Sarilumab is a human monoclonal antibody, which inhibits the binding of IL-6 to its 𝛂 receptor. It has been used in the treatment of inflammatory conditions like moderate to severe active rheumatoid arthritis and cytokine release syndrome after chimeric antigen receptor T- cell therapy. It was postulated to have an anti-inflammatory role in the treatment of COVID-19 by suppressing the pro-inflammatory signaling by pulmonary epithelial and immune cells and thus reduce the severity of pulmonary complications in COVID-19. WHO recommends the use of IL-6 inhibitors in the treatment of COVID-19 infections when there is systemic evidence of hyper-inflammation suggesting perhaps that there is clinical equivalence in the efficacy of Tocilizumab and Sarilumab1.
However, we evaluated Sarilumab efficacy and safety in the management of moderate to severe COVID-19 in at least 3 randomized trials. In these trials, Sarilumab had no impact on mortality, time to clinical improvement, did not decrease the ICU admission or decrease the need for NIV/MV or HFNO. Also, it did not impact the length of ICU stay and overall length of hospital stay. Thus, it did not demonstrate any efficacy in the treatment of moderate or severe COVID-19 infection. Additionally, the drug is prohibitively more expensive than other IL-6 antagonists and the availability of the drug is also an issue. While the drug seems to be safe in that it does not increase the risk of bacterial or fungal infections, there is no data on the other adverse reactions, especially the infusion reactions.
