CAPE COVID, CoDEX, COVD STEROID, DEXA COVID 19, Edalatifard M et al, GLUCOCOVID, Jamaati H et al, Metcovid, Recovery, REMAP-CAP, Steroids-SARI, Tang X et al.
- Downgraded by one level for serious imprecision; the clinical effect of reduction in all cause mortality is not appreciable(absolute effect 1/100-5/100)
- CAPE COVID, Jamaati H et al, Metcovid, Recovery
- Downgraded by one level for serious imprecision; the clinical effect on reduction to progression to IMV is not appreciable ( Absolute effect 0.4/100-3/100)
As of 31st May 2021, around 28,047,534 patients have been diagnosed with covid-19 in India according to the ministry of health and family welfare records. The pandemic has claimed around 3, 29,100 lives and there is a continuing surge in the covid-19 cases across India. SARS-CoV-2 binds to host cells through the ACE2 receptor, and after endocytosis and subsequent uncoating, the components of SARS-CoV-2 use host cells machinery to produce new viruses. Finally, the SARS-CoV-2 virions are released from the host cell by exocytosis. SARS-CoV-2 stimulates the host immune system to release the cytokines and subsequent inflammation and immune-dysfunction through activation or impairment of various immune cells, such, dendritic cells, NK cells, macrophages, and neutrophils. This process can lead to sepsis, septic shock, multiple organ failure, and death (1).
There have been many therapeutic interventions proposed for COVID 19, however steroids are the only drugs with a proven mortality benefit and also known to prevent progression to invasive mechanical ventilation. The use of steroids is based on the fact that the anti-inflammatory effects of steroids can help reduce systemic inflammatory response thus preventing multi-organ dysfunction. Hence steroids are widely being used all over the world for management of COVID19 patients in the moderate, severe and critical categories requiring oxygen. However,some areas of equipoise remain and we sought to answer these questions with our evidence synthesis.
• Does use of steroids lead to an increased incidence of secondary infections fuelling the epidemic of mucormycosis?
• Which is the most appropriate type, dose and duration of steroids?
• Can steroids be used for those with inflammation but no hypoxia which may be early in the disease and thus prevent progression?
We searched Epistemonikos and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language. We also reviewed reference lists of PAHO Ongoing Living of Covid 19 Therapeutics, the systematic review by WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group and included studies. We performed all searches up to 21 May 2021. We contacted researchers to identify unpublished and ongoing studies.
We included randomized controlled trials (RCTs) testing systemic steroids treatment of any formulation, dose or duration in people with acute COVID‐19, whether suspected or confirmed. Trials were included if the intervention arm did not combine systemic steroids with another experimental drug, and if the comparator arm included placebo or standard care. We excluded trials that did not report any outcomes that could provide usable data for the review and those lacking a comparator arm.
We planned to extract data for the following pre-defined outcomes:
- Primary outcome
- All-cause mortality
- Secondary outcomes
- Time to clinical recovery
- Need for non-invasive or mechanical ventilation
- Duration of ventilation
- Length of stay in hospital
- Need for other organ support
- Adverse events
-
- All adverse events
- Serious adverse events
- Nosocomial/opportunistic infections
- Hyperglycaemia or diabetes mellitus
- Hypertension
The working group felt that since the mortality benefit of steroids was irrevocably established and it was now standard of care we needed to do a focussed subgroup analysis to answer other questions that were pertinent to the use of steroids. Equipoise existed around the following
- Was there a benefit if used early in illness?
- Was there a benefit to using a higher dose of steroids in the critical ill patients?
- Was there a benefit to using a specific type of corticosteroid -e.g., methylprednisolone, dexamethasone, hydrocortisone or prednisolone?
- Was there a benefit in using a longer duration of steroids rather than the 10 days recommended?
FOCUSSED SUBGROUPS
- Type of corticosteroid:
- Dexamethasone
- Methylprednisolone
- Hydrocortisone
- Dose per day:
- High dose (≥10mg/day dexamethasone or equivalent)
- Low dose (<10mg/day dexamethasone or equivalent)
- Duration:
- Longer course: >7 days (or until discharge)
- Shorter course: ≤7 days
- Time since onset of symptoms:
- Early <7 days
- Late ≥7 days
- Severity of disease
- Non-severe (Mild/Moderate severity with no hypoxia)
- Severe/Critical
Two reviewers independently assessed eligibility of search results. One reviewer extracted data from each included study which was checked by the second reviewer. Risk of bias assessment was taken from PAHO Ongoing Living of Covid 19 Therapeutics and WHO REACT systematic review, where Cochrane Risk of bias (RoB) v2.0 tool was used.
We used RevMan 5.4 to perform meta‐analysis using a fixed‐effects model or a random-effects model for outcomes where pooling of effect estimates was appropriate. We used risk ratios (RR) for dichotomous outcomes with 95% confidence intervals (CIs).
