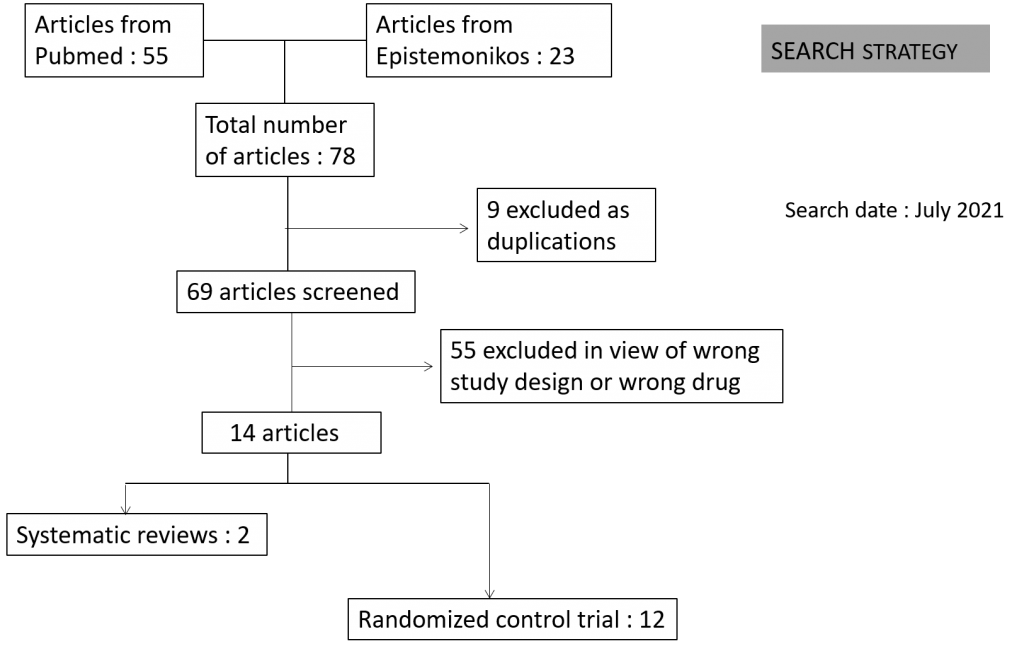
We found 12 RCTs that matched the PICO question as pre-defined by the expert working group. These included 4 studies with ambulatory patients who did not need hospitalization, 6 studies with patients with non-severe COVID 19 but were hospitalized during study period and 2 studies that included patients who had severe to critical COVID 19. Hence recommendation being considered for: Symptomatic confirmed COVID 19 infection
Categories:
- Ambulatory,
- Hospitalised non severe
- Hospitalised severe to critical
Azithromycin in Comparison to Standard of Care in Covid 19
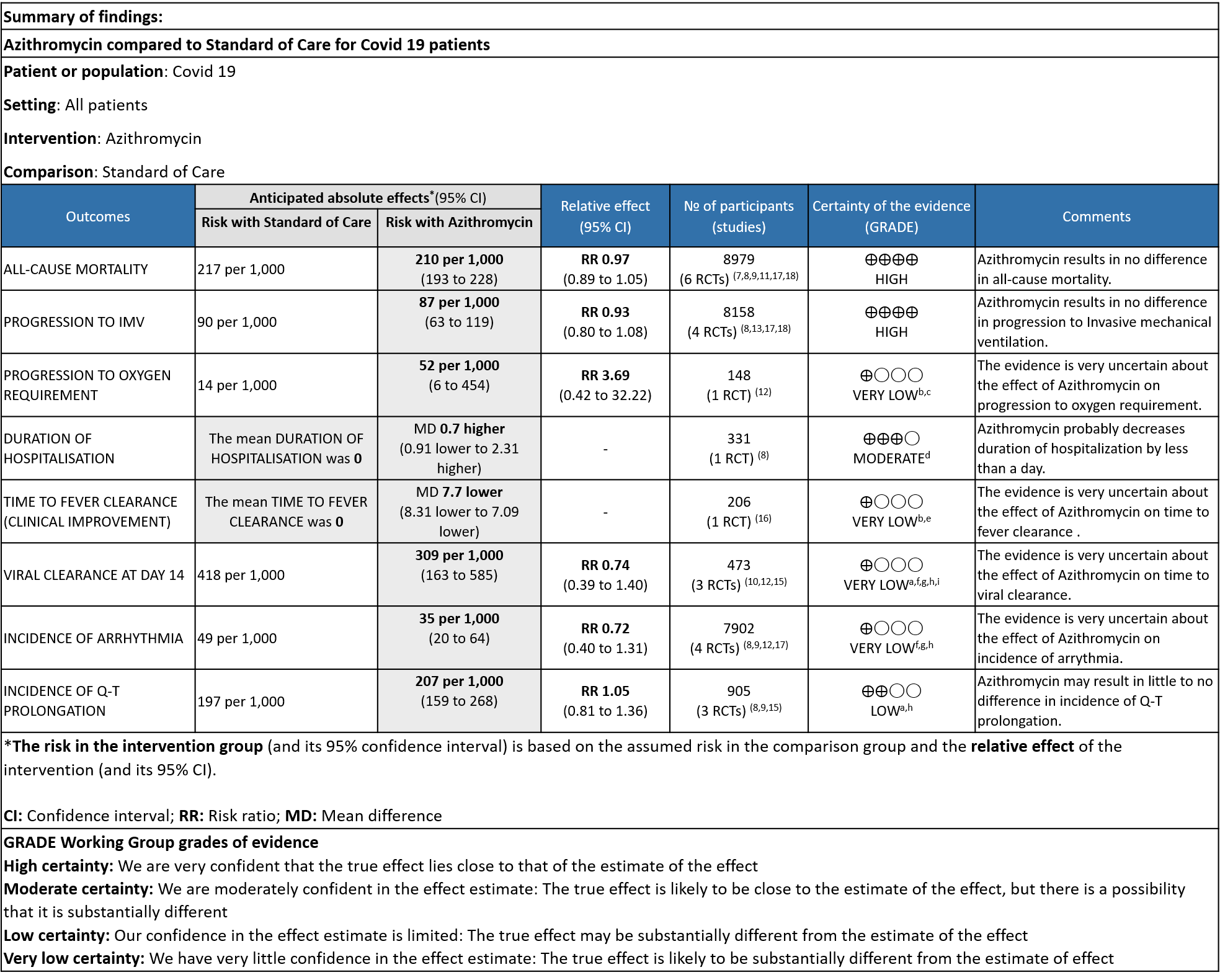
- ALL-CAUSE MORTALITY: High certainty of evidence from 6 RCT’s in a total of 8979 patients reveals that Azithromycin does not reduce all-cause mortality (RR 0.97; 95% CI 0.89 to 1.05).
- PROGRESSION TO INVASIVE MECHANICAL VENTILATION (IMV): High certainty evidence from 4 RCT’s in 8158 patients revealed that Azithromycin does not reduce progression to invasive mechanical ventilation (RR 0.93; 95% CI of 0.80 to 1.08).
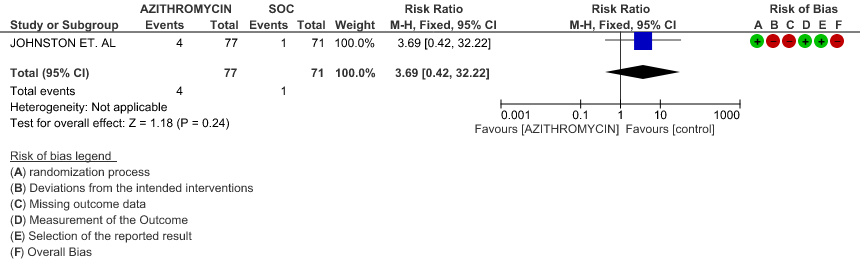
- PROGRESSION TO OXYGEN REQUIREMENT: Low certainty evidence from 1 RCT in 148 patients revealed a very uncertain effect of Azithromycin on progression to oxygen requirement (RR 3.69 with a 95% CI of 0.42 to 32.22).
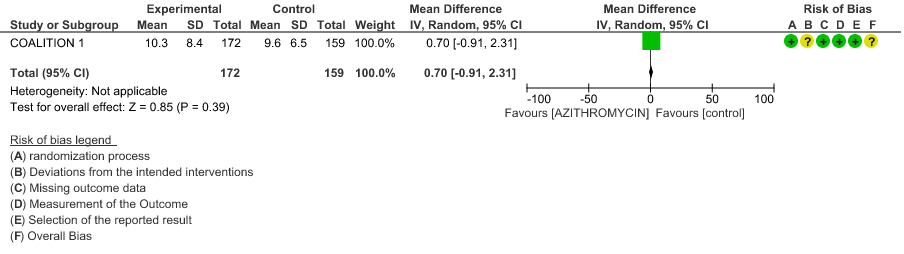
- DURATION OF HOSPITALISATION: Moderate certainty evidence from 1 RCT in 331 patients revealed that Azithromycin probably decreases duration of hospitalization by less than a day (MD 0.7 higher; 95% CI 0.91 lower to 2.31 higher).