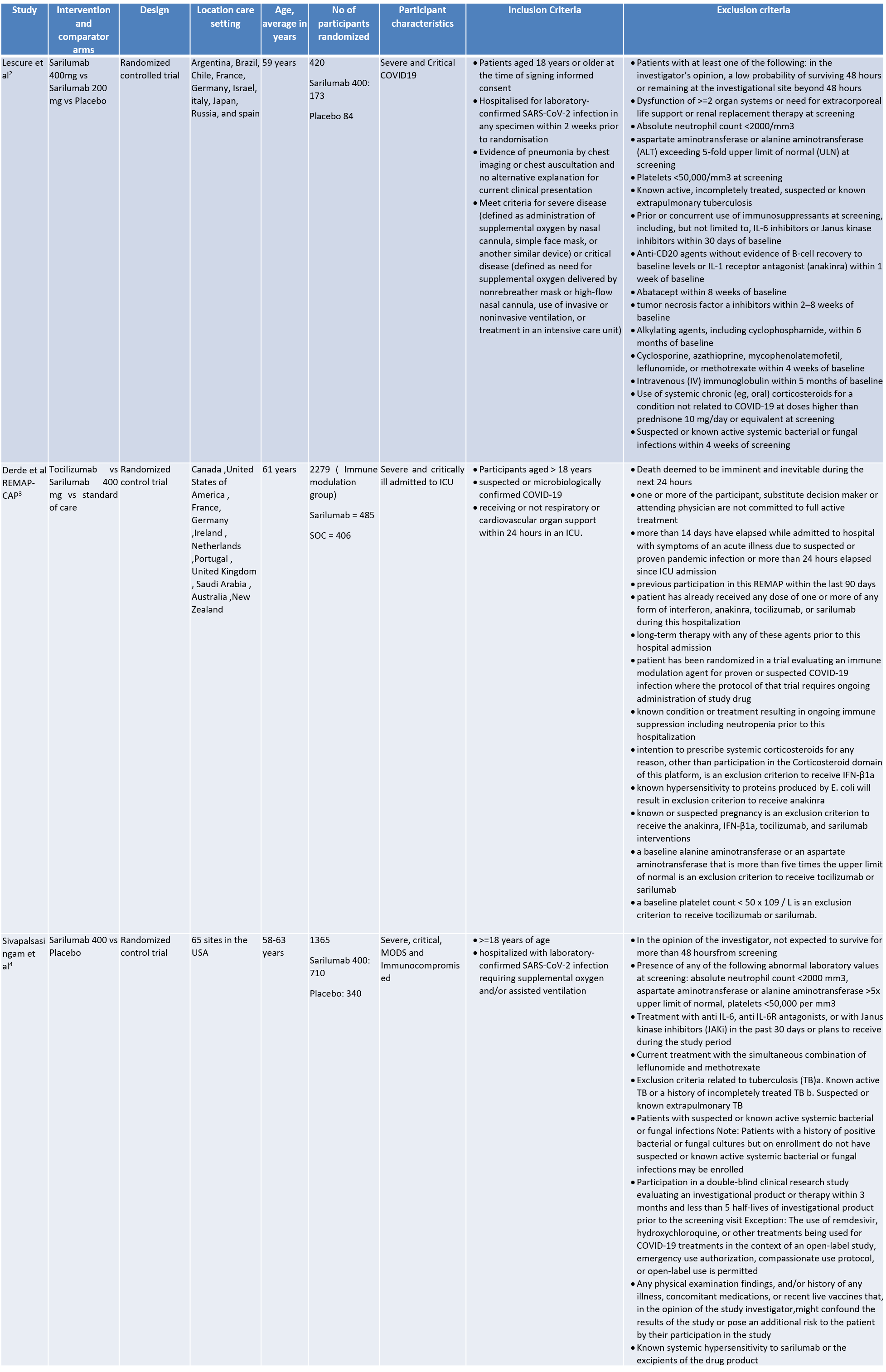
Hence although there is no study with a head to head comparison between Sarilumab and Tocilizumab, the overall available evidence did not suggest that the drug could be recommended for use as an alternative to Tocilizumab. In view of all of the above, the panel strongly recommends against the use of Sarilumab for moderate to severe COVID-19 infection.
Date of latest search: 30th August 2021
Date of completion & presentation to Expert Working Group: 3rd September 2021
Date of planned review: 30th February 2022
Evidence synthesis team: Tejinder Pal Singh Grewal, Navneet Arora, Jane Miracline, Bhagteshwar Singh, Richard Kirubakaran and Priscilla Rupali.
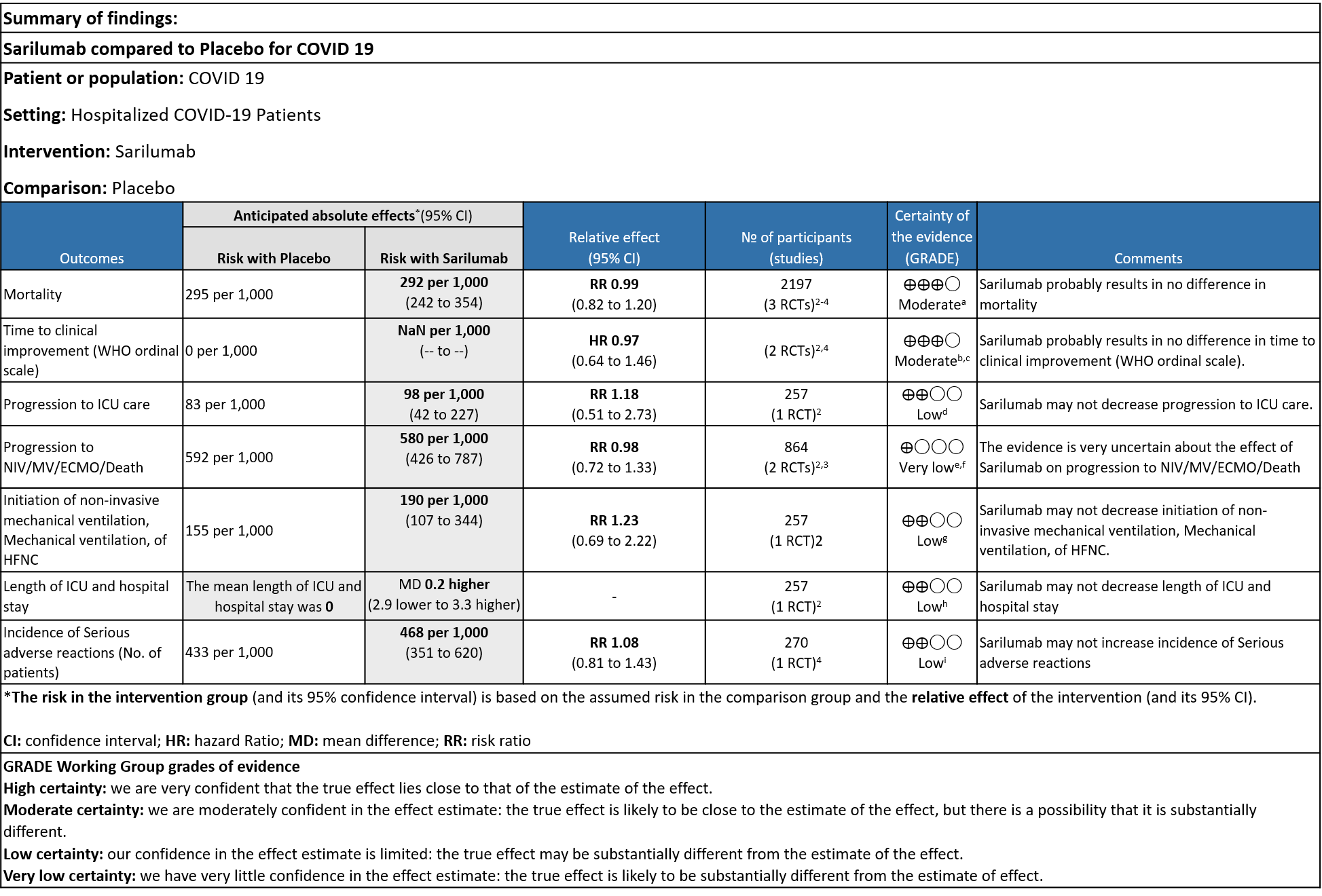
Sarilumab compared to standard of care for severe COVID-19
Explanations
a. Downgrade by 1 level, serious imprecision as the confidence interval is wide (0.82 to 1.20)
b. We will not downgrade it as the direction of the effect is on the same side, despite the i square of 49%, showing no statistical difference
c. Downgraded by 1 level, serious imprecision as the confidence interval is wide (0.64-1.46)
e. Downgraded by 2 levels for very serious imprecision as the event rate was low, with OIS criteria not met
f. Downgraded by 2 levels, very serious inconsistency as I Square value is 89%
g. Downgrade by 1 level for serious imprecision as the confidence interval is wide (0.72 to 1.33)
h. Downgraded by 2 levels for very serious imprecision, as the event rate was low and the confidence interval is wide (0.69 to 2.22)