We planned to perform subgroup analysis in the predefined focused subgroups as mentioned earlier. We used GRADE methodology to assess the certainty in the evidence, and documented this in a ‘Summary of findings’ table using GradeProGDT (hyperlink)
Results
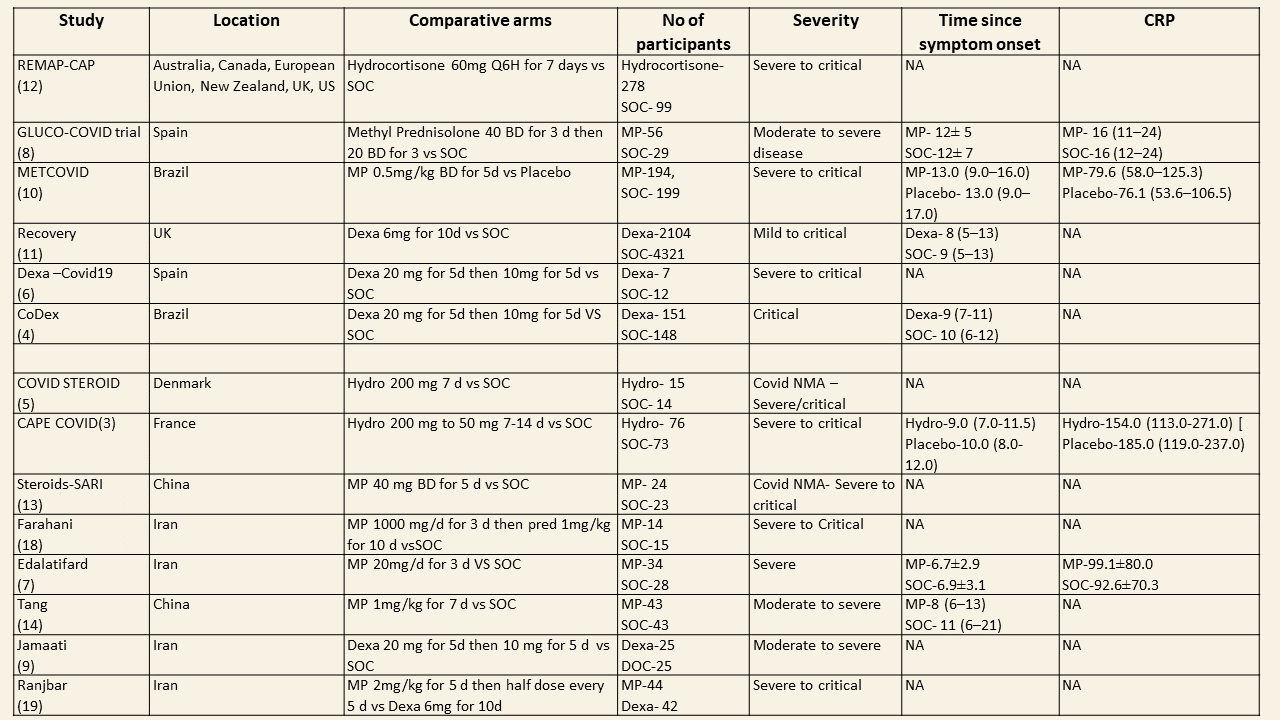
We found 13 trials which compared steroids against standard of care or placebo. Varying steroid types, doses and duration were used in different categories of severity of COVID19 and these studies were done in different parts of the world. One study compared two different types and doses of steroids in severe and critical disease. The median CRP was at least 2-3 times the upper limit of normal and steroids were instituted at least 5 days after onset of symptoms. The doses for steroids varied widely with Methylprednisolone doses ranging from 20mg to 1000mg, Dexamethasone doses from 6mg to 20mg, Hydrocortisone from 60mg to 240mg/day. In addition, the duration of steroids varied from 3 days to 14 days.
Equivalent doses of steroids
| Dexamethasone | Methylprednisolone | Prednisolone | Hydrocortisone |
| 1.5mg | 8mg | 10mg | 40mg |
We first looked at the overall effects of steroids on various outcomes.