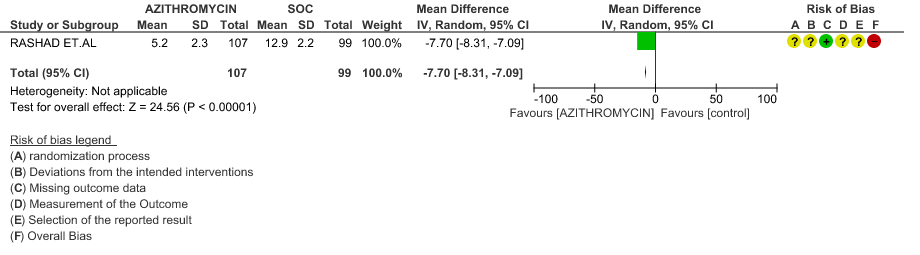
- TIME TO CLINICAL IMPROVEMENT (time to fever clearance): Very low certainty evidence from single study with high risk of bias is from 1 RCT in 206 patients revealed a very uncertain effect of Azithromycin on time to fever clearance (MD 7.7 lower; 95% CI 8.31 lower to 7.09 lower).
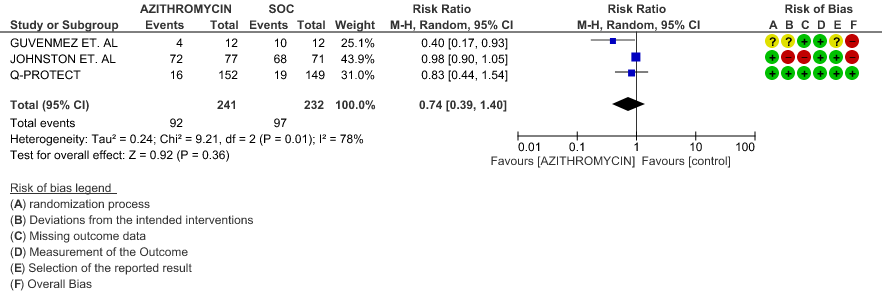
- VIRAL CLEARANCE ON DAY 14: Very low certainty evidence from 3 RCT’s in 473 patients revealed a very uncertain effect of Azithromycin on time to viral clearance (RR 0.74; 95 % CI 0.39 to 1.40).
- INCIDENCE OF ARRHYTHMIA: Very low certainty evidence from 4 RCT’s in 7902 patients revealed a very uncertain effect of Azithromycin on incidence of arrhythmia (RR 0.72; 95% CI 0.40 to 1.31).
- INCIDENCE OF QT-PROLONGATION: Low certainty evidence from 4 RCT’s in 905 patients revealed that Azithromycin may result in no difference in incidence of Q-T prolongation (RR 1.05; 95% CI 0.81 to 1.3).
SUBGROUP ANALYSIS
Azithromycin in Comparison to Standard of Care in Ambulatory Patients with Covid 19
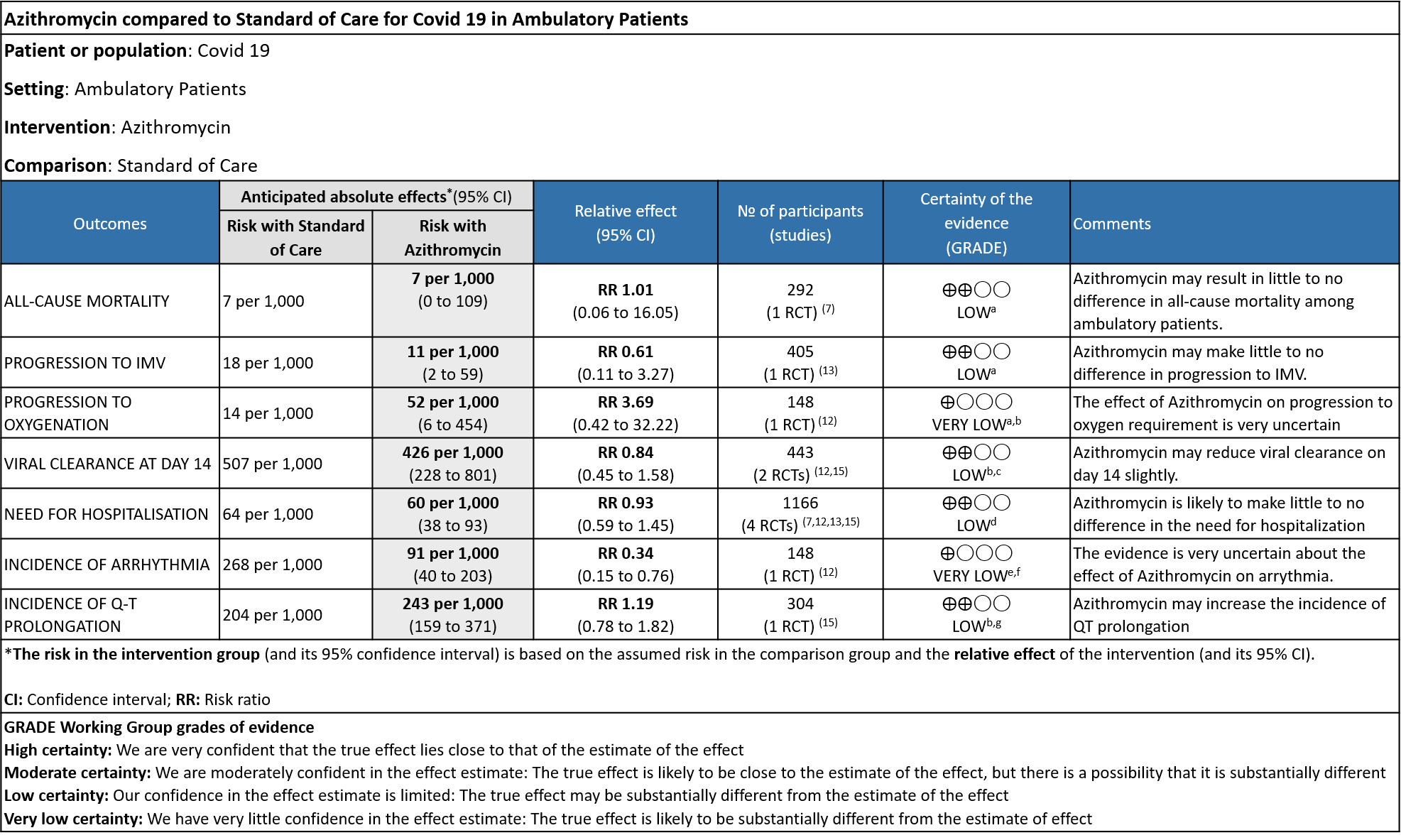
- ALL-CAUSE MORTALITY: Low certainty evidence from 1 RCT in 292 patients revealed that Azithromycin may result in no difference to all-cause mortality in ambulatory patients (RR 1.01 with a 95% CI of 0.06 to 16.05).
- PROGRESSION TO IMV: Low certainty evidence from 1 RCT in 405 patients revealed that Azithromycin may reduce progression to IMV slightly (RR 0.61 with 95% CI of 0.11 to 3.27).
- PROGRESSION TO OXYGEN REQUIREMENT: Low certainty evidence from 1 RCT in 148 patients, revealed a very uncertain effect of Azithromycin on progression to oxygen requirement (RR 3.69 with 95% CI of 0.42 to 32.22).
- DURATION OF HOSPITALISATION: Low certainty evidence from 4 RCT’s in 1066 patients revealed that Azithromycin may make no difference in the need for hospitalization (RR 0.93 with 95% CI of 0.59 to 1.45).
- VIRAL CLEARANCE ON DAY 14: Low certainty evidence from 2 RCT in 443 patients revealed that Azithromycin may reduce viral clearance on day 14 slightly (RR 0.84 with 95% CI of 0.45 to 1.58).
- INCIDENCE OF ARRHYTHMIA: Very low certainty evidence from 1 RCT in 148 patients revealed a very uncertain effect of Azithromycin on arrhythmia (RR 0.34 with 95% CI of 0.15 to 0.76).
