The evidence from the RCTs indicates that systemic steroids were associated with decrease in the all-cause mortality and need for mechanical ventilation to a moderate extent. This benefit was observed in patients with severe or critical COVID-19, who had need for oxygen therapy. There was evidence against the use of systemic steroids in non-hypoxic patients; subgroup analysis from a large trial did not show any benefit and there was also concern about potential increase in mortality.
No significant increase in the adverse events, especially secondary infections, was observed in the trials. The panel believes that appropriate and responsible use of systemic steroids with strict sugar control would lead only to a small risk of undesirable effects but the same could be moderate if used in high doses or for longer duration.
There was no statistically significant difference in terms of mortality, progression to mechanical ventilation, serious adverse events, nosocomial infections, or hyperglycemia, with respect to the use of equivalent doses of different classes of steroids. The data available on the outcomes, with respect to the timing of steroids after the symptom onset (≤7 days vs. 7 days or more), the dose (< or ≥ 10 mg of dexamethasone or equivalent) as well as the total duration of steroids ( 7 days) was weak. However, no statistically significant difference was observed amongst these different subgroups suggesting that using steroids too early in higher doses or longer duration was not proven to be beneficial and may be contributing to harm like hyperglycemia, higher risk of nosocomial infections and mucormycosis where inappropriate use of steroids is emerging as an important risk factor.
The panel concluded that moderate amount benefit of systemic steroids supports their use in severe to critically ill COVID-19 patients, who have hypoxia.
Date of latest search: 28th April 2021.
Date of completion of Summary of findings table and presentation to Expert Working Group: 29th May 2021.
Date of planned review: 27th November 2021
Evidence synthesis team: Anupa Thampy, Richard Kirubakaran, Bhagteshwar Singh and Priscilla Rupali
CAPE COVID, CoDEX, COVD STEROID, DEXA COVID 19, Edalatifard M et al, GLUCOCOVID, Jamaati H et al, Metcovid, Recovery, REMAP-CAP, Steroids-SARI, Tang X et al.
- Downgraded by one level for serious imprecision; the clinical effect of reduction in all cause mortality is not appreciable(absolute effect 1/100-5/100)
- CAPE COVID, Jamaati H et al, Metcovid, Recovery
- Downgraded by one level for serious imprecision; the clinical effect on reduction to progression to IMV is not appreciable ( Absolute effect 0.4/100-3/100)
As of 31st May 2021, around 28,047,534 patients have been diagnosed with covid-19 in India according to the ministry of health and family welfare records. The pandemic has claimed around 3, 29,100 lives and there is a continuing surge in the covid-19 cases across India. SARS-CoV-2 binds to host cells through the ACE2 receptor, and after endocytosis and subsequent uncoating, the components of SARS-CoV-2 use host cells machinery to produce new viruses. Finally, the SARS-CoV-2 virions are released from the host cell by exocytosis. SARS-CoV-2 stimulates the host immune system to release the cytokines and subsequent inflammation and immune-dysfunction through activation or impairment of various immune cells, such, dendritic cells, NK cells, macrophages, and neutrophils. This process can lead to sepsis, septic shock, multiple organ failure, and death (1).
There have been many therapeutic interventions proposed for COVID 19, however steroids are the only drugs with a proven mortality benefit and also known to prevent progression to invasive mechanical ventilation. The use of steroids is based on the fact that the anti-inflammatory effects of steroids can help reduce systemic inflammatory response thus preventing multi-organ dysfunction. Hence steroids are widely being used all over the world for management of COVID19 patients in the moderate, severe and critical categories requiring oxygen. However,some areas of equipoise remain and we sought to answer these questions with our evidence synthesis.
• Does use of steroids lead to an increased incidence of secondary infections fuelling the epidemic of mucormycosis?
• Which is the most appropriate type, dose and duration of steroids?
• Can steroids be used for those with inflammation but no hypoxia which may be early in the disease and thus prevent progression?
We searched Epistemonikos and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language. We also reviewed reference lists of PAHO Ongoing Living of Covid 19 Therapeutics, the systematic review by WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group and included studies. We performed all searches up to 21 May 2021. We contacted researchers to identify unpublished and ongoing studies.
We included randomized controlled trials (RCTs) testing systemic steroids treatment of any formulation, dose or duration in people with acute COVID‐19, whether suspected or confirmed. Trials were included if the intervention arm did not combine systemic steroids with another experimental drug, and if the comparator arm included placebo or standard care. We excluded trials that did not report any outcomes that could provide usable data for the review and those lacking a comparator arm.
We planned to extract data for the following pre-defined outcomes:
- Primary outcome
- All-cause mortality
- Secondary outcomes
- Time to clinical recovery
- Need for non-invasive or mechanical ventilation
- Duration of ventilation
- Length of stay in hospital
- Need for other organ support
- Adverse events
-
- All adverse events
- Serious adverse events
- Nosocomial/opportunistic infections
- Hyperglycaemia or diabetes mellitus
- Hypertension
The working group felt that since the mortality benefit of steroids was irrevocably established and it was now standard of care we needed to do a focussed subgroup analysis to answer other questions that were pertinent to the use of steroids. Equipoise existed around the following
- Was there a benefit if used early in illness?
- Was there a benefit to using a higher dose of steroids in the critical ill patients?
- Was there a benefit to using a specific type of corticosteroid -e.g., methylprednisolone, dexamethasone, hydrocortisone or prednisolone?
- Was there a benefit in using a longer duration of steroids rather than the 10 days recommended?
FOCUSSED SUBGROUPS
- Type of corticosteroid:
- Dexamethasone
- Methylprednisolone
- Hydrocortisone
- Dose per day:
- High dose (≥10mg/day dexamethasone or equivalent)
- Low dose (<10mg/day dexamethasone or equivalent)
- Duration:
- Longer course: >7 days (or until discharge)
- Shorter course: ≤7 days
- Time since onset of symptoms:
- Early <7 days
- Late ≥7 days
- Severity of disease
- Non-severe (Mild/Moderate severity with no hypoxia)
- Severe/Critical
Two reviewers independently assessed eligibility of search results. One reviewer extracted data from each included study which was checked by the second reviewer. Risk of bias assessment was taken from PAHO Ongoing Living of Covid 19 Therapeutics and WHO REACT systematic review, where Cochrane Risk of bias (RoB) v2.0 tool was used.
We used RevMan 5.4 to perform meta‐analysis using a fixed‐effects model or a random-effects model for outcomes where pooling of effect estimates was appropriate. We used risk ratios (RR) for dichotomous outcomes with 95% confidence intervals (CIs).