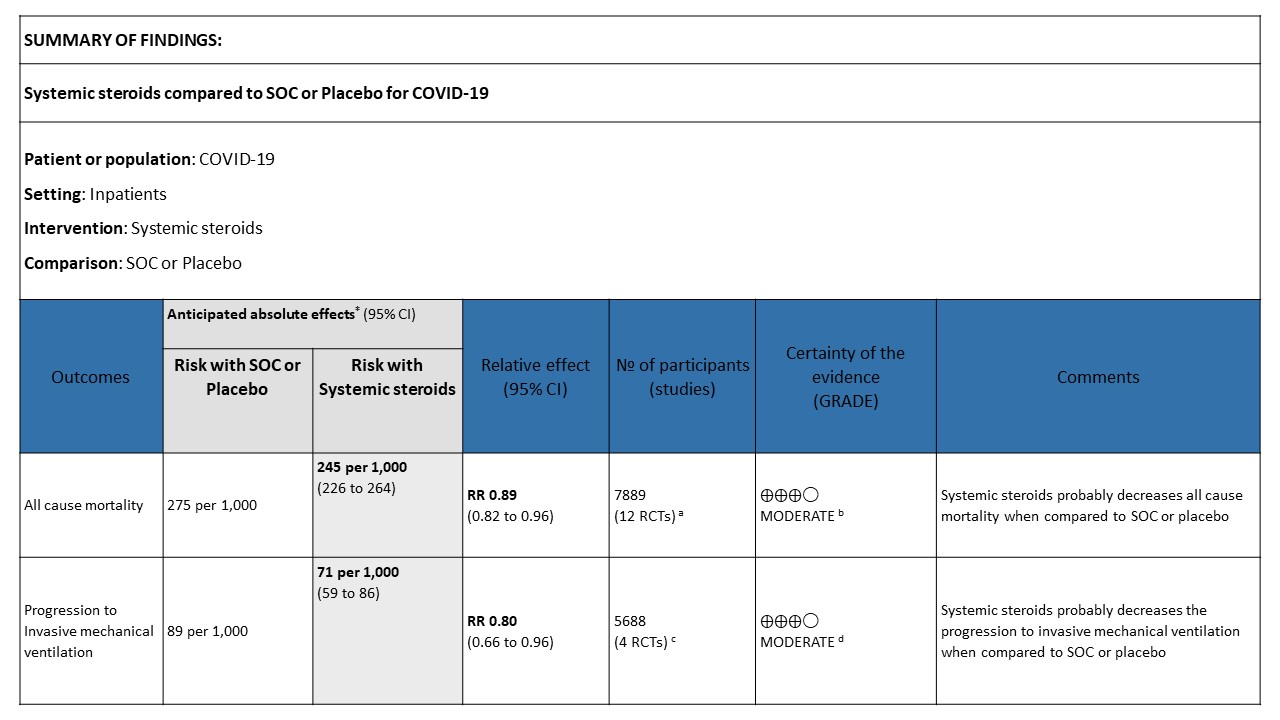
[A] All-cause mortality: Moderate certainty evidence in 12 RCTs (3-14) done in 7889 patients revealed that systemic steroids probably reduced all-cause mortality when compared to standard of care (SOC) or placebo RR=0.89 (95%CI 0.82-0.96). This suggested a mortality benefit of up to 11% (95% CI 4%-18%) with an absolute risk reduction of 0.018 and a number needed to treat of 55.
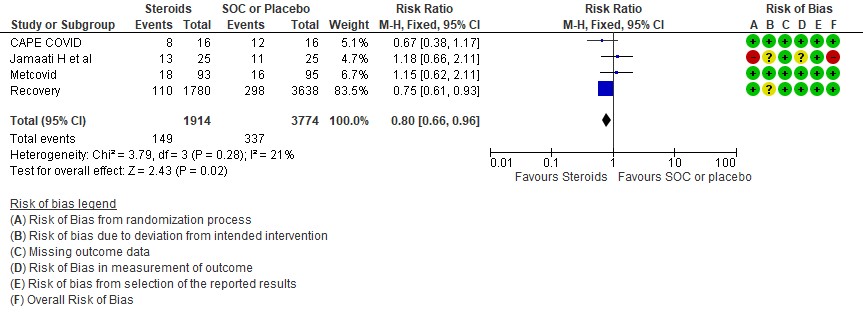
[B] Progression to invasive mechanical ventilation: Moderate certainty of evidence in 4 RCTs (3,9-11) in 5688 patients revealed that systemic steroids probably reduced progression to invasive mechanical ventilation vs SOC by 20% RR=0.80 (95% CI 0.66-0.96).
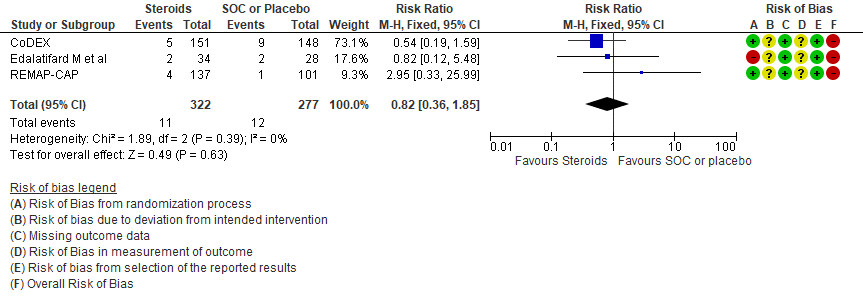
[C] Serious adverse events: Very low certainty of evidence suggesting an uncertain effect in 3 RCTs(4,7,12) in 599 patients revealed no increase in adverse events in the steroid group as compared to SOC/placebo RR=0.82 (95% CI 0.36-1.85)
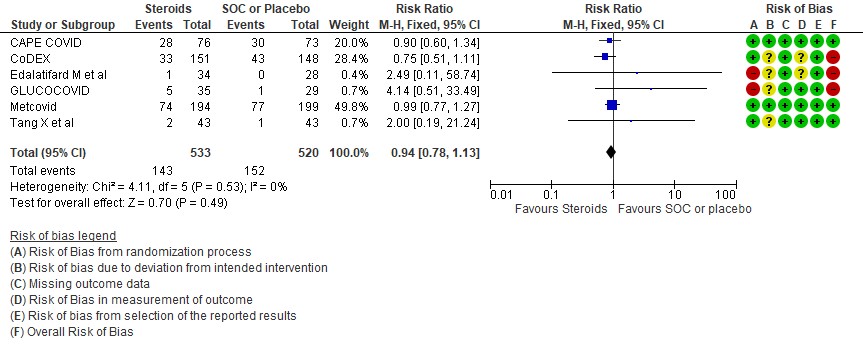
[D] Nosocomial/Opportunistic infections: Low certainty evidence in 6 RCTs (3,4,7,8,10,14)in 1053 patients revealed that steroids made little to no difference to an increase in nosocomial or opportunistic infections as compared to SOC or placebo RR=0.94 (95% CI 0.78-1.13).