- INCIDENCE OF QT-PROLONGATION: Low certainty evidence from 1 RCT in 304 patients revealed that Azithromycin may increase the incidence of QT prolongation (RR 1.19 with 95% CI of 0.78 to 1.82)
Azithromycin in Comparison to Standard of Care in Hospitalized Non-Severe Patients with Covid 19
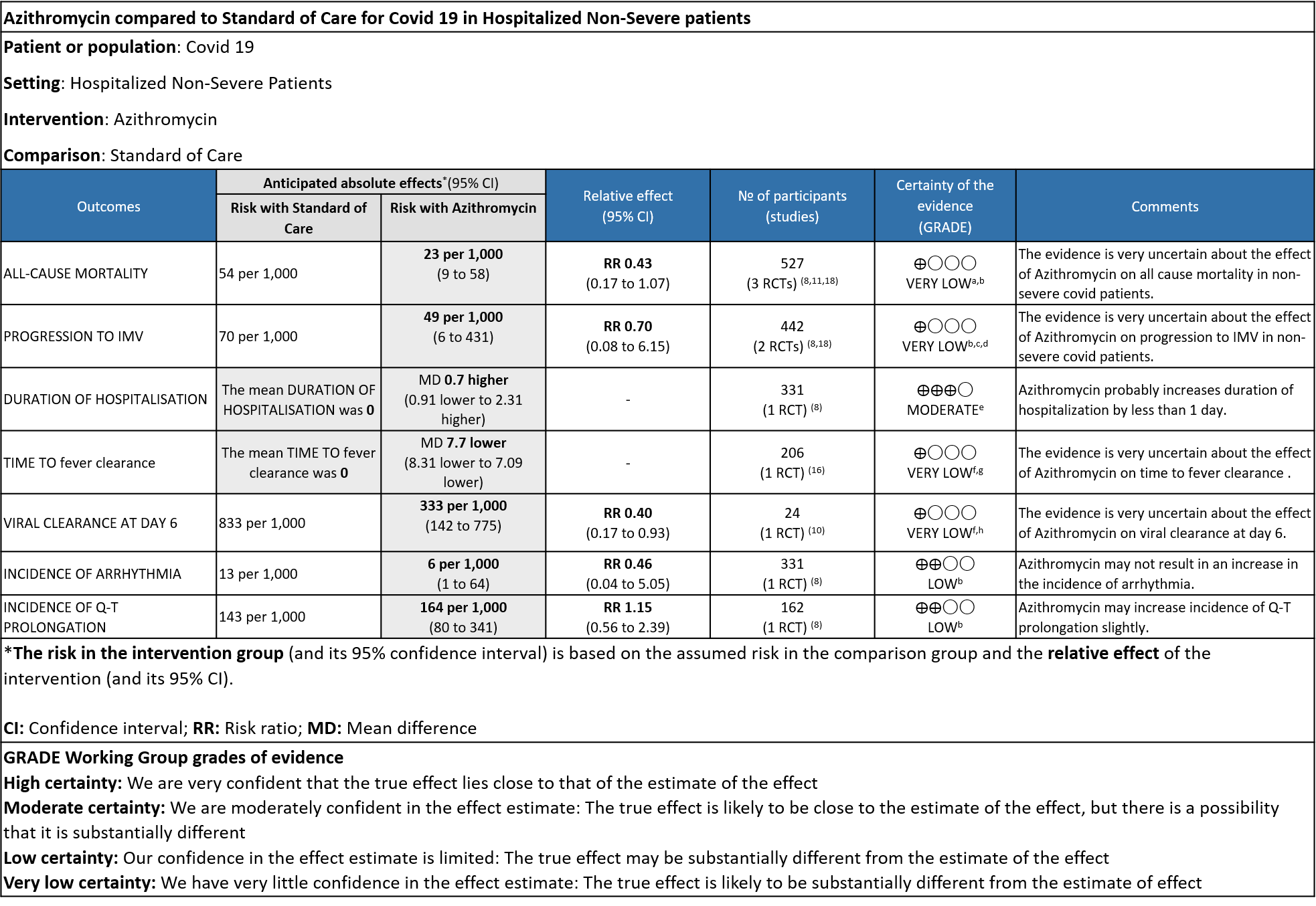
- ALL-CAUSE MORTALITY: Very low certainty evidence from 3 RCT’s in 527 patients, revealed a very uncertain effect of Azithromycin on all-cause mortality (RR 0.43 with 95% CI of 0.17 to 1.07).
- PROGRESSION TO IMV: Very low certainty evidence from 2 RCT’s in 442 patients revealed a very uncertain effect of Azithromycin on progression to IMV (RR 0.70 with a 95% CI of 0.08 to 6.15).
- DURATION OF HOSPITALISATION: Moderate certainty evidence from 1 RCT in 331 patients found that Azithromycin probably increases duration of hospitalization by a 1 day (MD 0.7 higher; 95% CI 0.91 lower to 2.31 higher).
- TIME TO CLINICAL IMPROVEMENT (time to fever clearance): Very low certainty evidence (single study with high risk of bias) from 1 RCT in 206 patients revealed a very uncertain effect of Azithromycin on time to fever clearance (MD 7.7 lower; 95% CI 8.31 lower to 7.09 lower).
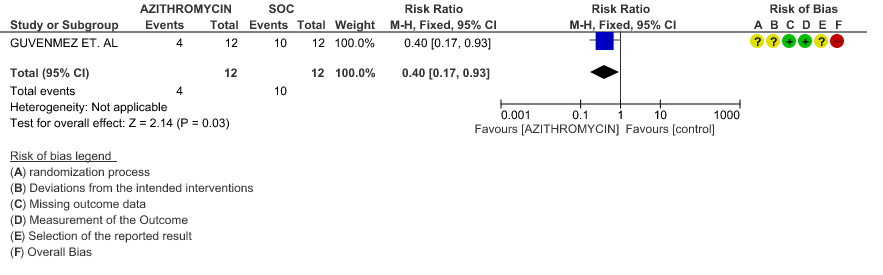
- VIRAL CLEARANCE ON DAY 6: Very low certainty evidence from 1 RCT (high risk of bias) in 24 patients revealed a very uncertain effect of Azithromycin on viral clearance at day 6 (RR 0.40 with a 95% CI of 0.17 to 0.93).
- INCIDENCE OF ARRHYTHMIA: Low certainty evidence from 1 RCT in 331 patients revealed that Azithromycin may not cause an increase in the incidence of arrhythmia (RR 0.46 with a 95% CI of 0.04 to 5.05).
- INCIDENCE OF QT-PROLONGATION: Low certainty evidence from 1 RCT in 162 patients revealed that Azithromycin may increase incidence of Q-T prolongation slightly (RR 1.15 with a 95% CI of 0.56 to 2.39).
Azithromycin in Comparison to Standard of Care in Hospitalized Severe to Critical Patients with Covid 19
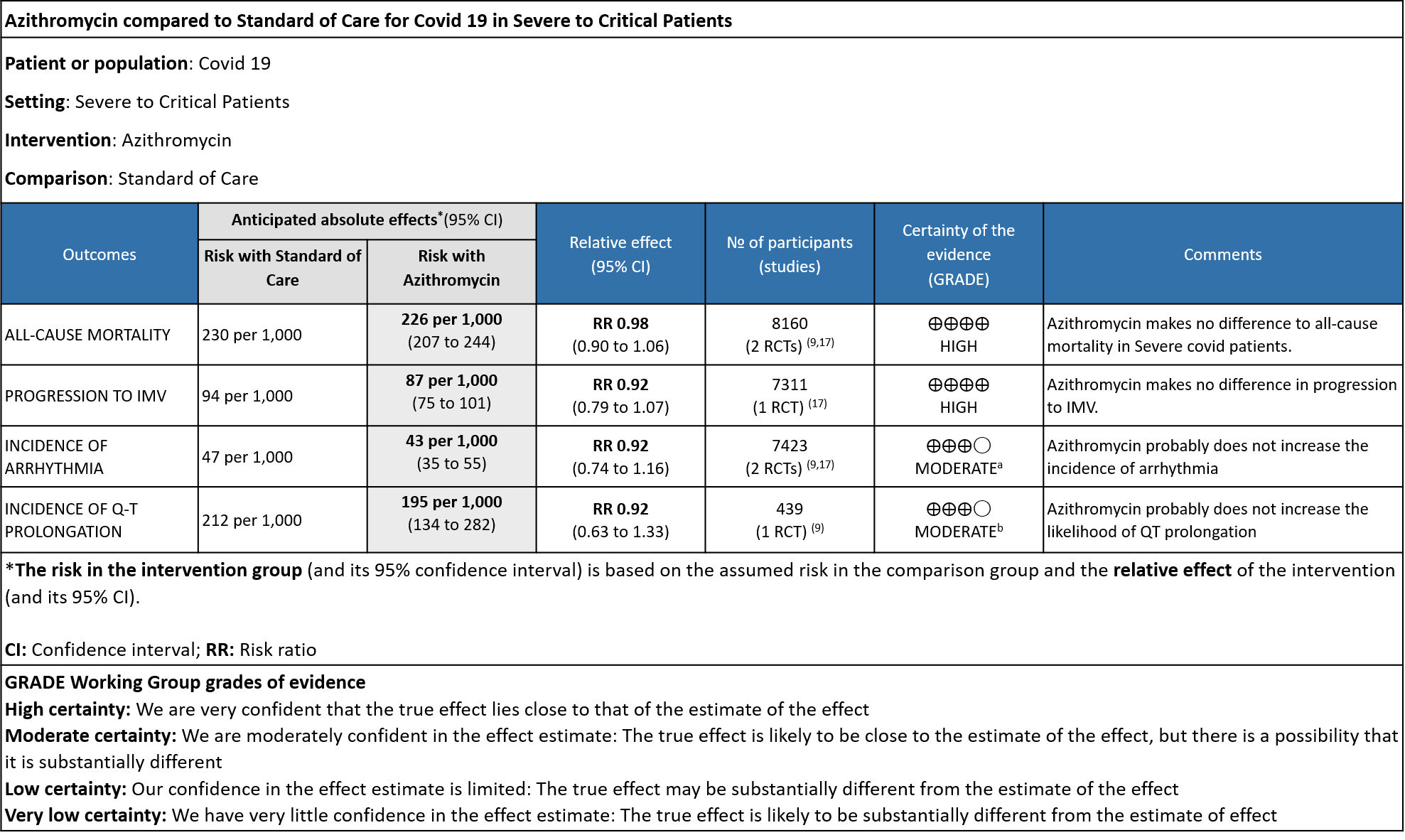
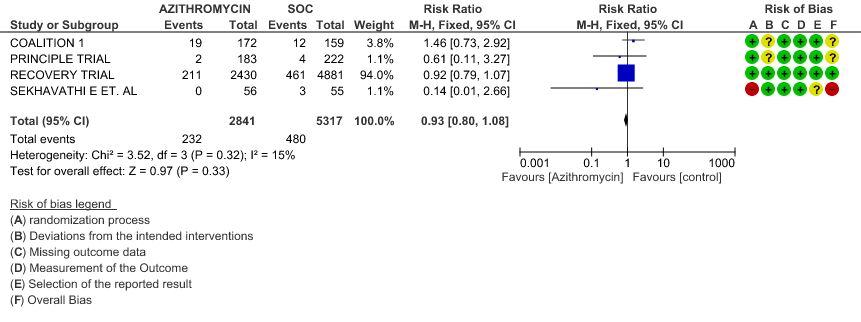
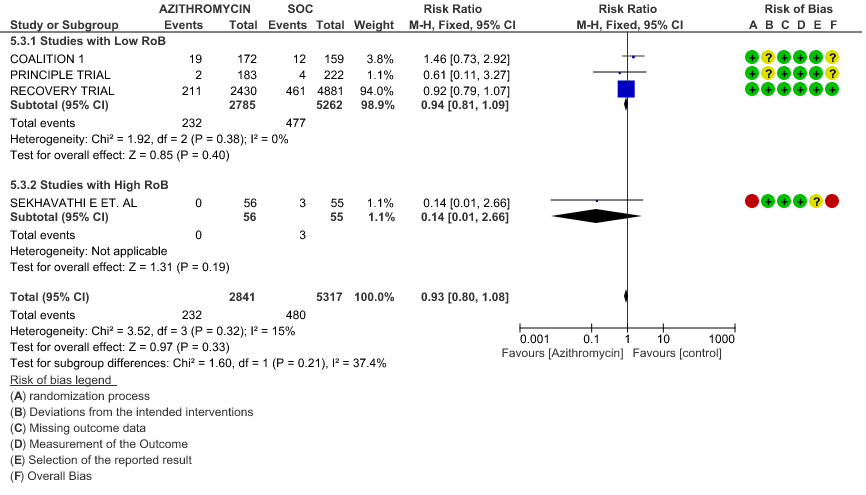
- ALL-CAUSE MORTALITY: High certainty evidence from 2 large RCT’s with a total number of 8160 patients revealed that Azithromycin did not reduce all-cause mortality in patients with severe to critical COVID 19 (RR 0.98; 95% CI 0.90-1.06).