We planned to perform subgroup analysis in the predefined focused subgroups as mentioned earlier. We used GRADE methodology to assess the certainty in the evidence, and documented this in a ‘Summary of findings’ table using GradeProGDT (hyperlink)
Results
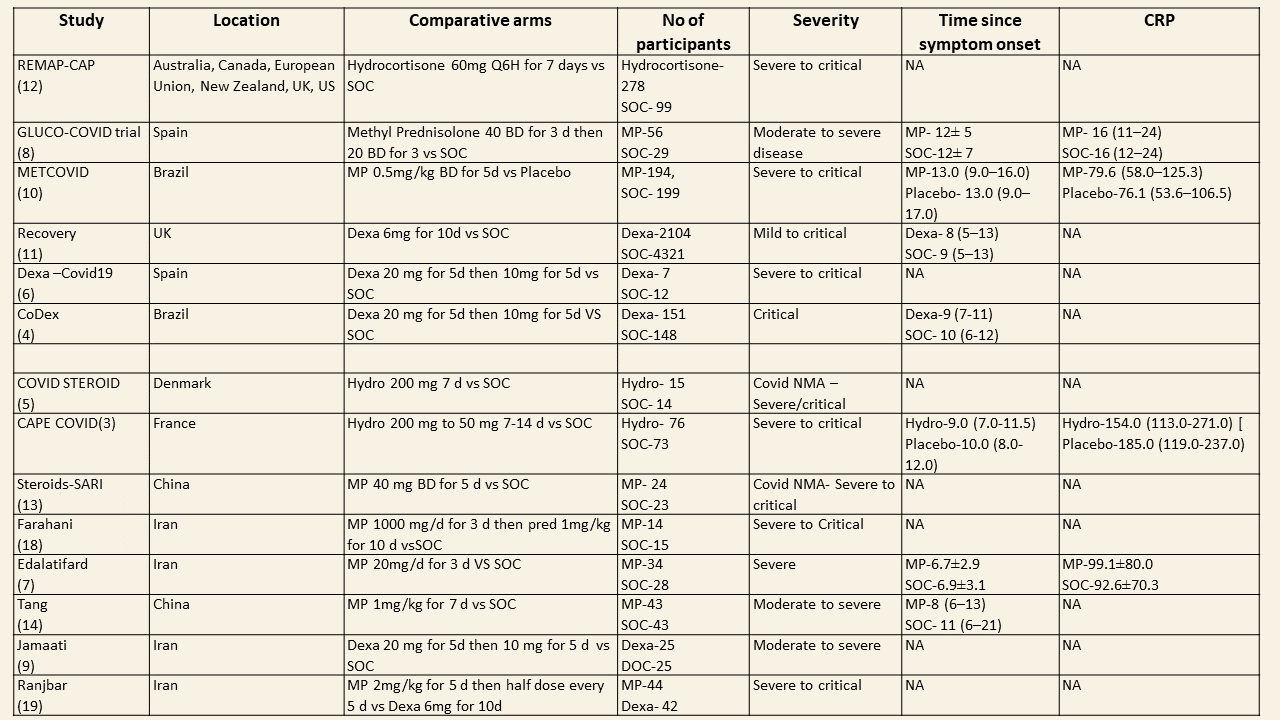
We found 13 trials which compared steroids against standard of care or placebo. Varying steroid types, doses and duration were used in different categories of severity of COVID19 and these studies were done in different parts of the world. One study compared two different types and doses of steroids in severe and critical disease. The median CRP was at least 2-3 times the upper limit of normal and steroids were instituted at least 5 days after onset of symptoms. The doses for steroids varied widely with Methylprednisolone doses ranging from 20mg to 1000mg, Dexamethasone doses from 6mg to 20mg, Hydrocortisone from 60mg to 240mg/day. In addition, the duration of steroids varied from 3 days to 14 days.
Equivalent doses of steroids
| Dexamethasone | Methylprednisolone | Prednisolone | Hydrocortisone |
| 1.5mg | 8mg | 10mg | 40mg |
We first looked at the overall effects of steroids on various outcomes.
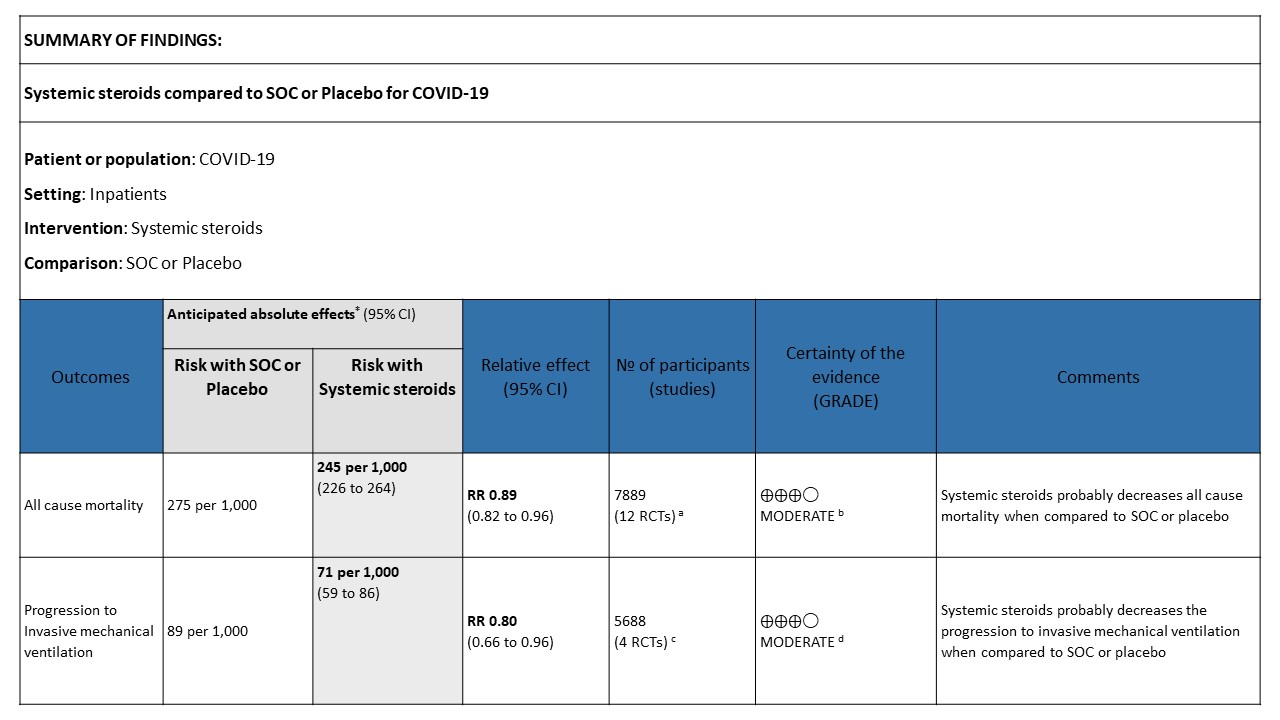
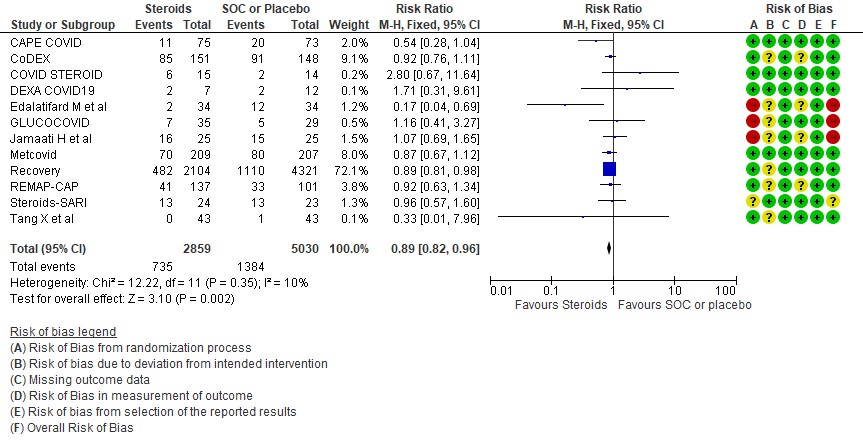
[A] All-cause mortality: Moderate certainty evidence in 12 RCTs (3-14) done in 7889 patients revealed that systemic steroids probably reduced all-cause mortality when compared to standard of care (SOC) or placebo RR=0.89 (95%CI 0.82-0.96). This suggested a mortality benefit of up to 11% (95% CI 4%-18%) with an absolute risk reduction of 0.018 and a number needed to treat of 55.
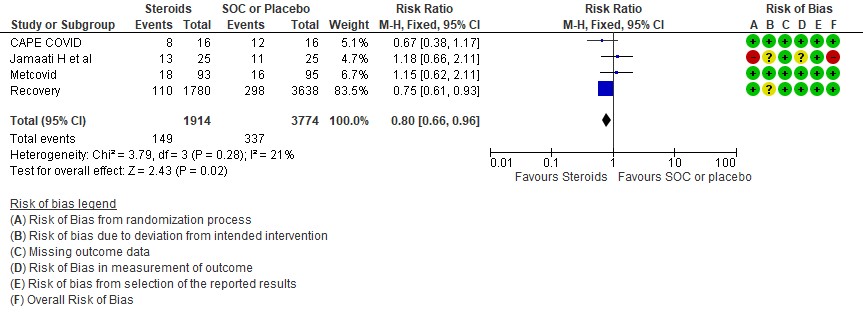
[B] Progression to invasive mechanical ventilation: Moderate certainty of evidence in 4 RCTs (3,9-11) in 5688 patients revealed that systemic steroids probably reduced progression to invasive mechanical ventilation vs SOC by 20% RR=0.80 (95% CI 0.66-0.96).