i. Downgraded by 2 levels for very serious imprecision, as the mean difference of 0.2 days is clinically insignificant.
j. Downgraded by 2 levels for very serious imprecision, as the event rate was low and the confidence interval is wide (0.81 to 1.43)
Interleukin-6 is released in response to infection and stimulates inflammatory pathways as part of the acute-phase response. Studies suggest that interleukin-6 (IL-6) levels are elevated in cases of complicated Covid-19 infection. Sarilumab is a monoclonal antibody that inhibits both membrane-bound and soluble interleukin-6 receptors. It has been previously used to treat inflammatory conditions, such as rheumatoid arthritis, as well as cytokine release syndrome after chimeric antigen receptor (CAR) T-cell therapy.
To identify all available systematic reviews pertaining to our PICO question, a systematic search of PubMed, covid-nma.org and the Epistemonikos databases was conducted. The search strategy was designed and validated by the Group’s information specialist. The search results were screened independently by two reviewers against the pre-defined criteria set out above.
There were total 47 studies available out of which 37 did not fulfil the inclusion criteria. The remaining 10 studies were screened and 3 RCTs were included.
For the risk of bias, Cochrane ROB2 tool was used. For all outcomes, one review author performed the assessment, and cross-checked it against the assessment by another review author. If there was a difference in more than one domain, it was assessed by a third independent author.