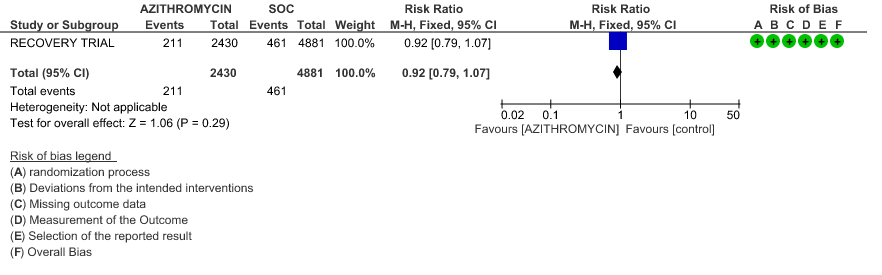
- PROGRESSION TO IMV: High certainty evidence from 1 large RCT in 7311 patients revealed that Azithromycin did not reduce progression to IMV (RR 0.92 with 95% CI of 0.79 to 1.07).
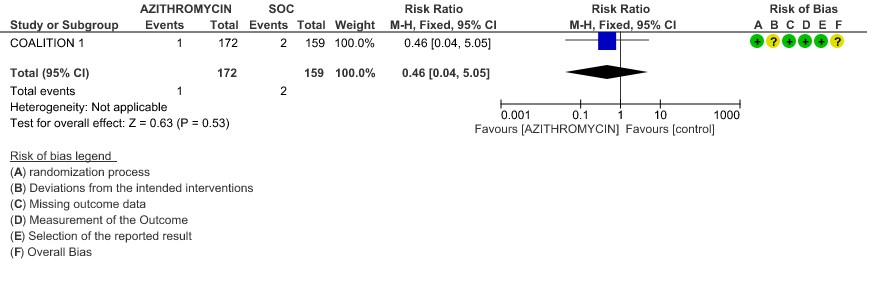
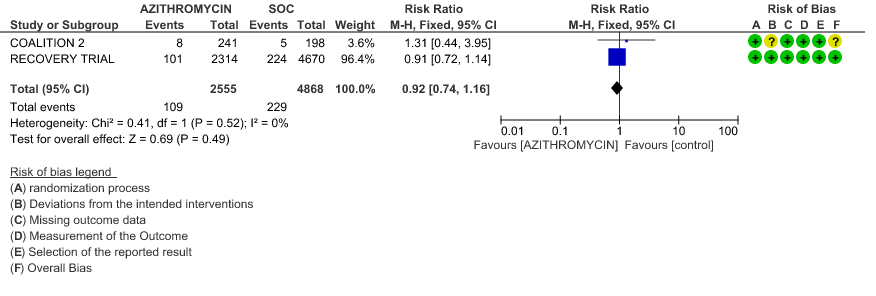
- INCIDENCE OF ARRHYTHMIA: Moderate certainty evidence from 2 RCT’s in 7423 patients revealed that Azithromycin probably does not increase the incidence of arrhythmia in patients with severe to critical COVID 19 (RR 0.92 with 95% CI of 0.74 to 1.16).
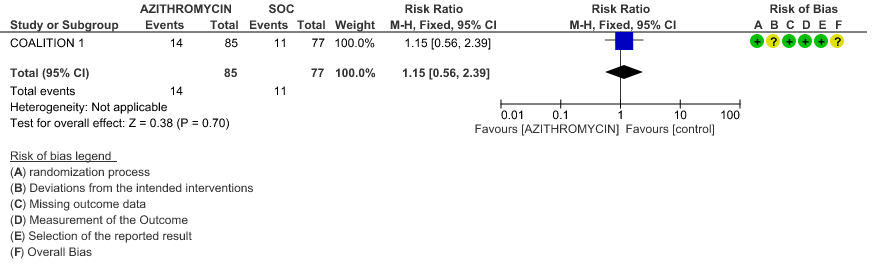
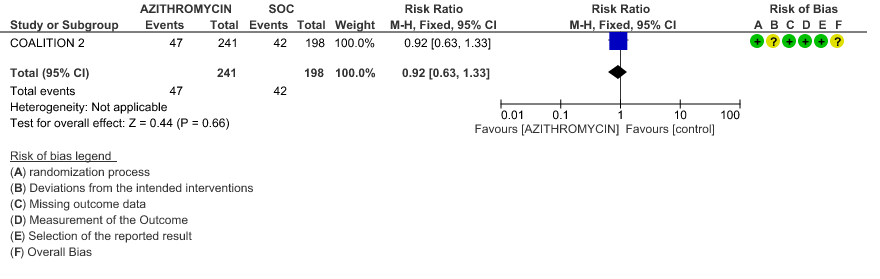
- INCIDENCE OF QT-PROLONGATION: Moderate certainty evidence from 1 RCT in 439 patients revealed that Azithromycin probably does not increase the likelihood of QT prolongation (RR 0.92 with a 95% CI of 0.63 to 1.33).
1. Azithromycin compared to standard of care in Covid 19
1a. All-Cause Mortality
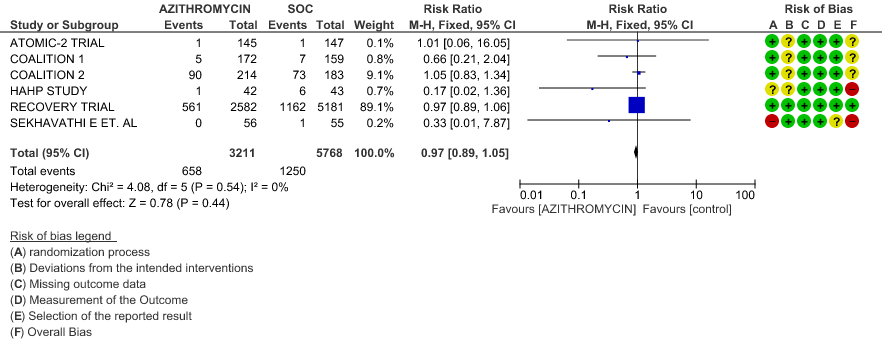
1b. All-Cause Mortality – Stratified with RoB
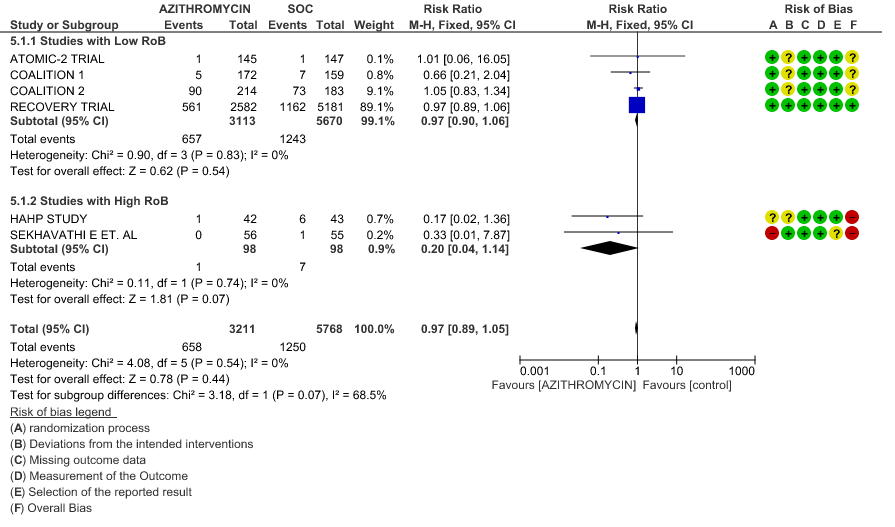
1c. Progression to IMV
1d. Progression to IMV – Stratified with RoB
1e. Progression to oxygen requirement
1f. Duration of Hospitalization
1g. Time to clinical improvement
1h. Viral clearance on day 14
1i. Incidence of Arrhythmias
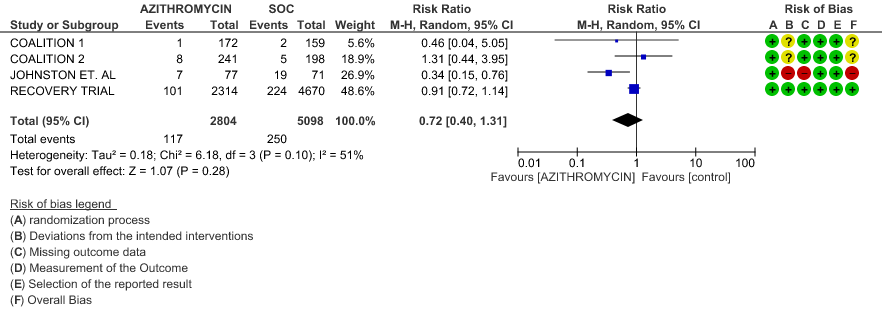
1j. Incidence of QT prolongation
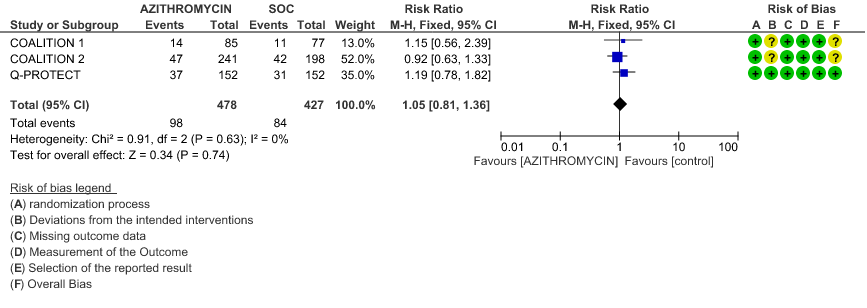
Sub Group Analysis
2. Ambulatory patients
2a. All-Cause Mortality
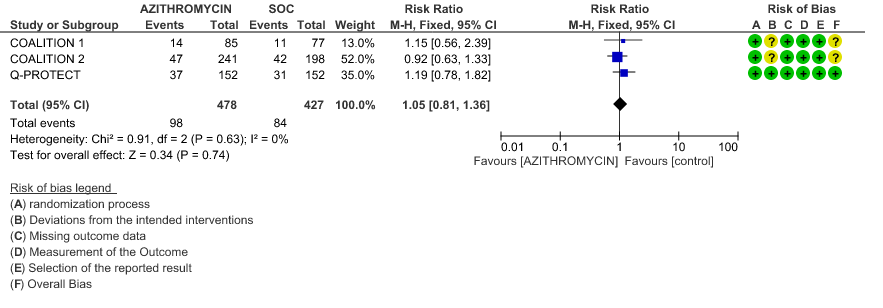
2b. Progression to IMV
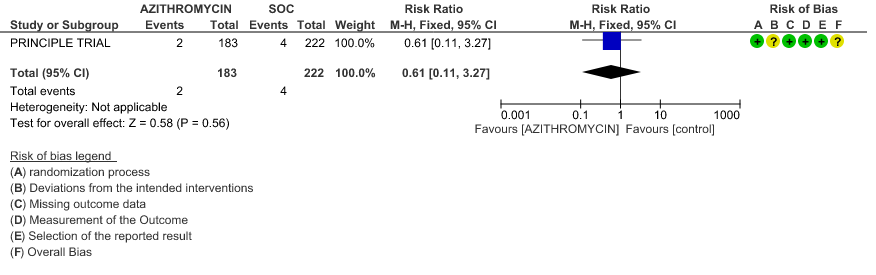
2c. Progression to oxygen requirement
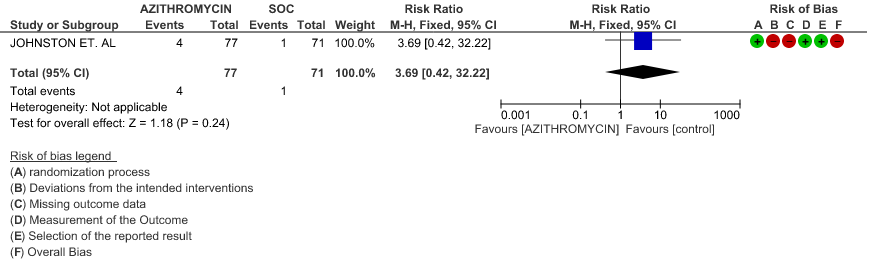
2d. Viral clearance on day 14
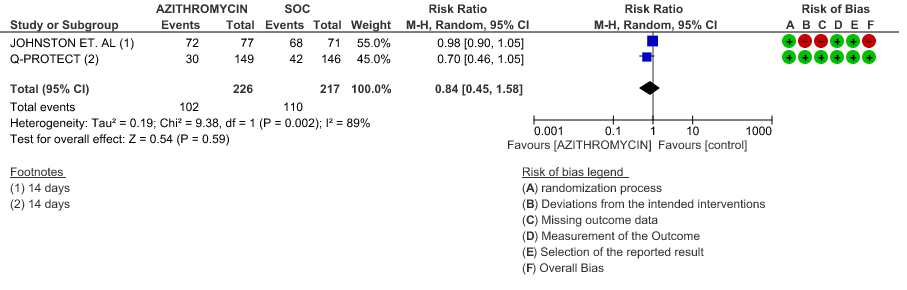
2e. Need for Hospitalisation
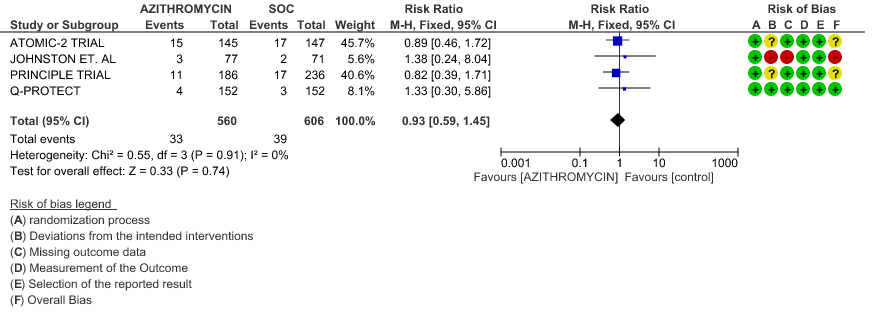
2f. Incidence of Arrhythmias
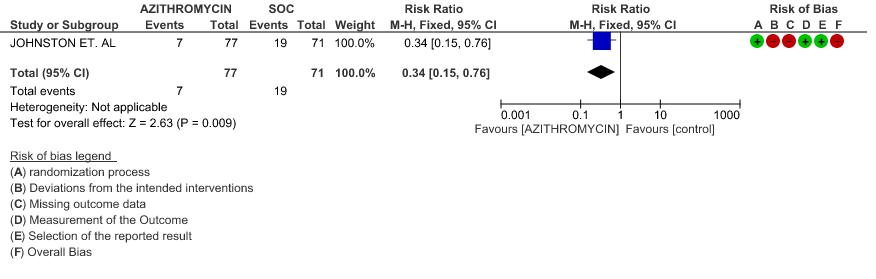
2g. Incidence of QT Prolongation
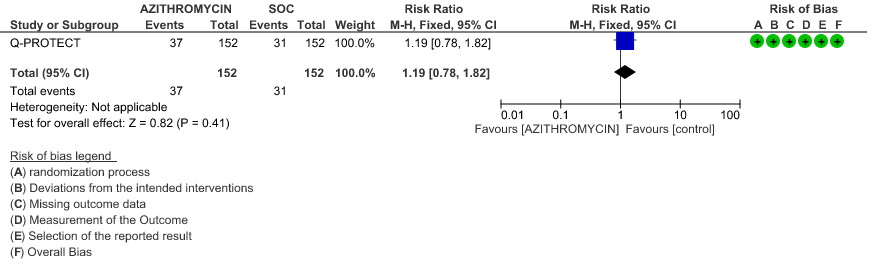
3. Hospitalised Non-severe patients
3a. All-cause mortality
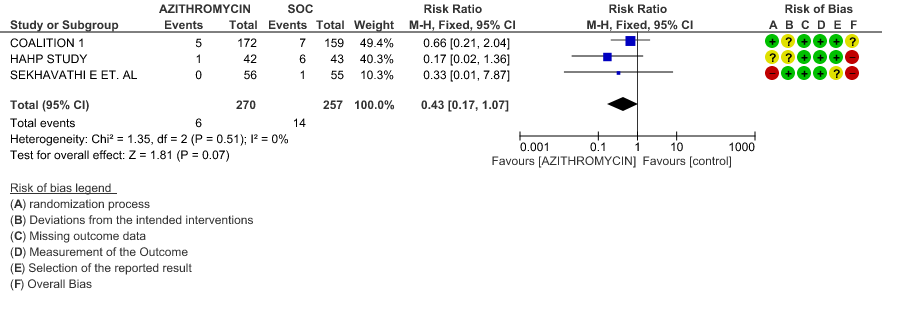
3b. Progression to IMV
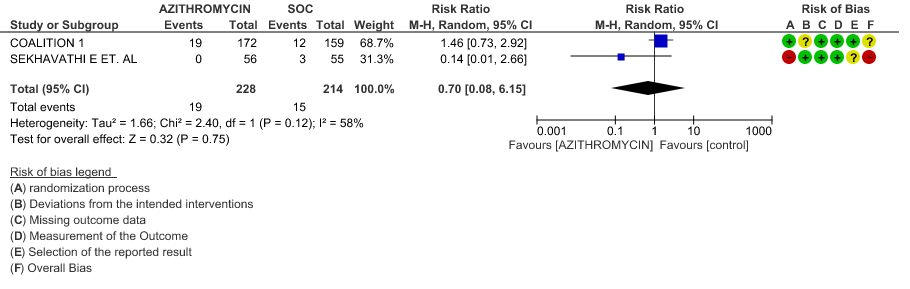
3c. Duration of Hospitalization
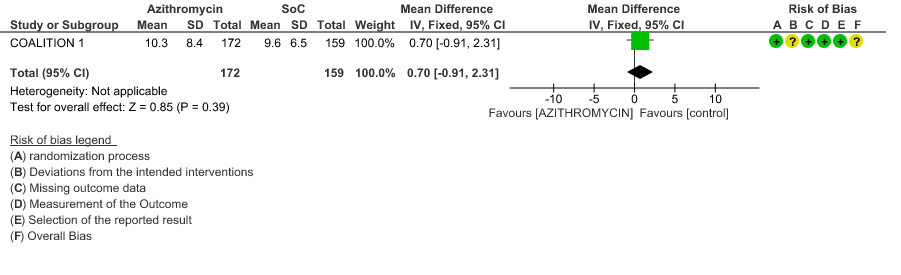
3d. Time to Clinical Improvement
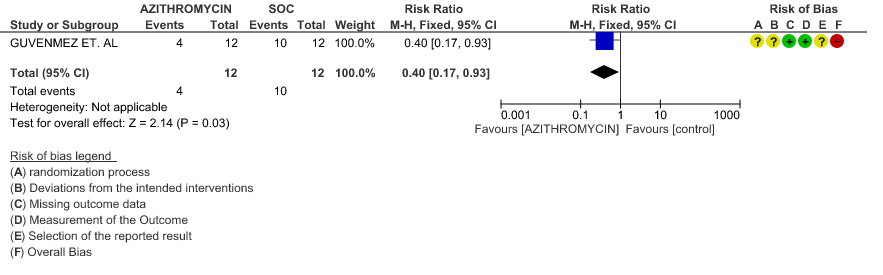
3e. Viral clearance at day 6
3f. Incidence of Arrhythmias
3g. Incidence of QT prolongation
4. Hospitalised severe to critical patients
4a. All-cause mortality
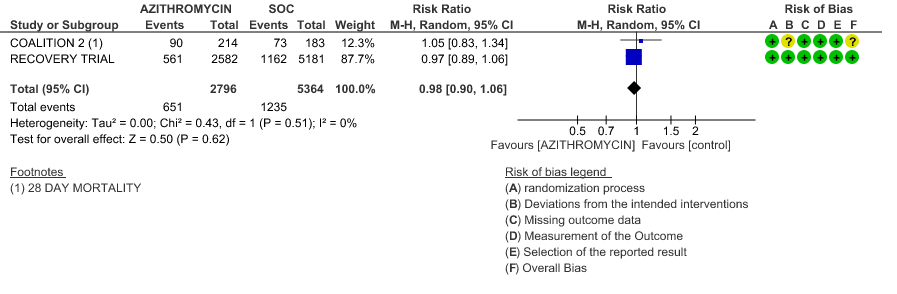
4b. Progression to IMV
4c. Incidence of Arrhythmias
4d. Incidence of QT prolongation
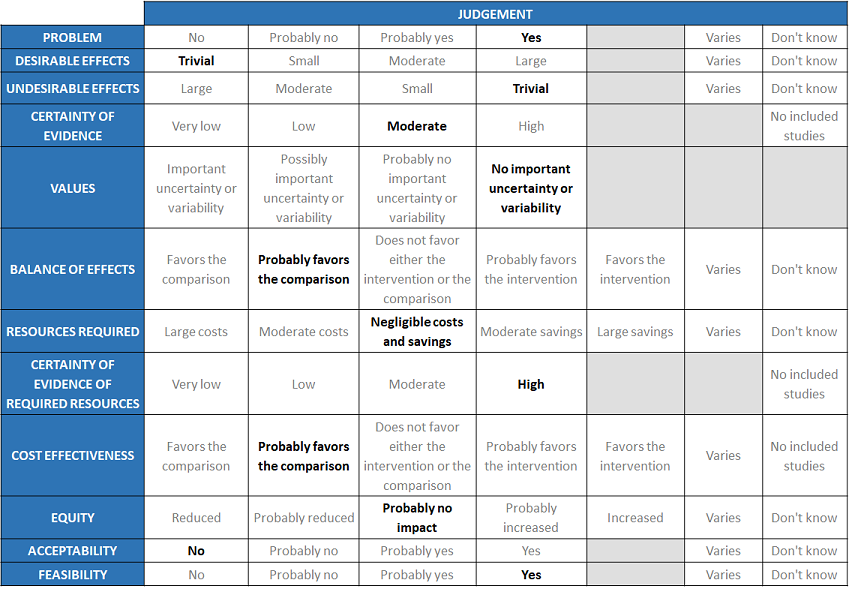
The Anti-inflammatory Expert Working Group met on 12th August 2021 to consider Azithromycin as a treatment for COVID-19. Conflict of interest declarations were reviewed by the Steering Committee; none were found to be relevant to Colchicine
A summary and then more detailed explanations of the Expert Working Group's judgements follow.
Problem
The COVID-19 pandemic in India with more than 30 million cases and over 0.39 million deaths has significantly impacted and stressed the health structure of the country. With a shortage of intensive care unit beds, oxygen and trained personnel the country is facing a major health crisis. Already there is a widespread use of Azithromycin for COVID patients.