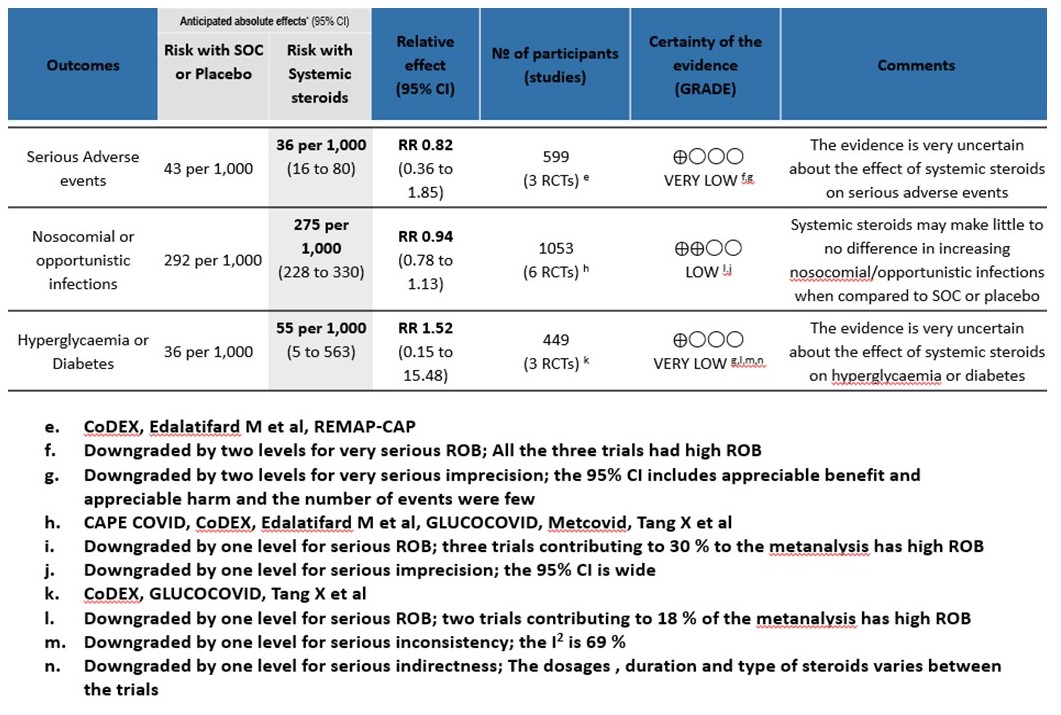
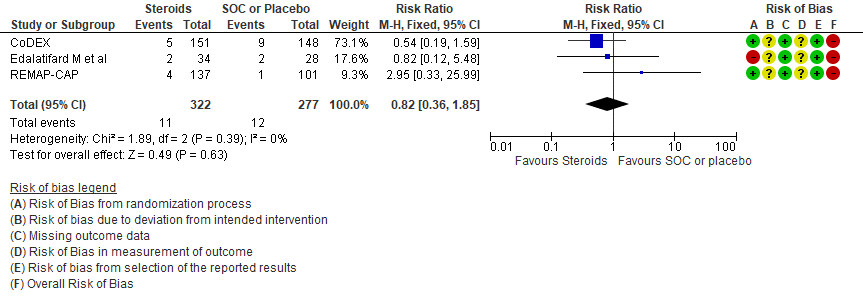
[C] Serious adverse events: Very low certainty of evidence suggesting an uncertain effect in 3 RCTs(4,7,12) in 599 patients revealed no increase in adverse events in the steroid group as compared to SOC/placebo RR=0.82 (95% CI 0.36-1.85)
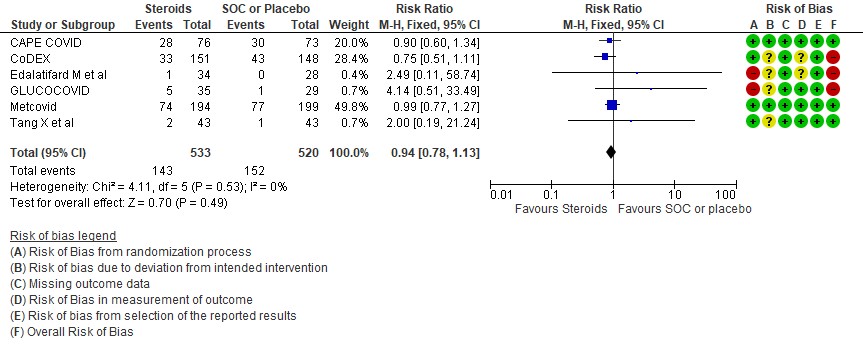
[D] Nosocomial/Opportunistic infections: Low certainty evidence in 6 RCTs (3,4,7,8,10,14)in 1053 patients revealed that steroids made little to no difference to an increase in nosocomial or opportunistic infections as compared to SOC or placebo RR=0.94 (95% CI 0.78-1.13).
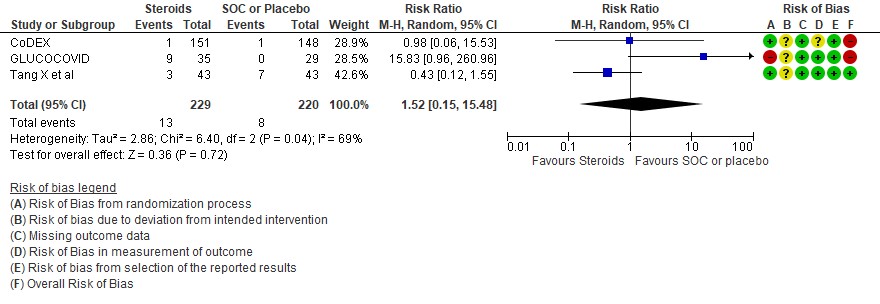
[E] Hyperglycemia: Very low certainty evidence in 3 RCTs (4,8,14)in 449 patients revealed a very uncertain effect of steroids on hyperglycemia or diabetes as compared to SOC/placebo RR=1.52 (95% CI 0.15 – 15.48).
Subgroup analysis
The group also wanted to explore the optimal usage of steroids in different clinical settings.
[1] Utility of steroids in early disease <7 days of symptoms
Disaggregated data was not available in most of the trials assessed by us. However, this was available in the WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group (2). This was a within trial post hoc comparisons of 2 populations. There appeared to be no difference in terms of all-cause mortality when symptom of patients was less than 7 days and greater than 7 days.
On review of the population in the studies, majority had oxygen or ventilation requirement in first 7 days. However, we noted inconsistency and imprecision across studies and we were unable to determine the balance of baseline characteristics within subgroups. In addition, the studies were not randomized into severity strata and hence it was probably difficult to adjust for the confounders.