[E] Hyperglycemia: Very low certainty evidence in 3 RCTs (4,8,14)in 449 patients revealed a very uncertain effect of steroids on hyperglycemia or diabetes as compared to SOC/placebo RR=1.52 (95% CI 0.15 – 15.48).
Subgroup analysis
The group also wanted to explore the optimal usage of steroids in different clinical settings.
[1] Utility of steroids in early disease <7 days of symptoms
Disaggregated data was not available in most of the trials assessed by us. However, this was available in the WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group (2). This was a within trial post hoc comparisons of 2 populations. There appeared to be no difference in terms of all-cause mortality when symptom of patients was less than 7 days and greater than 7 days.
On review of the population in the studies, majority had oxygen or ventilation requirement in first 7 days. However, we noted inconsistency and imprecision across studies and we were unable to determine the balance of baseline characteristics within subgroups. In addition, the studies were not randomized into severity strata and hence it was probably difficult to adjust for the confounders.
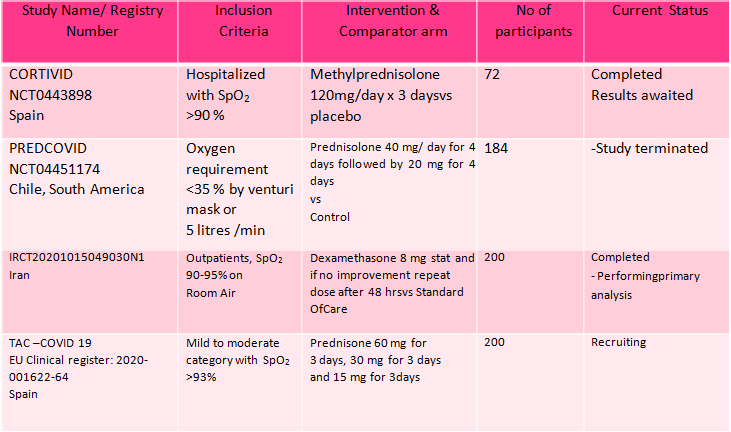
[2] Utility of steroids in mild/moderate disease with no hypoxia
We found 4 studies (2,15,16,17) attempting to answer this question, however 1 was terminated, 2 have been completed and we are awaiting publication and 1 trial was still recruiting. In the mild to moderate illness with no hypoxia, the only data available is from the Recovery trial (11) with patients not on O2 where it demonstrated no clear benefit or harm with 28-day mortality RR 1.19 (95% CI 0.92-1.55) or the composite of progression to ventilation or death RR 1.20(95% CI 0.96-1.50).
[3] Utility of a different type of steroid like methylprednisolone or hydrocortisone in severe or critical illness as compared to Dexamethasone
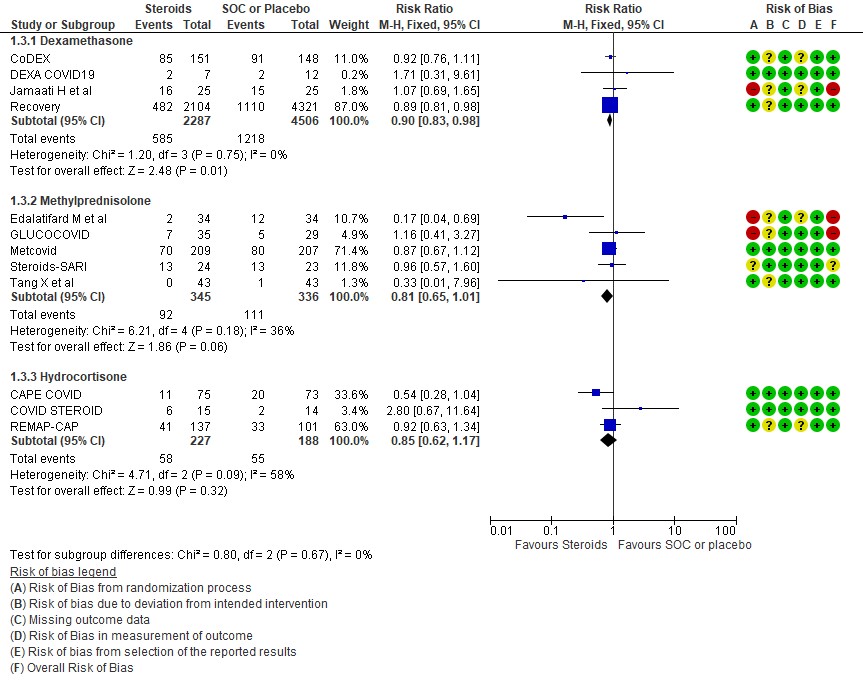
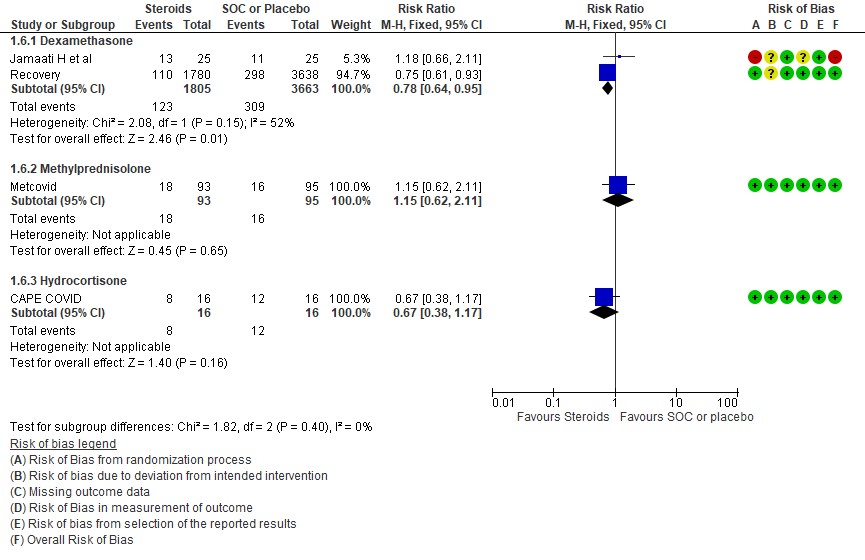