Desirable effects
The panel agreed that the evidence suggests that Azithromycin doesn’t have a clinically significant effect on all-cause mortality, progression to IMV, progression to oxygen requirement, duration of hospitalisation, time to fever clearance and viral clearance at day 14 in overall category whereas azithromycin may result in reducing all-cause mortality and progression to IMV. Panel also discussed that azithromycin has a trivial effect over desirable outcomes.
Undesirable effects
The pooled data suggested that adverse events like incidence of arrhythmias and incidence of QT prolongation were not substantially different between the two groups and were comparatively lesser patients had experienced adverse events has been seen in Azithromycin group. However, the panel felt that the undesirable effect may be varying when considering patients with heart disease and elderly patients. The panel also discussed that Antibiotic resistance is not measured in most of the trials. So they concluded that azithromycin has a trivial effect over undesirable outcomes.
Certainty of evidence
Using GRADE methodology, the evidence synthesis team rated Certainty of evidence was found to be high that Azithromycin did not make a beneficial impact on critical outcomes like all-cause mortality, progression to IMV, moderate certainty evidence that Azithromycin did not reduce duration of hospitalization by even a day.
Low to very low certainty evidence revealed that time to clinical improvement (time to fever clearance), progression to oxygen requirement, viral clearance was not found to be different between the 2 groups in overall category. Low to very low certainty evidence revealed that time to clinical improvement (time to fever clearance), progression to oxygen requirement, viral clearance was not found to be different between the 2 groups. In subgroup analysis for ambulatory patients, certainty of evidence was moderate for duration of hospitalisation and very low for all-cause mortality, progression ton IMV, time for fever clearance, viral clearance at day 6, whereas low for incidence of arrhythmia and incidence of QT-prolongation. Certainty of evidence was moderate for all outcomes in hospitalised severe to critical patients. The expert working agreed with these judgements and rated the overall certainty of evidence as moderate certainty that this drug does not work for the main outcomes of mortality or progression to IMV. Macrolides do have an anti-inflammatory effect and there is biological plausibility for evidence of effect but the evidence for other outcomes is less certain.
Values
The EWG felt that all the outcomes including those of mortality, progression to mechanical ventilation, progression to oxygen requirement and adverse were expressed variably in the studies. However, there is no important uncertainty or variability on how people would value the main outcomes.