[2] Utility of steroids in mild/moderate disease with no hypoxia
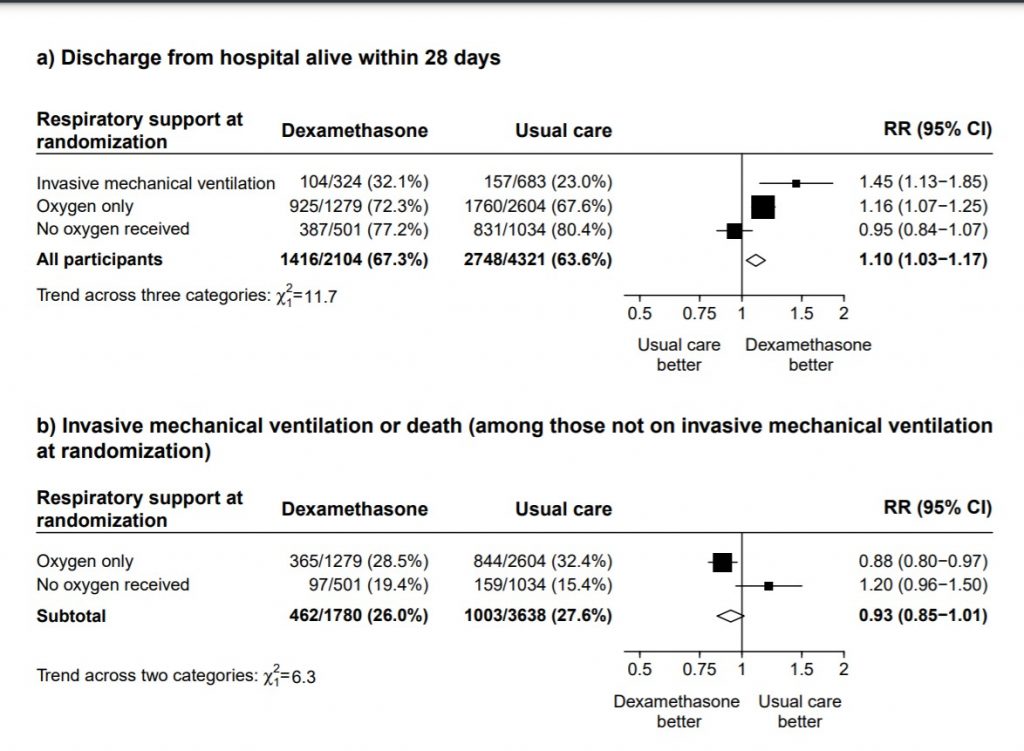
We found 4 studies (2,15,16,17) attempting to answer this question, however 1 was terminated, 2 have been completed and we are awaiting publication and 1 trial was still recruiting. In the mild to moderate illness with no hypoxia, the only data available is from the Recovery trial (11) with patients not on O2 where it demonstrated no clear benefit or harm with 28-day mortality RR 1.19 (95% CI 0.92-1.55) or the composite of progression to ventilation or death RR 1.20(95% CI 0.96-1.50).
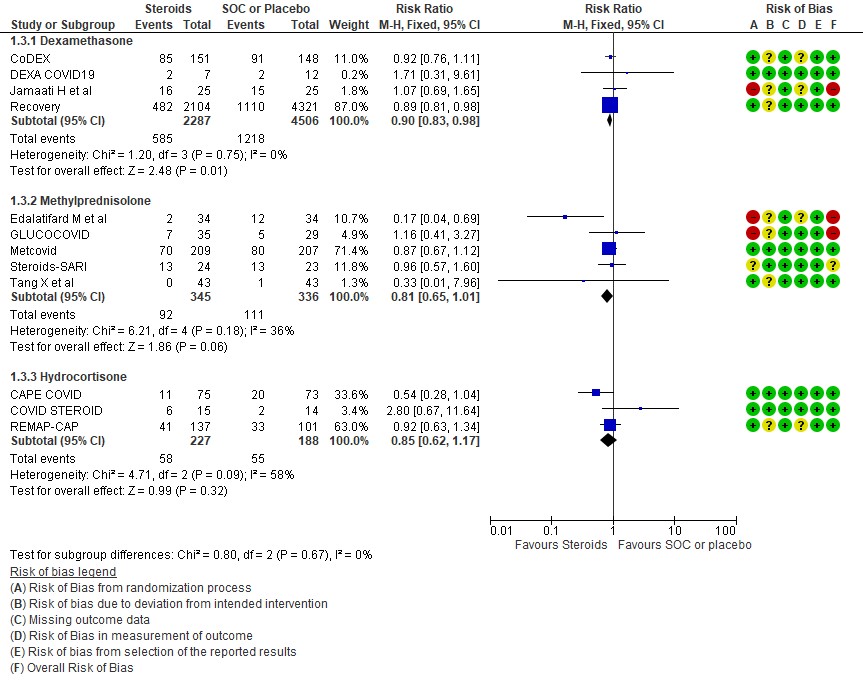
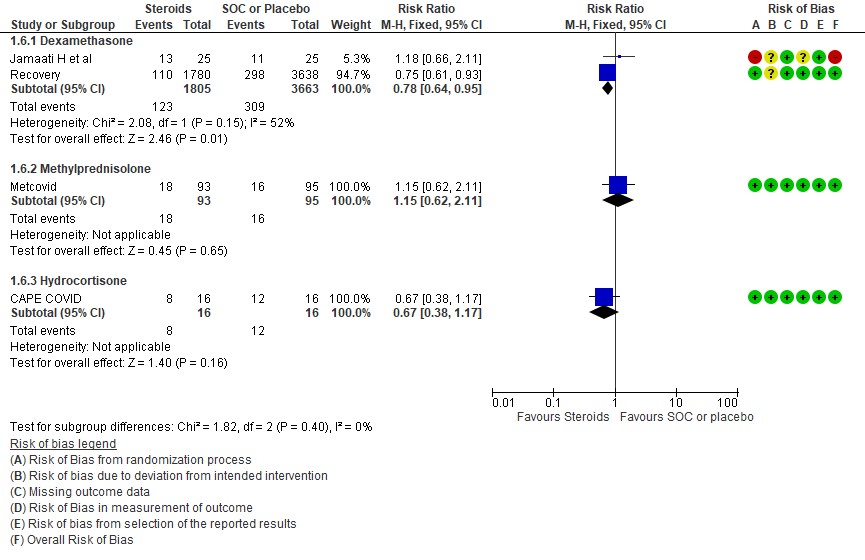
[3] Utility of a different type of steroid like methylprednisolone or hydrocortisone in severe or critical illness as compared to Dexamethasone
We found 4 studies (4,6,9,11) which used Dexamethasone in 6793 patients, 5 studies (7,8,10,13,14) which used Methylprednisolone in 681 patients and 3 studies (3,5,12) which used Hydrocortisone in 415 patients as the primary intervention for evaluation of mortality. The RR for each subtype of steroids Dexamethasone RR=0.9 (95% CI 0.83-0.98); Methylprednisolone RR=0.81 (95% CI 0.65-1.01), Hydrocortisone RR=0.85 (95% CI 0.62-1.17). There were no subgroup differences identified suggesting that these differences between subgroups were not significant and both Methylprednisolone and Hydrocortisone seemed to have an effect on mortality similar to Dexamethasone. When progression to invasive mechanical ventilation was evaluated between each type of steroid, Dexamethasone n 2 trials (9,11) in 5468 patients RR=0.78 (95% CI 0.64-0.95), Methylprednisolone 1 trial (10) in 188 patients RR=1.15 (95% CI 0.62-2.11), Hydrocortisone 1 trial (3) 32 patients RR=0.67(95% CI 0.38-1.17).
Indirect post hoc between trial analysis revealed that there were no subgroup differences between dexamethasone, methylprednisolone and hydrocortisone for all outcomes of all-cause mortality, progression to mechanical ventilation, serious adverse events, nosocomial infections and hyperglycemia. This suggested that possibly there was no difference in efficacy between the 3 types of steroids.
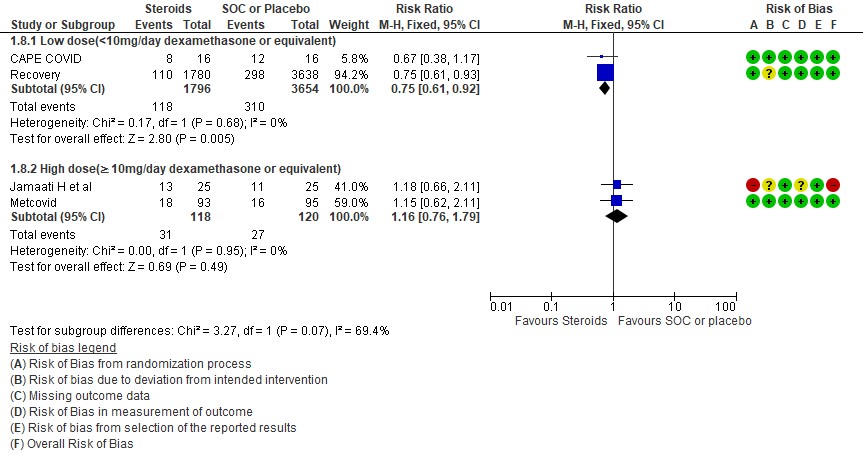
[4] Would a higher dose of steroids (≥ 10mg/day of Dexamethasone) be beneficial in severe/critical illness of COVID-19?