Data was entered into Review manager version 5.4 for meta-analysis. The results were entered into Grade ProGDT (online), to create the summary of findings (GRADE) table. We used risk ratios (RR) for dichotomous outcomes with 95% confidence intervals (CIs). Our PICO question is given below
Population – In-patients with severe Covid-19 infection
Intervention – Sarilumab
Control – standard of care/placebo
Following were the outcomes we wanted to extract data for:
1. Primary Outcomes:
a. Time to clinical improvement (WHO ordinal scale)
b. Proportion of patients progressing to severe respiratory failure, ICU admission or death
2. Secondary Outcomes:
a. Duration of ventilatory support
b. Time to non-invasive ventilation (NIV), invasive mechanical ventilation (IMV)
c. Length of ICU and hospital stay
d. All-cause mortality
e. Incidence of adverse reaction
We found 3 randomised controlled trials that met our search criteria
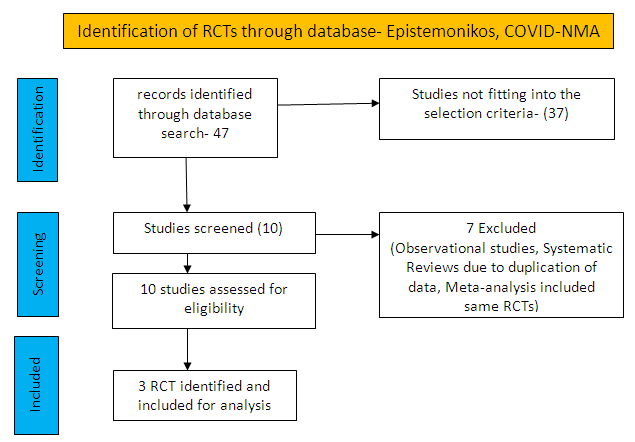
SARILUMAB COMPARED TO STANDARD OF CARE FOR COVID-19
Critical (primary) Outcomes: As presented in the Summary of findings table, the evidence is of low or very low certainty about the effect of Sarilumab on progression to ICU Care, non-invasive ventilation, invasive mechanical ventilation, length of ICU stay and adverse events. Initiation of NIV / MV / HFNC also had low evidence of certainty. Whereas time to clinical improvement and mortality had a moderate certainty of evidence.
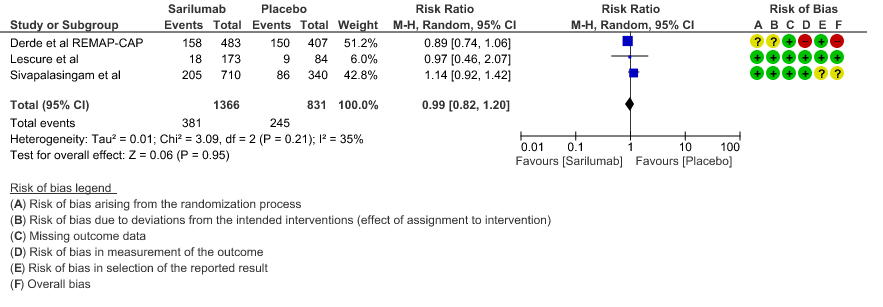
a. All-Cause mortality: Moderate certainty of evidence in 2197 patients from 3 RCTs2-4 found that there is probably no difference between Sarilumab and standard of care RR 0.99 (95 % CI 0.82 to 1.20)
b. Time to clinical improvement: Moderate certainty of evidence in 2 RCTs2,4 found that there was probably no difference in time to clinical improvement (WHO Ordinal Scale) between Sarilumab and Standard of care HR 0.97 (95% CI 0.64 to 1.46).
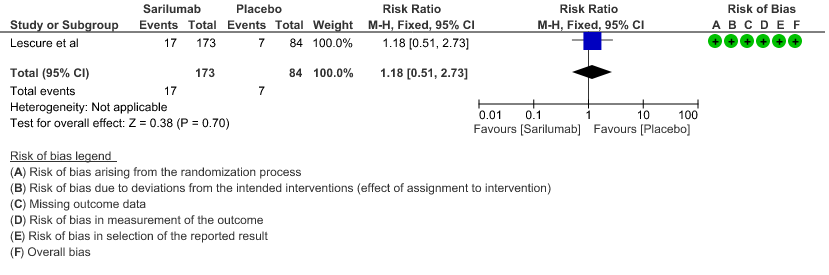
c. Progression to ICU Care: Low certainty of evidence in 257 patients from 1 RCT2 found that Sarilumab may not decrease progression to ICU when compared to standard of care RR 1.18 (95% CI 0.51 to 2.73).