Balance of effects
The expert working group felt that there was a lot of heterogeneity in the trials. No actual harm but a lot of indirect harms are associated with the drug. It is an antibiotic and not recommended as an antiviral and hence concluded that the balance of effects probably favor the comparison.
Resources required
The group felt that the costs were small. The panel discussed and concluded that the requirement of resource is negligible cost and savings. The group includes clinicians in different types of Indian hospitals who have a good idea of drug and hospitalization costs.
Certainty of evidence of required resources
The panel discussed that since it is a commonly used drug which is inexpensive and can be orally delivered and easily available and hence felt that there was high certainty of evidence for required resources to implement this intervention.
Cost effectiveness
The panel discussed that even though there was no research evidence that evaluated cost of Colchicine in an Indian context, it probably favors the comparison, as there is no effectiveness of the drug so difficult to assess this.
Equity
At this point in time this intervention would probably no impact on equity even though the cost are low.
Acceptability
The group felt that this intervention is likely to have wide acceptance by all the relevant stakeholders (policy-makers, patients and clinicians) as it is an oral drug that is probably quite safe, here it is not effective. So this intervention should be considered unacceptable for use in COVID-19 patients as an intervention
Feasibility
This is a feasible intervention if found efficacious as it is easy to deliver and available easily over the counter in the country.
Azithromycin is one of the most common antibiotics prescribed in outpatient practice. It is easily and widely available, relatively inexpensive and has low risk of adverse drug reactions. The drug has been indiscriminately used in the treatment of COVID-19 citing its anti-inflammatory activity or treatment of a secondary infection despite no clear evidence of its utility. However, its use may lead to a false sense of security for the patient leading to delays in seeking medical attention and the implementation of other interventions for which evidence of benefit exists.
Azithromycin is often used as a prophylactic or empiric choice for suspected secondary bacterial pneumonias. However, the incidence of secondary bacterial pneumonia in mild to moderate COVID 19 is found to be low and hence the use of Azithromycin in all patients cannot be justified.
The evidence to date does not justify the use of Azithromycin in COVID 19, though this may be updated as new evidence emerges. If Azithromycin is subsequently assessed to be effective and safe in the treatment of COVID-19, it would be feasible to implement in management protocols.
While subgroup meta-analyses were conducted to assess utility across various severity of illness, these did not show substantial differences in the evidence synthesized thus far. Therefore the recommendation applies to all severity subgroups.