We found 5 studies (11,12,5,3,7) which evaluated outcomes in 6908 patients with a lower dose of steroids (<10mg/day of Dexamethasone equivalent) and 7 studies (3,6,8,9,10,13,14) in 981 patients which evaluated outcomes with a higher dose of steroids (≥ 10mg/day of Dexamethasone equivalent) for the outcome of mortality. Low dose steroids (< 10mg/day of Dexamethasone or dexamethasone equivalent) had a mortality benefit RR=0.88 (95% CI 0.80-0.96) vs high dose steroids ((≥ 10mg/day of Dexamethasone equivalent) RR=0.92 (95% CI 0.80-1.06). There were no subgroup differences suggesting there was no real difference between a higher dose or lower dose of steroids with regard to mortality.
For progression to invasive mechanical ventilation (IMV) 2 studies (3,11) in 5450 patients given low dose steroids were evaluated vs high dose steroids also given in 2 studies (9,10) in 238 patients. Low dose steroids had a RR=0.75 (95% CI 0.61 -0.92) suggesting a benefit and high dose steroids had a RR=1.16 (95% CI 0.76-1.79). Tests for subgroup differences was found to be significant with an I2 =69.4% suggesting that there was a possible difference between the two groups. Hence, we did a relative risk ratio for each of these subgroups and found that the difference in effect on progression to IMV between low dose and high dose steroid subgroups across trials is not significant statistically suggesting these doses seem to be similar in prevention of progression to invasive mechanical ventilation.
Indirect post hoc between trial analysis revealed that there are no subgroup differences between high dose and low dose steroids with regard to all-cause mortality, serious adverse events, nosocomial infections and hyperglycemia. We explored a potential difference in effect in progression to IMV but we found this to be insignificant.
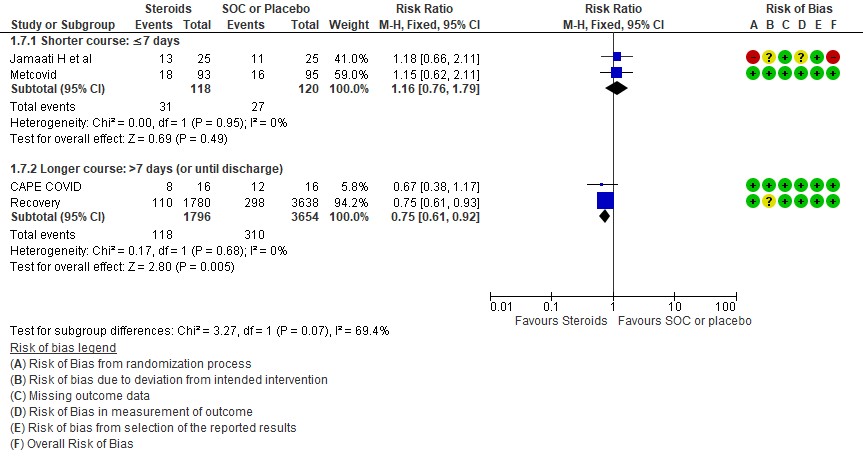
[5] Would a longer dose of steroids be beneficial in severe to critical COVID19 infection?
We found 8 trials(5,7,8,9,10,12,13,14) done in 998 patients which evaluated use of steroids for ≤ 7 days and 4 trials (3,4,6,11) done in 6891 patients which evaluated use of steroids for > 7 days for outcomes of mortality and progression to invasive mechanical ventilation. In the trials using a shorter duration of steroids RR=0.88(95% CI 0.74-1.05) but those using a longer duration were found to have RR=0.89 (95% CI 0.82-0.97). However there seemed to be no subgroup differences with an I2 = 0% suggesting that there was no difference in subgroups using a short duration of steroids vs a longer duration of steroids.
Progression to invasive mechanical ventilation was studied in 2 trials (9,10) with 238 patients which gave steroids for < 7 days vs 2 trials (3,11) in 5450 patients in which a longer duration (> 7 days) of steroids was given revealing a RR=1.16 (95% CI 0.76-1.79) and RR=0.75(95% CI 0.61-0.92) respectively. The I2 was 69.4% suggesting a possible difference between subgroups and hence a relative risk ratio was done between which seemed to suggest that there was no statistically significant difference between the subgroups of < 7days or > 7 days with regard to progression to invasive mechanical ventilation.
An indirect post hoc between trial analysis revealed that there are no subgroup differences between short course and long course steroids with regard to outcomes of all-cause mortality, serious adverse events, nosocomial infections, hyperglycemia. We explored a potential difference in effect in progression to IMV but we found this to be insignificant. In the long duration group, the mean duration of steroid use in Recovery trial was 7 days and in CAPE-COVID it was 10.5 days.
1 All cause mortality
2.Progression to invasive mechanical ventilation
3. Serious adverse events
4. Occurrence of nosocomial/opportunistic infection
5. Hyperglycemia or diabetes
SUBGROUP ANALYSIS
[A] Do steroids make a difference if used early in illness
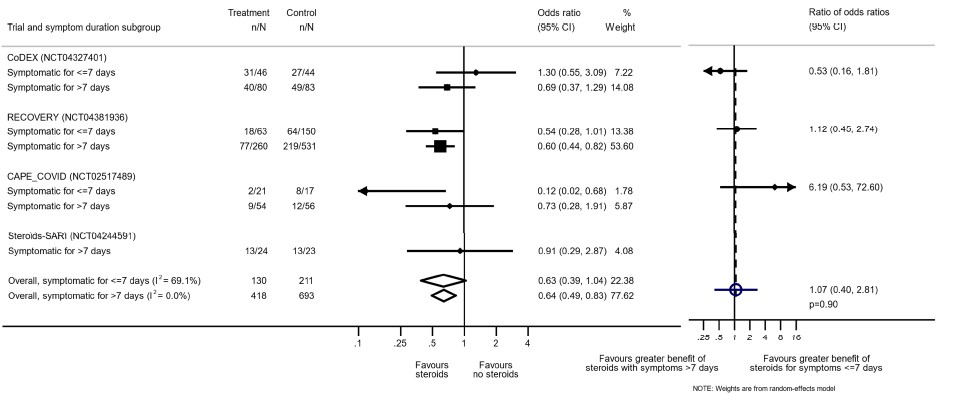
[Reference: The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group -supplementary material. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA. 2020;324(13):1330–1341. doi:10.1001/jama.2020.17023]
[B] Are steroids beneficial in mild-moderate illness with no hypoxia
B.1 : Data adapted from the RECOVERY Trial(11) as below
(a) Panel shows Mortality at 28 days with RR=0.95 (95% CI 0.85-1.07) in the group with no oxygen
(b) Panel shows a composite outcome of invasive mechanical ventilation or death in the group with no oxygen with RR= 1.02 (95% CI 0.96-1.05)
[C] Which type of steroid is preferred in severe to critical COVID 19 – a comparison of 3 formulations
C.1 Mortality
C.2: Progression to invasive mechanical ventilation
[D] What dose (<10 mg/day OR >10 mg/day) of steroids would be appropriate for treatment of severe to critical COVID 19?