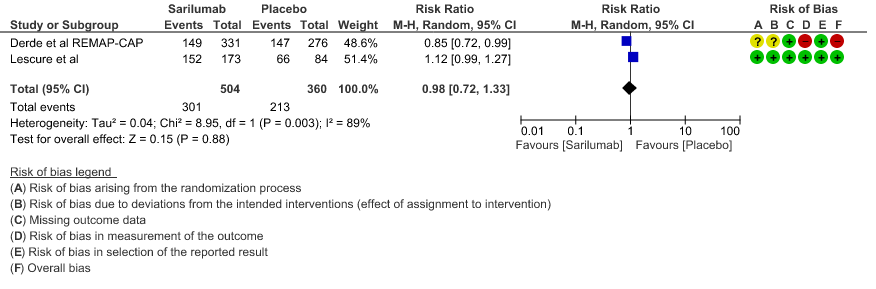
d. Progression to NIV/MV/ECMO/Death: Very low evidence of certainty in 864 patients from 2RCTs2,3 revealed that the effect of Sarilumab on progression to NIV/ MV / ECMO or death was very uncertain RR 0.98 (95% CI 0.72 to 1.33).
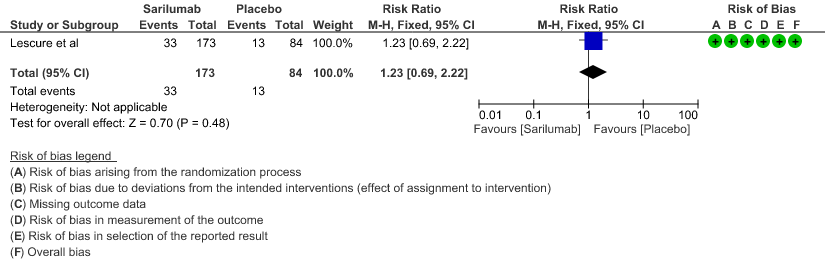
e. Initiation of NIV, IMV or HFNC (high flow nasal cannula): Low certainty of evidence in 257 patients from 1 RCT2 found that Sarilumab may not decrease initiation of non-invasive ventilation, invasive mechanical ventilation, or HFNC RR 1.23 (95% CI 0.69 to 2.22).
f. Length of ICU Stay: Low certainty of evidence in 257 patients from 1 RCT2 found that Sarilumab may not decrease the length of ICU and hospital stay with a mean difference MD 0.20 (95% CI 2.90 lower to 3.30 higher).