As with trials for many interventions, key sub-populations of concern who may respond differently, or may require more cautious use like those with heart disease were not included, or data for their outcomes were not available separately. However, with no overall benefit being found, it would be undesirable to pursue further examination of use of azithromycin in these subgroups.
The recommendation against the use of Azithromycin will be reviewed as and when any new evidence emerges. The present evidence dictates that this drug is not useful for the treatment of COVID-19 infection. Although the evidence for discontinuation of the drug because of the undesirable effects reported with widespread use such as arrhythmias, QT prolongation & gastrointestinal effects have so far been low, in the studies available so far, this needs careful monitoring as the potential for drug-related harm cannot be ruled out.
Azithromycin is useful in the treatment of acute COVID-19 infection. Its use as a prophylactic or empiric drug is likely to propagate antimicrobial resistance and limit its activity in endemic tropical diseases where it is often the drug of choice. Further research is needed to
- Document if indiscriminate use of Azithromycin pre-admission led to development of multi drug resistant infection during ICU or hospital stay.
- Document if indiscriminate use led to the development of more cardiac morbidity like arrhythmias, sudden cardiac deaths in high risk patients with multiple co morbidities.
- Define the possible immunomodulatory role of Azithromycin in long COVID or post-acute COVID especially with regard to lung fibrosis or bronchiolitis obliterans in COVID-19 infection.
- Touret F, Gilles M, Barral K, Nougairède A, van Helden J, Decroly E, de Lamballerie X, Coutard B. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci Rep. 2020 Aug 4;10(1):13093. doi: 10.1038/s41598-020-70143-6. PMID: 32753646; PMCID: PMC7403393.
- Oliver ME, Hinks TSC. Azithromycin in viral infections. Rev Med Virol. 2021 Mar;31(2):e2163. doi: 10.1002/rmv.2163. Epub 2020 Sep 23. PMID: 32969125; PMCID: PMC7536932.
- Youth for homecare. COVID-19 home care kits. https://insider.in/covid-19-homecare-kits--youth-for-seva-covid-relief/event
- Poschet JF, Perkett EA, Timmins GS, Deretic V. Azithromycin and ciprofloxacin have a chloroquine-like effect on respiratory epithelial cells. bioRxiv [Preprint]. 2020 Mar 31:2020.03.29.008631. doi: 10.1101/2020.03.29.008631. PMID: 32511331; PMCID: PMC7239066.
- Min JY, Jang YJ. Macrolide therapy in respiratory viral infections. Mediators Inflamm. 2012;2012:649570. doi: 10.1155/2012/649570. Epub 2012 Jun 6. PMID: 22719178; PMCID: PMC3375106.
- Giorgia Sulis,Brice Batomen,Anita Kotwani,Madhukar Pai,Sumanth Gandra, Sales of antibiotics and hydroxychloroquine in India during the COVID-19 epidemic: An interrupted time series analysis, PLOS Medicine, 18(7), e1003682 - July 2021, https://doi.org/10.1371/journal.pmed.1003682
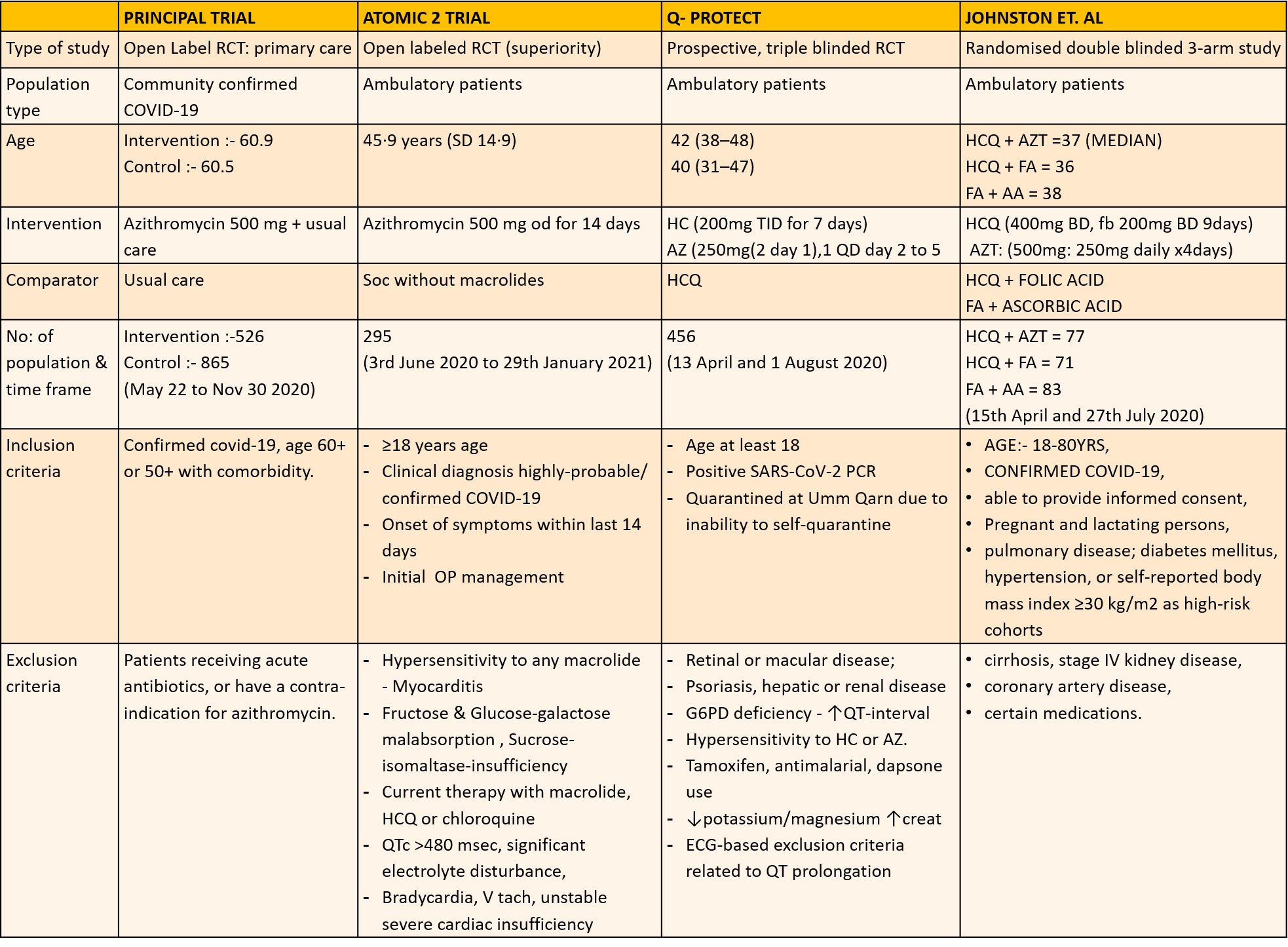
- Hinks TSC, Barber VS, Black J, Dutton SJ, JabeenM et. al. A multi-centre open-label two-arm randomised superiority clinical trial of azithromycin versus usual care in ambulatory COVID-19: study protocol for the ATOMIC2 trial. Trials. 2020 Aug 17;21(1):718. doi: 10.1186/s13063-020-04593-8. PMID: 32807209; PMCID: PMC7429453.
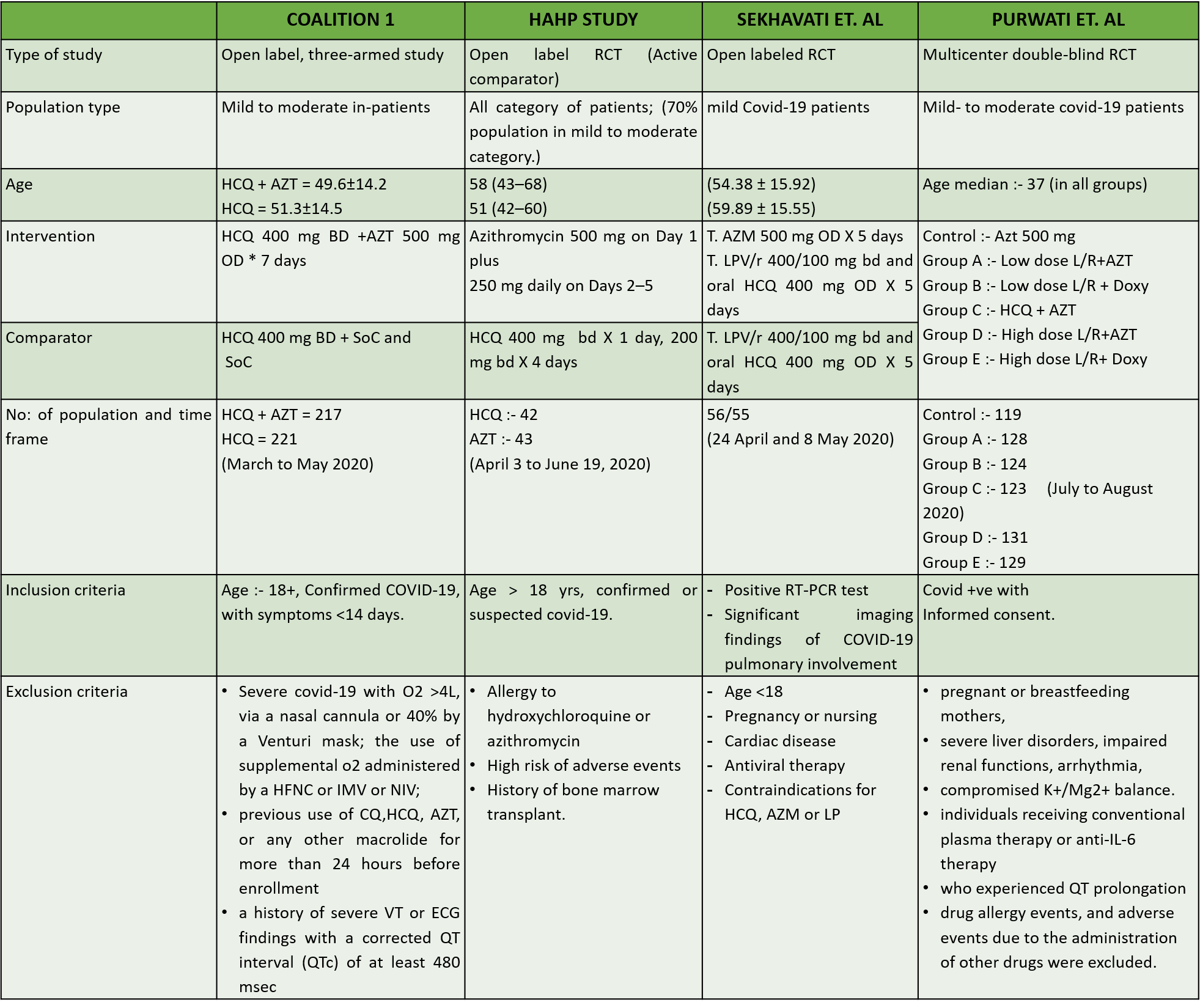
- Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC et.al.; Coalition Covid-19 Brazil I Investigators. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med. 2020 Nov 19;383(21):2041-2052. doi: 10.1056/NEJMoa2019014. Epub 2020 Jul 23. Erratum in: N Engl J Med. 2020 Nov 19;383(21):e119. PMID: 32706953; PMCID: PMC7397242.