D.1 Mortality
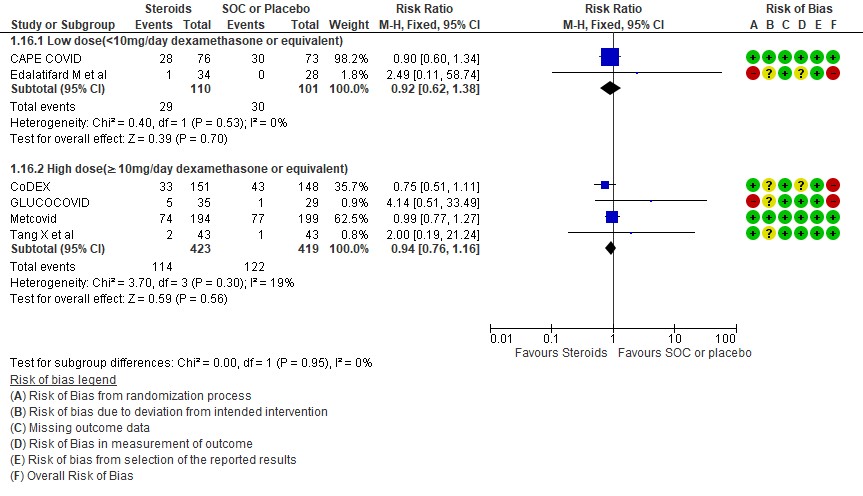
D2. Progression to invasive mechanical ventilation
[E] What duration (<10 days OR > 10 days) of steroids is advised for people with severe to critical COVID 19?
E.1 Mortality
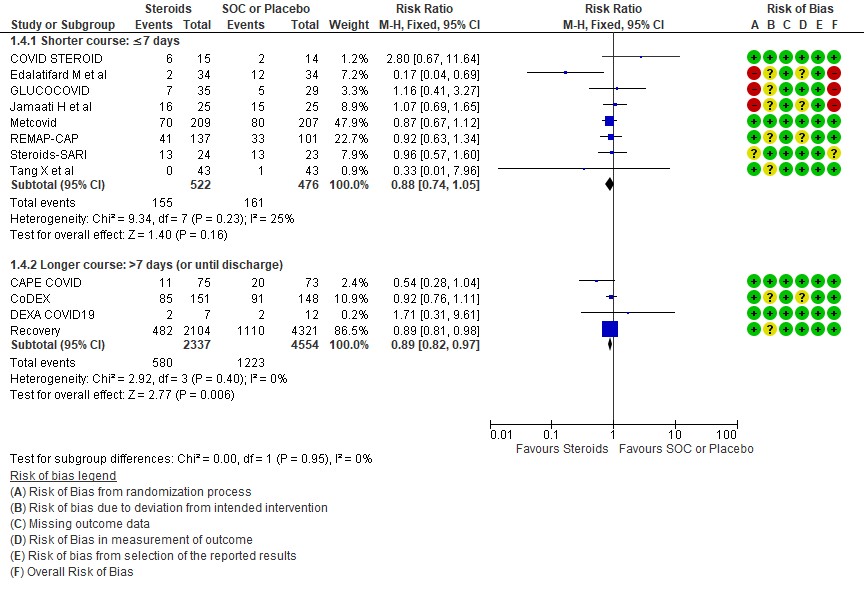
E.2 Progression to invasive mechanical ventilation
Problem
COVID 19 infection produces significant morbidity and mortality and this is well known. Hence low cost accessible therapeutic interventions for clinical management are a priority. Today with the oxygen needs climbing and paucity of intensive care beds everywhere in the country, we need resources and drugs which will prevent progression of disease and in turn reduce the duration of severe and critical disease. Steroids have been shown to be beneficial in multiple trials, however gaps in evidence exist with regard to dose, duration, drug choice and optimal timing of steroids in the hypoxic patients. There are also concerns about inappropriate use in the non-hypoxic patients, and questionable benefit with higher doses and prolonged courses (>10 days) in the hypoxic patients. The burgeoning epidemic of mucormycosis has been variably attributed to the immunosuppressive effect of steroids and the ensuing hyperglycemia even if given for a shorter duration as steroids have a long half-life.
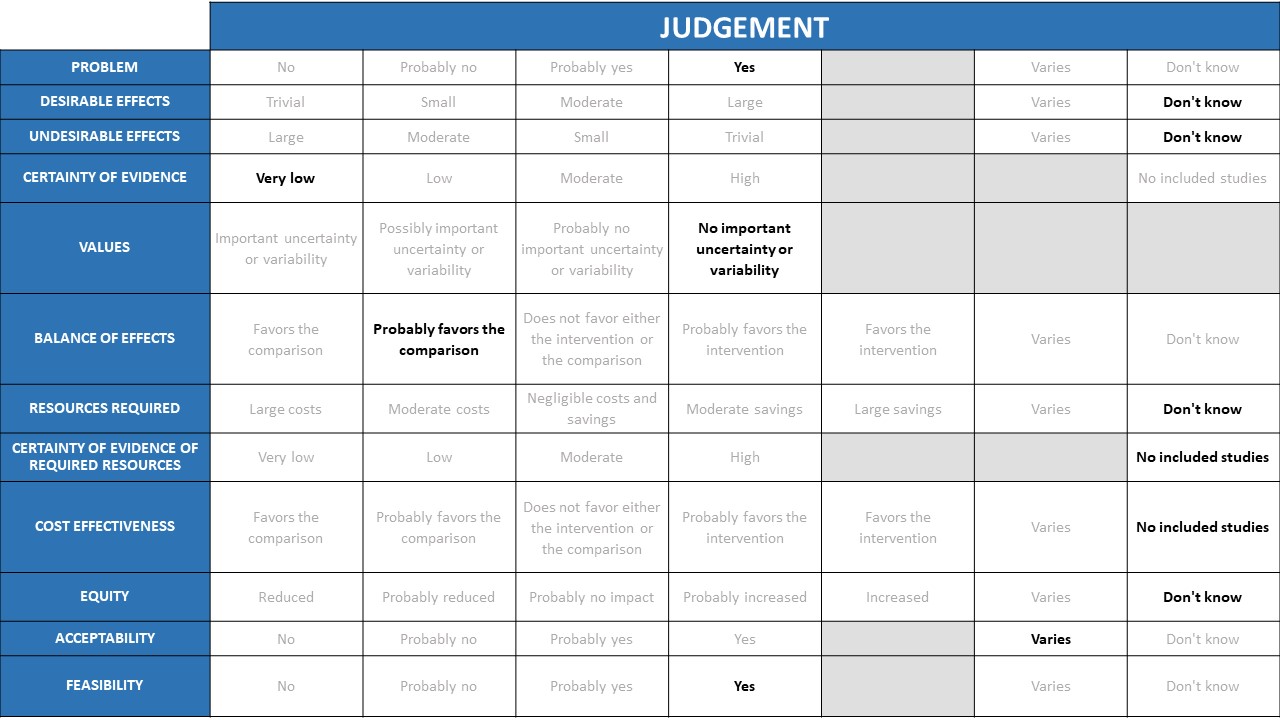
Desirable effects
The group felt that in the moderate, severe & critical illness COVID19 categories there were moderate desirable effects in terms of reduction in mortality and progression to invasive ventilation in the group given steroids. The group debated at length whether this would be small vs. moderate desirable effects considering the results are likely to vary according to drug choice, even though this is not reflected in the available evidence. The group also noted that since most of the evidence comes from the Recovery trial, in which most received dexamethasone it would be heavily weighted towards giving Dexamethasone rather than Hydrocortisone or methylprednisolone. This choice was also felt to reflect biological plausibility as the latter two drugs have more mineralocorticoid effect than dexamethasone and the anti-inflammatory effect of steroids is mostly from the glucocorticoid component.
For non-hypoxic patients, the evidence for desirable effects is limited. Subgroup analysis did not show benefit in this group in the recovery trial, and there was concern about potential increase in mortality and need for invasive mechanical ventilation (not statistically significant). Hence at present no desirable effects have been noted in this category. In exceptional circumstances if a decision was made to give the drug, that decision should left to the treating physician based on inflammatory markers and patients clinical condition and attendant comorbidities.