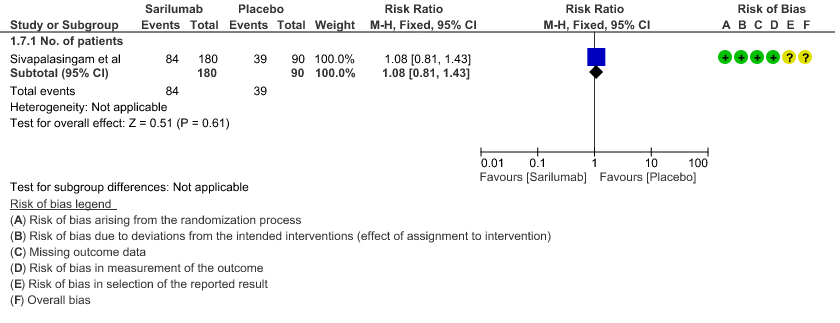
g. Incidence of Serious adverse reactions (No. of patients): Low certainty of evidence from in 270 patients from 1 RCT4 found that Sarilumab may not increase the incidence of serious adverse reactions RR 1.08 (95% CI 0.81 to 1.43)
1. All Cause Mortality
2. Time to clinical improvement
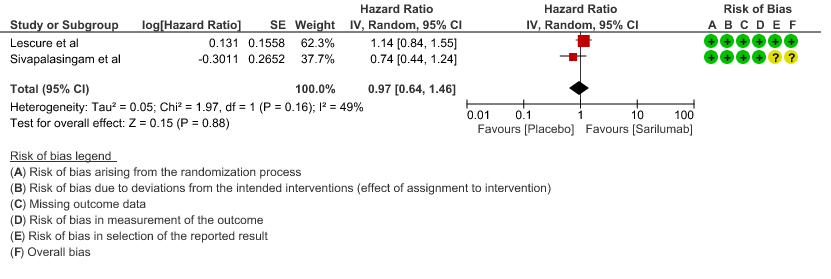
3. Progression to ICU care
4. Progression to NIV/MV/ECMO/Death
5. Initiation of NIV, IMV or HFNC
6. Length of ICU stay
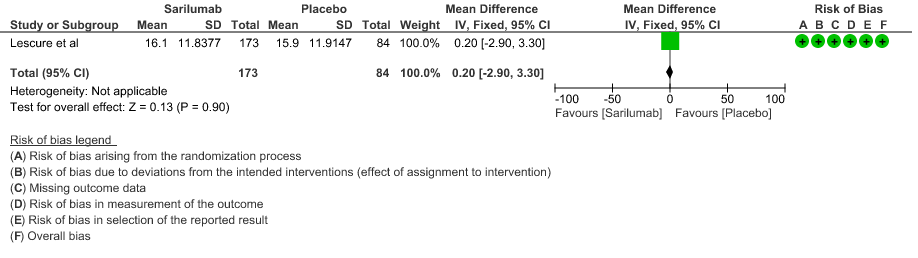
7. Adverse events:
The Antibody and Anti-inflammatory Expert Working Group met on 8th October 2021 to consider Sarilumab as a treatment for COVID-19. Conflict of interest declarations were reviewed by the Steering Committee; none were found to be relevant to Sarilumab.
A summary and then more detailed explanations of the Expert Working Group's judgements follow.
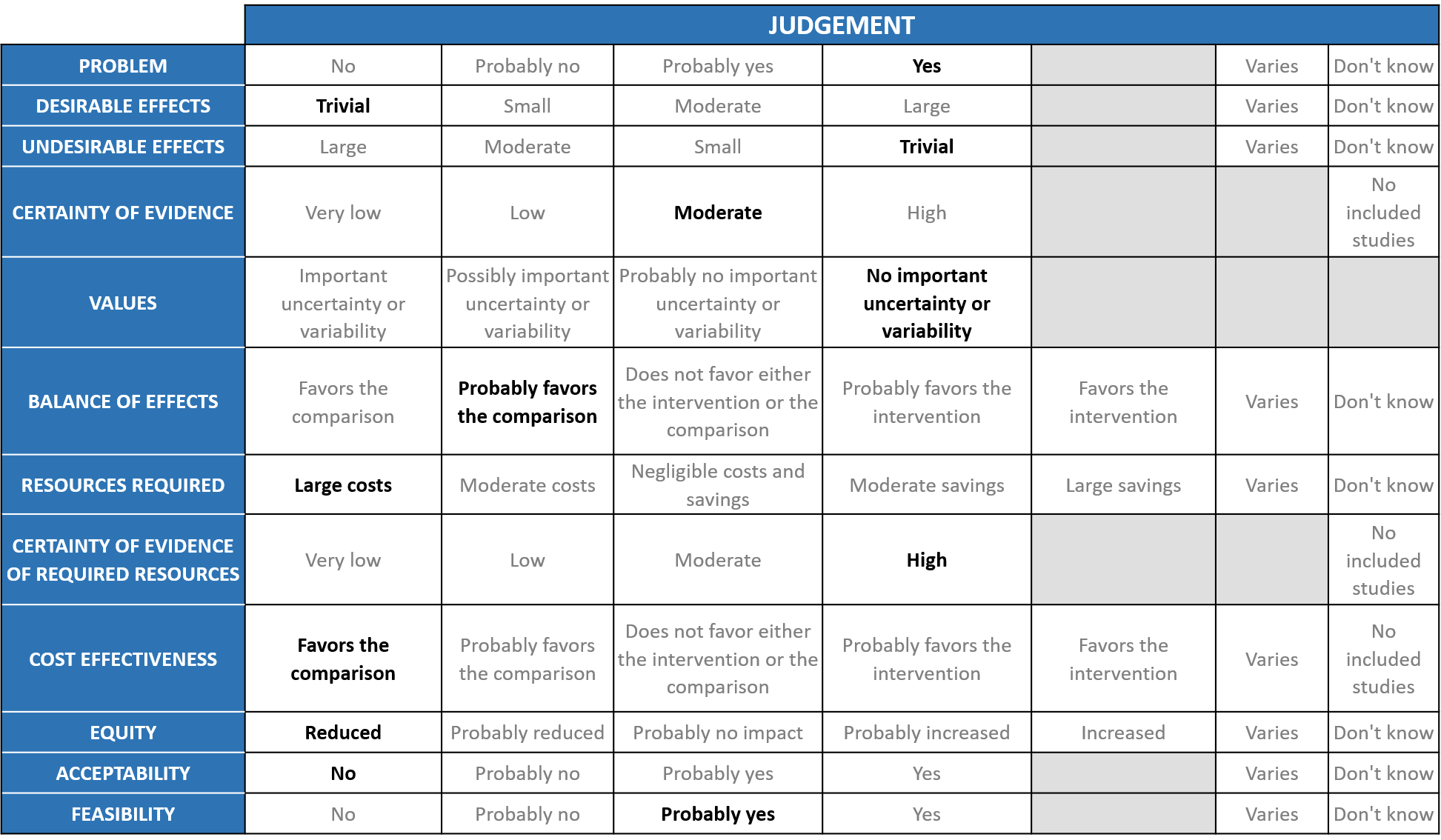
Problem
The COVID-19 pandemic in India with 30 million cases and 0.39 million deaths has significantly impacted and stressed the health structure of the country. During the second wave, there was a shortage of intensive care unit beds, oxygen and trained personnel leading to a major health crisis in the country. Sarilumab is an IL-6 inhibitor and was regarded to be equivalent to Tocilizumab as an adjunct to steroids in severe to critical COVID-19 as per the recommendation from WHO in July 2021. However, it is an expensive drug and not easily available.
Desirable effects
The group agreed that the evidence suggests that Sarilumab doesn’t have a clinically significant effect on mortality, time to clinical improvement, progression to NIV/IMV, length of ICU and hospital stay; the evidence was very uncertain about the effect of Sarilumab on NIV/MV/ECMO/Death. There was an increase in OSFD when compared to placebo. The group concluded that the desirable anticipated effects are trivial.