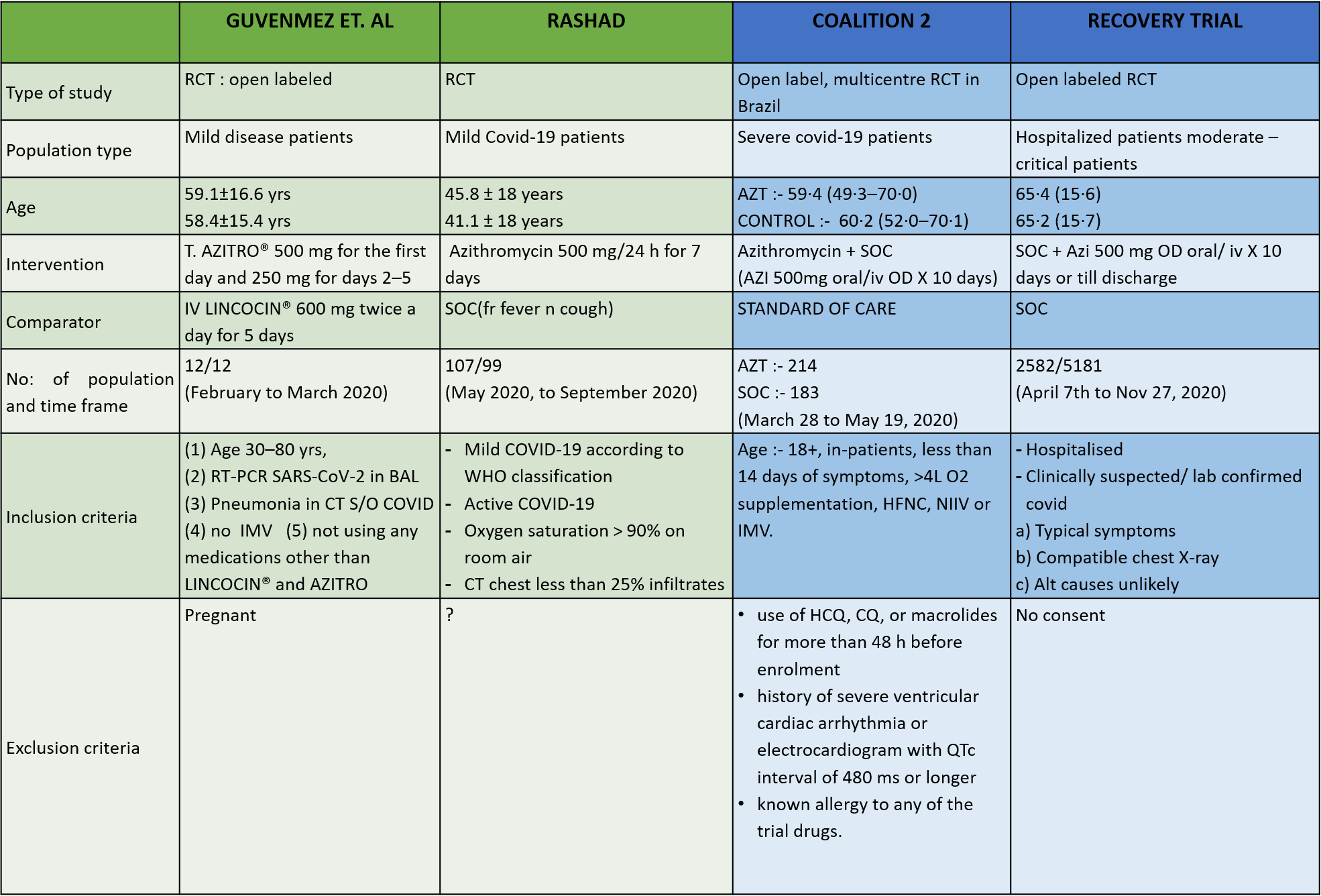
- Furtado RHM, Berwanger O, Fonseca HA, Corrêa TD, Ferraz LR et.al; COALITION COVID-19 Brazil II Investigators. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet. 2020 Oct 3;396(10256):959-967. doi: 10.1016/S0140-6736(20)31862-6. Epub 2020 Sep 5. PMID: 32896292; PMCID: PMC7836431.
- Guvenmez O, Keskin H, Ay B, Birinci S, Kanca MF. The comparison of the effectiveness of lincocin® and azitro® in the treatment of covid-19-associated pneumonia: A prospective study. J PopulTher Clin Pharmacol. 2020 Jun 3;27(S Pt 1):e5-e10. doi: 10.15586/jptcp.v27iSP1.684. PMID: 32543164.
- Brown SM, Peltan I, Kumar N, Leither L, Webb BJ et.al. Hydroxychloroquine vs. Azithromycin for Hospitalized Patients with COVID-19 (HAHPS): Results of a Randomized, Active Comparator Trial. Ann Am Thorac Soc. 2020 Nov 9;18(4):590–7. doi: 10.1513/AnnalsATS.202008-940OC. Epub ahead of print. PMID: 33166179; PMCID: PMC8009003.
- Johnston C, Brown ER, Stewart J, Karita HCS, Kissinger PJ et.al ; COVID-19 Early Treatment Study Team. Hydroxychloroquine with or without azithromycin for treatment of early SARS-CoV-2 infection among high-risk outpatient adults: A randomized clinical trial. EClinicalMedicine. 2021 Mar;33:100773. doi: 10.1016/j.eclinm.2021.100773. Epub 2021 Feb 27. PMID: 33681731; PMCID: PMC7912360.
- PRINCIPLE Trial Collaborative Group. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021 Mar 20;397(10279):1063-1074. doi: 10.1016/S0140-6736(21)00461-X. Epub 2021 Mar 4. PMID: 33676597; PMCID: PMC7972318.
- Purwati, Budiono, Rachman BE, Yulistiani, Miatmoko A, Nasronudin, Lardo S, Purnama YI, LaelyM et. al. A Randomized, Double-Blind, Multicenter Clinical Study Comparing the Efficacy and Safety of a Drug Combination of Lopinavir/Ritonavir-Azithromycin, Lopinavir/Ritonavir-Doxycycline, and Azithromycin-Hydroxychloroquine for Patients Diagnosed with Mild to Moderate COVID-19 Infections. Biochem Res Int. 2021 Feb 9;2021:6685921. doi: 10.1155/2021/6685921. PMID: 33628506; PMCID: PMC7881739.
- Omrani AS, Pathan SA, Thomas SA, Harris TRE, Coyle PV et.al. Randomized double-blinded placebo-controlled trial of hydroxychloroquine with or without azithromycin for virologic cure of non-severe Covid-19. EClinicalMedicine. 2020 Dec;29:100645. doi: 10.1016/j.eclinm.2020.100645. Epub 2020 Nov 20. PMID: 33251500; PMCID: PMC7678437.
- Rashad A, Nafady A, Hassan MH et al. Therapeutic efficacy of macrolides in management of patients with mild COVID-19, 01 February 2021, PREPRINT (Version 1) available at Research Square [https://doi.org/10.21203/rs.3.rs-181996/v1]
- RECOVERY Collaborative Group. Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021 Feb 13;397(10274):605-612. doi: 10.1016/S0140-6736(21)00149-5. Epub 2021 Feb 2. PMID: 33545096; PMCID: PMC7884931.
- Sekhavati E, Jafari F, SeyedAlinaghi S, Jamalimoghadamsiahkali S, Sadr S et.al. Safety and effectiveness of azithromycin in patients with COVID-19: An open-label randomised trial. Int J Antimicrob Agents. 2020 Oct;56(4):106143. doi: 10.1016/j.ijantimicag.2020.106143. Epub 2020 Aug 25. PMID: 32853672; PMCID: PMC7445147.
Covid Management Guidelines India Group – Antiviral working Group – Azithromycin. Covid Guidelines India; Published online on September 27th, 2021; URL: https://indiacovidguidelines.org/azithromycin/ ( ).