We found 4 studies (4,6,9,11) which used Dexamethasone in 6793 patients, 5 studies (7,8,10,13,14) which used Methylprednisolone in 681 patients and 3 studies (3,5,12) which used Hydrocortisone in 415 patients as the primary intervention for evaluation of mortality. The RR for each subtype of steroids Dexamethasone RR=0.9 (95% CI 0.83-0.98); Methylprednisolone RR=0.81 (95% CI 0.65-1.01), Hydrocortisone RR=0.85 (95% CI 0.62-1.17). There were no subgroup differences identified suggesting that these differences between subgroups were not significant and both Methylprednisolone and Hydrocortisone seemed to have an effect on mortality similar to Dexamethasone. When progression to invasive mechanical ventilation was evaluated between each type of steroid, Dexamethasone n 2 trials (9,11) in 5468 patients RR=0.78 (95% CI 0.64-0.95), Methylprednisolone 1 trial (10) in 188 patients RR=1.15 (95% CI 0.62-2.11), Hydrocortisone 1 trial (3) 32 patients RR=0.67(95% CI 0.38-1.17).
Indirect post hoc between trial analysis revealed that there were no subgroup differences between dexamethasone, methylprednisolone and hydrocortisone for all outcomes of all-cause mortality, progression to mechanical ventilation, serious adverse events, nosocomial infections and hyperglycemia. This suggested that possibly there was no difference in efficacy between the 3 types of steroids.
[4] Would a higher dose of steroids (≥ 10mg/day of Dexamethasone) be beneficial in severe/critical illness of COVID-19?
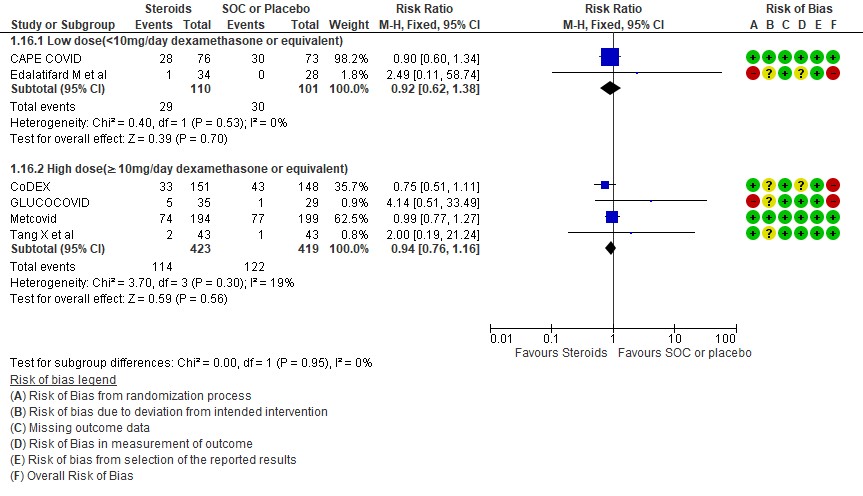
We found 5 studies (11,12,5,3,7) which evaluated outcomes in 6908 patients with a lower dose of steroids (<10mg/day of Dexamethasone equivalent) and 7 studies (3,6,8,9,10,13,14) in 981 patients which evaluated outcomes with a higher dose of steroids (≥ 10mg/day of Dexamethasone equivalent) for the outcome of mortality. Low dose steroids (< 10mg/day of Dexamethasone or dexamethasone equivalent) had a mortality benefit RR=0.88 (95% CI 0.80-0.96) vs high dose steroids ((≥ 10mg/day of Dexamethasone equivalent) RR=0.92 (95% CI 0.80-1.06). There were no subgroup differences suggesting there was no real difference between a higher dose or lower dose of steroids with regard to mortality.