Undesirable effects
In the hypoxic group pf patients in the moderate, severe and critical categories there seems to be no increase in secondary infections or adverse events. The group felt that this could vary; appropriate and responsible use with strict sugar control would lead to perhaps a small risk of undesirable effects but could be moderate if inappropriate use with higher doses or longer duration. Main concerns were that steroids in unnecessary high doses and duration with hyperglycemia could contribute to an increased risk of mucormycosis. Secondary bacterial infections though a theoretical risk were not seen in the studies evaluated, however there are anecdotal reports of increased incidence of nosocomial and community acquired secondary bacterial, viral and fungal infections after steroids were given for management of COVID19 infection. Therefore, the risk of undesirable effects will be higher in certain groups, such as those with diabetes mellitus.
Very few patients without hypoxia were included and hence a reasonable conclusion cannot be drawn with regard to undesirable effects in this group.
Certainty of evidence
There was consensus regarding moderate certainty of evidence for steroids preventing mortality and progression to invasive mechanical ventilation in moderate, severe and critical categories of illness. There was low/very low certainty of evidence for serious adverse events. The non-hypoxic patients were assessed as a subgroup in one trial. A separate summary of findings table was not made for this population, but likely to be very low certainty in the evidence overall.
Values
Outcomes considered for steroid recommendations were mortality, progression to invasive mechanical ventilation, serious adverse events, hyperglycemia and secondary infections, all of which were considered clinically relevant and important. The group felt there was no important uncertainty regarding the value applied to these outcomes by key stakeholders
Balance of effects
The group considered and acknowledged the overwhelming evidence of benefit of steroids among hypoxic patients producing a reduction in mortality & progression to invasive mechanical ventilation. When serious adverse events were evaluated there was no increase noted and there was very low certainty of evidence regarding hyperglycemia as well. Hence in this category of patients, the group favoured the intervention.
In the non-hypoxic group of patients, there was very low certainty of evidence but overall the group felt that the potential harms that are well-established for steroids are likely to outweigh benefits in this category of patients. The panel discussed that there are no trials focused on mucormycosis and the balance here favoured the intervention in hypoxic patients but did not favour in the non hypoxic patients.
Resources required
Clinicians in the group felt that considering the reduction in mortality and progression to mechanical ventilation this cheap intervention was lifesaving and would lead to large overall cost savings.
In non-hypoxic patients, the harms or benefits were unclear as it is not normally indicated in this category and hence it was not possible to comment on savings or costs. However, the potential for abuse was high and it is likely that there may be costs due to treatment required for secondary bacterial or opportunistic fungal infections.
Certainty of evidence of required resources
Experienced clinicians in the group felt that there was high certainty of evidence of required resources in implementing steroid use in hypoxic patients with COVID-19 as this was a drug widely used and available and clinicians were well aware of its benefits and harms.
In the patients with COVID-19 but no hypoxia, though the intervention itself was of low cost but there was an unclear indication for its use in this category of patients and potential harm outweighing the benefits here.
Cost effectiveness
Among the hypoxic patients, the panel feel that even though no cost effectiveness analyses were performed in the studies included, we can safely conclude that the intervention is cost effective considering its low cost and high savings from efficacy on mortality and progression to ventilation.
Among the non-hypoxic patients, it is unclear if there would be cost savings from efficacy as there is little evidence for efficacy in this category and possible harm when used inappropriately at high dose and longer duration in the absence of a clear indication.
Equity
This is an intervention that is cheap, widely available, equitable and accessible to all. However caution should be exercised towards its use due to the attendant side effects.
Acceptability
The group felt that the intervention was acceptable in hypoxic patients with COVID19 due to the overwhelming evidence that it saves lives, if it is given at the appropriate dose and duration with close monitoring for adverse effects.
In patients with mild disease with no hypoxia, in the absence of high-certainty evidence the group felt that the use should be strongly discouraged in this category. However, the group did recognize there are some situations where in the presence of intense systemic inflammation even without hypoxia clinician judgment may be exercised for use.
Feasibility
This is an oral and cheap intervention which could be easily implemented in hypoxic patients in any setting provided the indication was right and guidelines given for appropriate and responsible use. This should not be encouraged in patients without hypoxia.
Use of systemic corticosteroids in hypoxic patients was backed with significant data support from various trials, however risk of worsening hyperglycemia and risk of secondary infections needs equal consideration due to the attendant morbidity and sometimes mortality. In hypoxic patients, steroids even with or without evidence of systemic inflammation saves not just lives but resources. Though by itself this intervention is cheap, the ensuing side effects leading on to secondary bacterial nosocomial or opportunistic fungal infections are likely to be expensive and resource intensive taking up hospital beds and personnel that could be better used elsewhere. Hence steroid use in all hypoxic patients though warranted must be tempered with optimal and strict sugar control and stringent infection control precautions in definitely all but most especially in the immunocompromised individuals. India has an extremely high prevalence of diabetes and chronic invasive mucormycosis and hence steroid use or abuse when not indicated is likely to be detrimental.
There is no supportive evidence for use of steroids in non-hypoxic patients, however in the presence of overwhelming systemic inflammation with persistent fever and high inflammatory markers and change in high resolution computed tomography score judicious consideration with the input from a senior physician may be appropriate.
Extensive interrogation of potential differences in effect between subgroups was performed using an indirect between-trial approach, for mortality, progression to invasive mechanical ventilation, serious adverse events, infections and hyperglycemia. A detailed analyses of subgroup differences revealed that Methylprednisolone and Hydrocortisone had the same efficacy as Dexamethasone when equivalent doses were given. When equivalent doses of steroids were given, 10mg/day of equivalent doses of Dexamethasone no subgroup difference was found suggesting no superiority of higher vs lower dose. Longer vs shorter courses of steroids were also evaluated and again no subgroup difference was noted suggesting that longer duration had no benefit over shorter duration. Optimal timing of initiation of steroids 7 days after symptom onset was evaluated and no obvious differences were identified. Most of this evidence came from large trials like Recovery, REMAP-CAP etc which recruited large number of patients suggesting that in the arms where these trials were represented likely skewed the data to that side during a subgroup analysis. Principles of good clinical practice suggest that a steroid which has the maximal efficacy (glucorticoid rather than mineralocorticoid activity) with the minimal side effects, given in as low a dose as possible, for as short duration as possible and initiated when it is likely to be of maximal benefit (i.e., period of hyperinflammation) would be appropriate. A subgroup that is under-represented in trial evidence is patients who have raised inflammatory markers or persistent fever but not requiring oxygen and hence we were unable to draw conclusions or provide a suitable recommendation in these patients and at this point, may be left to clinician discretion to use steroids in this scenario in a very narrow subgroup of patients where the potential benefit likely outweighs the risk.
Pregnancy
The concerns over medications in pregnancy are the changes in physiology in the three trimesters and the degree of placental transfer of the active component of the drug and its effect on the foetus. Inhaled and systemic steroids in respiratory disease and pregnancy have mainly been studied in the context of Bronchial asthma. Use of oral Prednisolone and parenteral Hydrocortisone, both are considered safe in all trimesters as they undergo degradation by theplacental 11 β-hydroxysteroid dehydrogenase type 2 (11β-HSD2). Dexamethasone however is not metabolized by 11β-HSD2 and readily crosses the placenta and is useful for use in preterm labour to facilitate lung maturity. This raises the concern for use of Dexamethasone in hypoxic pregnant patients with Covid 19 however there is paucity of data on the use of steroids in pregnant patients
Hence Dexamethasone is recommended in the second, third trimesters of pregnancy and in post-partum patients with severe or critical Covid 19 pneumonia as rapid progression to severe disease is often seen in these patients.Oral prednisolone can be used in hypoxic pregnant patients in first trimester and second trimester for moderate Covid 19 pneumonia and in the first trimester only in severe Covid 19 pneumonia. Severe disease is often not encountered in the first trimester of pregnancy.
| Disease status | 1ST TRIMESTER | 2ND TRIMESTER | 3RD TRIMESTER | POST PARTUM |
| Moderate | Prednisolone 40mg | Prednisolone 40 mg | Dexamethasone 8mg | Dexamethasone 8mg |
| Severe | Prednisolone 40mg | Dexamethasone 8mg | Dexamethasone 8mg | Dexamethasone 8mg |
| Critical | Dexamethasone 8mg | Dexamethasone 8mg | Dexamethasone 8mg | Dexamethasone 8mg |
In all subjects, steroid doses, duration and preparation must follow as in the recommendation. Pulse steroids or higher dose or duration beyond the recommended doses are unlikely to be beneficial and perhaps even harmful even in hypoxic subjects. Higher doses and longer duration are not beneficial and can lead to toxicities like uncontrolled diabetes mellitus and superadded secondary infections like mucormycosis.