Undesirable effects
The pooled data suggested that the evidence is very uncertain about the effect of sarilumab on serious adverse events. Most studies looked at bacterial and fungal infections as SAE but there was no difference in the two groups. We are looking at only doses of 400mg not 200mg or 800mg. In Rheumatology patients dosing is different and given as 200mg S/C and at periodic intervals so it is unlikely we can extrapolate from rheumatological conditions to COVID-19. In the study which used the 800mg dose in the severe group, study was stopped due to safety vs benefit concerns in the meta-analysis, but the group notes that this is a higher dose than usually used, and specific details were not available.
As per the pre print (Siva et al), in the group with low flow oxygen the drug was withheld and the reason for this was unclear. This may have been due to the serious adverse events but there was no mention of infusion related side events suggesting the adverse outcomes may have been related to disease rather than the drug itself.
Certainty of evidence
Using GRADE methodology, the evidence synthesis team rated Certainty of evidence and it was found to be moderate for mortality and time to clinical improvement. Certainty was low for progression to ICU care, initiation of non-invasive mechanical ventilation, invasive mechanical ventilation, HFNC, Length of ICU and hospital stay and serious adverse events. Certainty was very low for a composite outcome of progression to NIV/MV/ECMO/Death. The group concluded that the overall certainty of evidence was moderate.