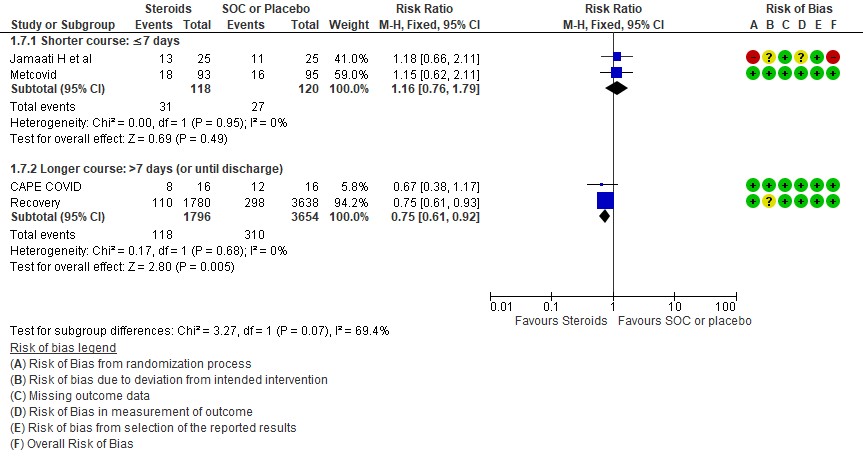
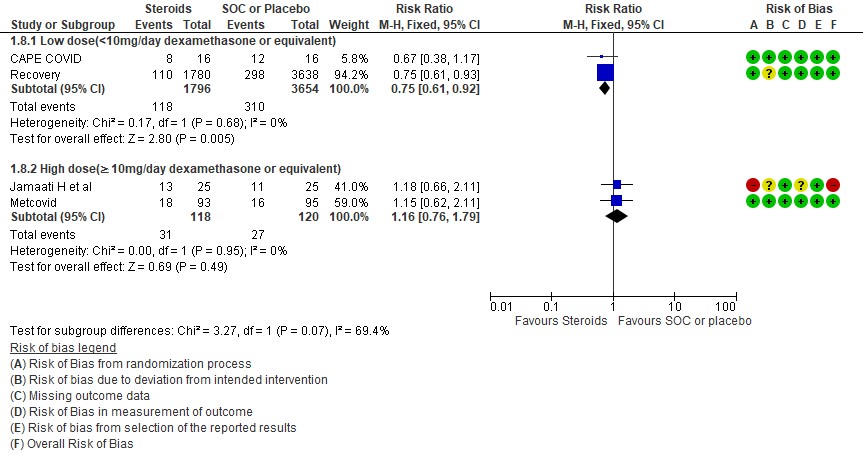
For progression to invasive mechanical ventilation (IMV) 2 studies (3,11) in 5450 patients given low dose steroids were evaluated vs high dose steroids also given in 2 studies (9,10) in 238 patients. Low dose steroids had a RR=0.75 (95% CI 0.61 -0.92) suggesting a benefit and high dose steroids had a RR=1.16 (95% CI 0.76-1.79). Tests for subgroup differences was found to be significant with an I2 =69.4% suggesting that there was a possible difference between the two groups. Hence, we did a relative risk ratio for each of these subgroups and found that the difference in effect on progression to IMV between low dose and high dose steroid subgroups across trials is not significant statistically suggesting these doses seem to be similar in prevention of progression to invasive mechanical ventilation.
Indirect post hoc between trial analysis revealed that there are no subgroup differences between high dose and low dose steroids with regard to all-cause mortality, serious adverse events, nosocomial infections and hyperglycemia. We explored a potential difference in effect in progression to IMV but we found this to be insignificant.
[5] Would a longer dose of steroids be beneficial in severe to critical COVID19 infection?
We found 8 trials(5,7,8,9,10,12,13,14) done in 998 patients which evaluated use of steroids for ≤ 7 days and 4 trials (3,4,6,11) done in 6891 patients which evaluated use of steroids for > 7 days for outcomes of mortality and progression to invasive mechanical ventilation. In the trials using a shorter duration of steroids RR=0.88(95% CI 0.74-1.05) but those using a longer duration were found to have RR=0.89 (95% CI 0.82-0.97). However there seemed to be no subgroup differences with an I2 = 0% suggesting that there was no difference in subgroups using a short duration of steroids vs a longer duration of steroids.