In non-hypoxic subjects, treating physician’s judgment should be strictly guided by the evidence and irresponsible use of steroid discouraged. Clinical features like fever, persistent cough, raised CRP and ground glass infiltrates on High Resolution Computed Tomography are non-specific and can be seen with other differential diagnoses like Influenza, Dengue fever etc. Steroids must be discouraged in 1st week of illness as cytokine storms / hyperinflammation is unlikely then.
Above all, close monitoring and strict control of blood sugar in all subjects, whether diabetics and nondiabetics is warranted, as many prediabetics may be pushed to diabetes even with this short course of steroids) on a daily basis. In those with leucopenia, apart from the diabetics; extra watch for superadded or opportunistic infections is a must.
Though corticosteroids have been unequivocally been shown to reduce mortality and progression to respiratory failure, there exist uncertainties with regard to the best steroid to be used, optimal dose, timing and duration of corticosteroid therapy. Steroids have been used for decades and the side effects whether in the short term or long term are well known. They are well known to produce increased susceptibility to infection, believed to be the most likely reason for the epidemic of covid associated mucormycosis. Though it is fairly certain that initiation of steroids in hypoxic COVID19 individuals is lifesaving, utility of steroids in non-hypoxic individuals with evidence of systemic inflammation is still unknown. In addition, the use of biomarkers like CRP, Ferritin in initiation, prognostication or discontinuation of steroids is still unknown.
- Kumar M and Al Khodor S. Pathophysiology and treatment strategies for COVID-19. J Transl Med (2020) 18:353 https://doi.org/10.1186/s12967-020-02520-8.
- The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. 2020;324(13):1330–1341. doi:10.1001/jama.2020.17023
- Dequin PF, Heming N, Meziani F at al. CAPE COVID Trial Group and the CRICS-TriGGERSep Network. Effect of Hydrocortisone on 21-Day Mortality or Respiratory Support Among Critically Ill Patients With COVID-19: A Randomized Clinical Trial. JAMA. 2020 Oct 6;324(13):1298-1306. doi: 10.1001/jama.2020.16761. PMID: 32876689; PMCID: PMC7489432.
- Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients with Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. 2020;324(13):1307–1316. doi:10.1001/jama.2020.17021
- Petersen MW, Meyhoff TS, Helleberg M et al. Low-dose hydrocortisone in patients with COVID-19 and severe hypoxia (COVID STEROID) trial-Protocol and statistical analysis plan. Acta Anaesthesiol Scand. 2020 Oct;64(9):1365-1375. doi: 10.1111/aas.13673. Epub 2020 Jul 30. PMID: 32779728; PMCID: PMC7404666 results given to WHO and were available in supplemental content. JAMA. doi:10.1001/jama.2020.17023.
- Villar J, Añón JM, Ferrando C, Aguilar G, Muñoz T, Ferreres J, Ambrós A, Aldecoa C, Suárez-Sipmann F, Thorpe KE, Jüni P, Slutsky AS; DEXA-COVID19 Network. Efficacy of dexamethasone treatment for patients with the acute respiratory distress syndrome caused by COVID-19: study protocol for a randomized controlled superiority trial. Trials. 2020 Aug 16;21(1):717. doi: 10.1186/s13063-020-04643-1. Accessed URL https://clinicaltrials.gov/ct2/show/NCT04325061 on 5 July 2021
- Edalatifard M, Akhtari M, Salehi M, Naderi Z, Jamshidi A, Mostafaei S, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial [Preprint]. Eur Respir J 2020; published online 17 September 2020. Available from: https://doi.org/10.1183/13993003.02808-2020
- GLUCOCOVID investigators, Corral-Gudino L, Bahamonde A, Arnaiz-RevillasF, Gómez-Barquero J, Abadía-Otero J, et al. Methylprednisolone in adults hospitalizedwith COVID-19 pneumonia: An open-label randomized trial (GLUCOCOVID). WienKlin Wochenschr [Internet]. 2021 Feb 3 [cited 2021 Feb 11]; Available from: http://link.springer.com/10.1007/s00508-020-01805-8
- Jamaati H, Hashemian SM, Farzanegan B, Malekmohammad M, Tabarsi P,Marjani M, et al. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: A preliminary report of a randomized clinical trial. European Journal of Pharmacology. 2021 Apr;897: 173947.
- Jeronimo CMP, Farias MEL, Almeida Val FF, Sampaio VS, Alexandre MAA, Melo GC, et al. Methylprednisolone as adjunctive therapy for patients hospitalized withCOVID-19 (METCOVID): a randomised, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis 2020: ciaa1177. Available from: https://doi.org/10.1093/cid/ciaa1177.253
- The Recovery Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 2021; 384:693-704 DOI: 10.1056/NEJMoa2021436.
- The Writing Committee for the REMAP-CAP Investigators, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA 2020; 324(13):1317-29. https://doi.org/10.1001/jama.2020.17022
- Li Weng, Jian‐feng Xie, Zhi‐yong Peng, Ai‐hua Qin et al. Corticosteroids therapy in adult patients with COVID‐19 and ARDS (Steroids‐SARI) trial. The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis -supplemental content. JAMA. doi:10.1001/jama.2020.17023
- Tang X, Feng Y-M, Ni J-X, Zhang J-Y, Liu L-M, Hu K, et al. Early Use of Corticosteroid May Prolong SARS-CoV-2 Shedding in Non-Intensive Care Unit Patients with COVID-19 Pneumonia: A Multicenter, Single-Blind, Randomized Control Trial. Respiration. 2021 Jan 22;1–11.
- Salinas M, Andino P, Palma L, Valencia J, Figueroa E, Ortega J. Early use of corticosteroids in non-critical patients with COVID-19 pneumonia (PREDCOVID): a structured summary of a study protocol for a randomised controlled trial. Trials. 2021 Jan 26;22(1):92. doi: 10.1186/s13063-021-05046-6. PMID: 33499887; PMCID: PMC7835442.
- MohammedReza Salehi et al. Study of Dexamethasone effects on the improvement of clinical symptoms and laboratory signs of Iranian COVID-19 patients: a clinical trial study. IRCT20201015049030N1
- TAC –COVID 19 EU Clinical register: 2020-001622-64. Spain. Outpatient treatment of COVID-19 with early pulmonary corticosteroids as an opportunity to modify the course of the disease. https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001622-64/ES#C
- Farahani RH, Mosaed R, Nezami-Asl A, Chamanara N, Soleiman-Meigooni S, Kalantar S, et al. Evaluation of the efficacy of methylprednisolone pulse therapy in treatment of Covid-19 adult patients with severe respiratory failure: randomized, clinical trial [Preprint]. ResearchSquare 2020. Available from: https://doi.org/10.21203/rs.3.rs-66909/v1.
- Ranjbar K, Shahriarirad R, Erfani A, Khodamoradi Z, Saadi MHG, Mirahmadizadeh A, et al. Methylprednisolone or Dexamethasone, Which One Is the Superior Corticosteroid in the Treatment of Hospitalized COVID-19 Patients: A Triple-Blinded Randomized Controlled Trial [Internet]. In Review; 2021 Feb [cited 2021Feb 14]. Available from: https://www.researchsquare.com/article/rs-148529/v
Covid Management Guidelines India Group - Anti-inflammatory Working Group. Systemic Corticosteroids. Covid Guidelines India; Published online on July 15, 2021; URL: https://indiacovidguidelines.org/systemic-corticosteroids/ (accessed ).