Values
The outcomes studied in this study were to assess mortality, time to clinical improvement, progression to ICU care, initiation of NIV/MV/HFNC, length of ICU and hospital stay, progression to NIV/MV/ECMO/Death. The group concluded that there was no important uncertainty or variability in what outcomes were chosen in the trial.
Balance of effects
Sarilumab even though it is an IL-6 inhibitor similar to tocilizumab does not seem to have a beneficial effect. In addition Tocilizumab now is freely available and hence the overwhelming opinion of the group was that the balance between desirable and undesirable effects of this drug probably favors the comparison.
Resources required
The group felt that this drug is expensive with little evidence of efficacy, suggesting large costs. Cost of Sarilumab: $700 (Rs.52000-60,000) for 200mg but we need 400mg so around 1.2 lakhs per therapeutic dose.
Certainty of evidence of required resources
The group discussed that since the costs are known due to the drug being available for use in indications other than COVID-19 the certainty of evidence for required resources was high.
Cost effectiveness
The group discussed that there are no studies looking at this. However, this is an expensive intervention and evidence did not favor its use.
Equity
At this point in time this intervention would reduce impact on equity as this is an expensive intervention which is not available freely.
Acceptability
The group felt that this intervention has no data available for efficacy and hence unacceptable.
Feasibility
The feasibility of implementation of this intervention varies. This drug if found efficacious can be administered, however may not be widely used as it is expensive.
Sarilumab was not found to be beneficial when critical outcomes were evaluated. There was also a wide variation in the doses used in the various studies. The resources required for implementation include huge cost of sarilumab. Considering the trivial desirable effects and large costs required for implementation, this group is of the opinion that Sarilumab is not equivalent to Tocilizumab and should not be considered for use in hospitalized severe or critically ill covid19 patients. The current recommendation is based on evidence to date and will be updated based on new evidence.
The expert group carefully considered the effect across patients with different levels of oxygen and ventilation requirement of sarilumab and strongly recommends against the use of sarilumab for all subgroups of patients with COVID-19.
Sarilumab was considered for use in COVID-19 patients considering the similarity of the molecule with Tocilizumab, both being IL-6 inhibitors. However, currently there is no evidence to support the use of Sarilumab in any patient group for COVID-19 treatment. This recommendation against the use of Sarilumab will be reviewed as and when any new evidence emerges.
It is surprising that sarilumab was not found to be beneficial since it is also an IL-6 receptor antagonist similar to tocilizumab which is efficacious in treatment of severe and critical COVID-19. At present it is unclear as to whether the lack of efficacy was because of use of a lower dose. So there may be a role to study a higher dose in the management of severe to critical COVID-19. However, it is more expensive than tocilizumab and hence a RCT with Sarilumab being evaluated for efficacy at a higher dose may not be worth the while at the moment. However, we understand that our present conclusion is based on limited data and hence this could possibly be studied at a later date in the future.
- Therapeutics and COVID-19: living guideline [Internet]. [cited 2021 Nov 25]. Available from: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-therapeutics-2021.3
- Lescure F-X, Honda H, Fowler RA, Lazar JS, Shi G, Wung P, et al. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Respiratory Medicine. 2021 May;9(5):522–32.
- Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med. 2021 Feb 25;NEJMoa2100433.
- Sivapalasingam S, Lederer DJ, Bhore R, Hajizadeh N, Criner G, Hosain R, et al. A Randomized Placebo-Controlled Trial of Sarilumab in Hospitalized Patients with Covid-19 [Internet]. Infectious Diseases (except HIV/AIDS); 2021 May [cited 2021 Nov 25]. Available from: http://medrxiv.org/lookup/doi/10.1101/2021.05.13.21256973
Covid Management Guidelines India Group –Anti-inflammatory Working Group - Sarilumab . Covid Guidelines India; Published online on November 29th, 2021; URL: https://indiacovidguidelines.org/sarilumab/ (accessed )