Progression to invasive mechanical ventilation was studied in 2 trials (9,10) with 238 patients which gave steroids for < 7 days vs 2 trials (3,11) in 5450 patients in which a longer duration (> 7 days) of steroids was given revealing a RR=1.16 (95% CI 0.76-1.79) and RR=0.75(95% CI 0.61-0.92) respectively. The I2 was 69.4% suggesting a possible difference between subgroups and hence a relative risk ratio was done between which seemed to suggest that there was no statistically significant difference between the subgroups of < 7days or > 7 days with regard to progression to invasive mechanical ventilation.
An indirect post hoc between trial analysis revealed that there are no subgroup differences between short course and long course steroids with regard to outcomes of all-cause mortality, serious adverse events, nosocomial infections, hyperglycemia. We explored a potential difference in effect in progression to IMV but we found this to be insignificant. In the long duration group, the mean duration of steroid use in Recovery trial was 7 days and in CAPE-COVID it was 10.5 days.
1 All cause mortality
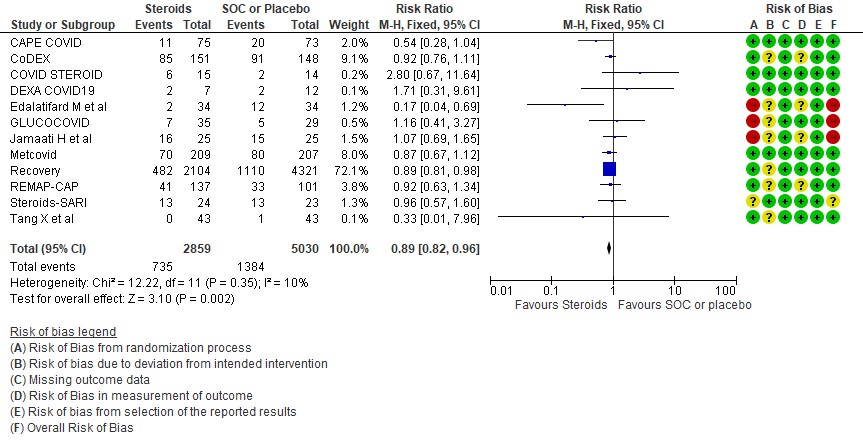
2.Progression to invasive mechanical ventilation
3. Serious adverse events
4. Occurrence of nosocomial/opportunistic infection
5. Hyperglycemia or diabetes
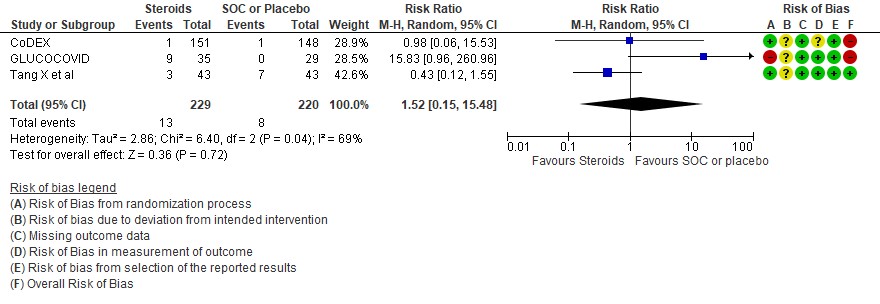
SUBGROUP ANALYSIS
[A] Do steroids make a difference if used early in illness
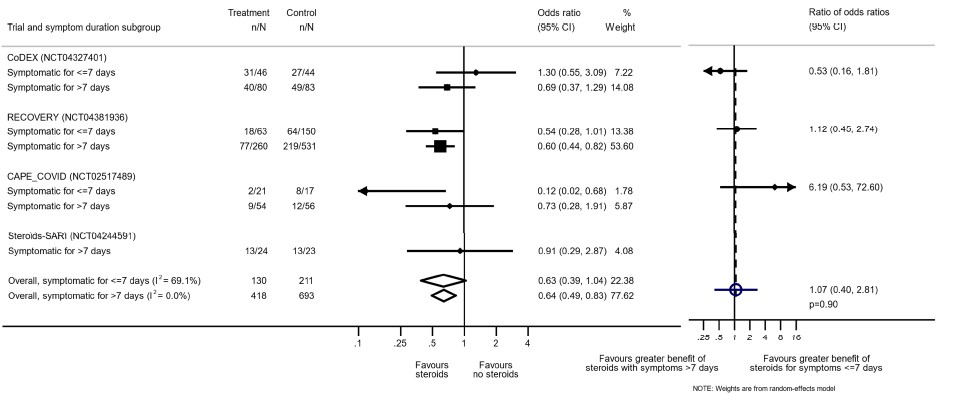
[Reference: The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group -supplementary material. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA. 2020;324(13):1330–1341. doi:10.1001/jama.2020.17023]
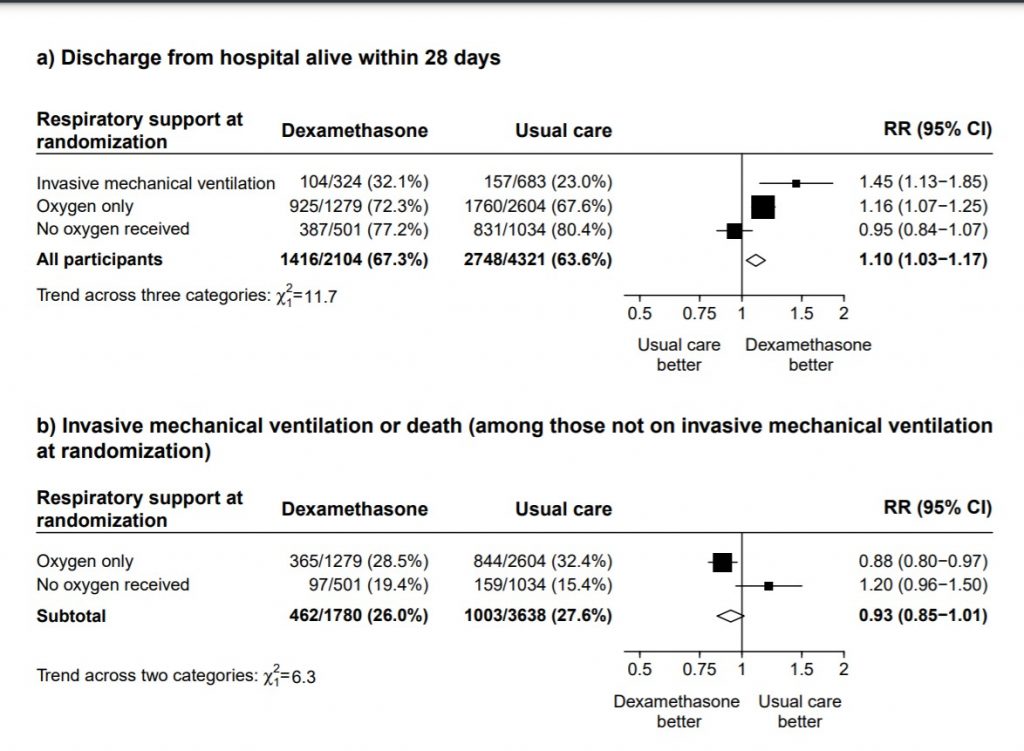
[B] Are steroids beneficial in mild-moderate illness with no hypoxia
B.1 : Data adapted from the RECOVERY Trial(11) as below
(a) Panel shows Mortality at 28 days with RR=0.95 (95% CI 0.85-1.07) in the group with no oxygen
(b) Panel shows a composite outcome of invasive mechanical ventilation or death in the group with no oxygen with RR= 1.02 (95% CI 0.96-1.05)
[C] Which type of steroid is preferred in severe to critical COVID 19 – a comparison of 3 formulations
C.1 Mortality
C.2: Progression to invasive mechanical ventilation
[D] What dose (<10 mg/day OR >10 mg/day) of steroids would be appropriate for treatment of severe to critical COVID 19?
D.1 Mortality
D2. Progression to invasive mechanical ventilation
[E] What duration (<10 days OR > 10 days) of steroids is advised for people with severe to critical COVID 19?
E.1 Mortality
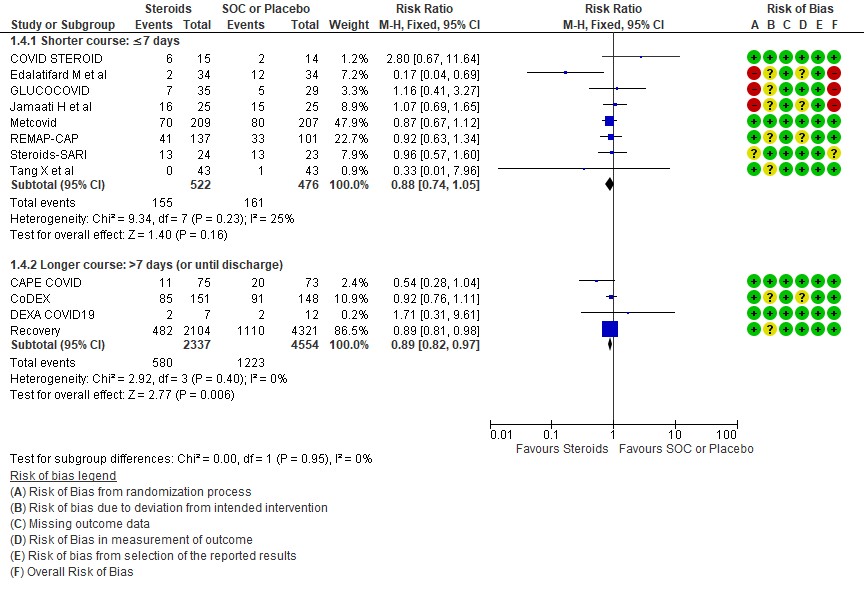
E.2 Progression to invasive mechanical ventilation