Sarilumab compared to standard of care for severe COVID-19
Explanations
a. Downgrade by 1 level, serious imprecision as the confidence interval is wide (0.82 to 1.20)
b. We will not downgrade it as the direction of the effect is on the same side, despite the i square of 49%, showing no statistical difference
c. Downgraded by 1 level, serious imprecision as the confidence interval is wide (0.64-1.46)
e. Downgraded by 2 levels for very serious imprecision as the event rate was low, with OIS criteria not met
f. Downgraded by 2 levels, very serious inconsistency as I Square value is 89%
g. Downgrade by 1 level for serious imprecision as the confidence interval is wide (0.72 to 1.33)
h. Downgraded by 2 levels for very serious imprecision, as the event rate was low and the confidence interval is wide (0.69 to 2.22)
i. Downgraded by 2 levels for very serious imprecision, as the mean difference of 0.2 days is clinically insignificant.
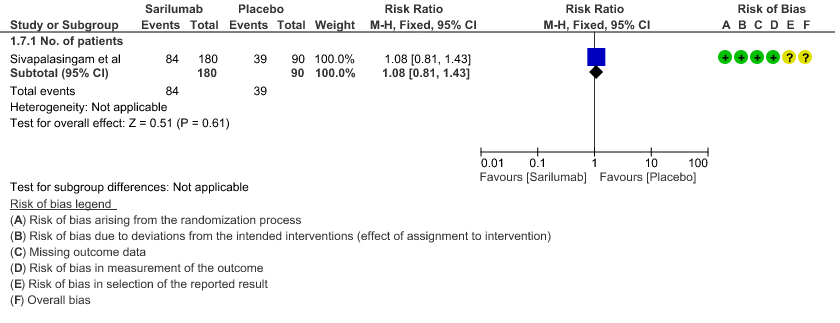
j. Downgraded by 2 levels for very serious imprecision, as the event rate was low and the confidence interval is wide (0.81 to 1.43)
Interleukin-6 is released in response to infection and stimulates inflammatory pathways as part of the acute-phase response. Studies suggest that interleukin-6 (IL-6) levels are elevated in cases of complicated Covid-19 infection. Sarilumab is a monoclonal antibody that inhibits both membrane-bound and soluble interleukin-6 receptors. It has been previously used to treat inflammatory conditions, such as rheumatoid arthritis, as well as cytokine release syndrome after chimeric antigen receptor (CAR) T-cell therapy.
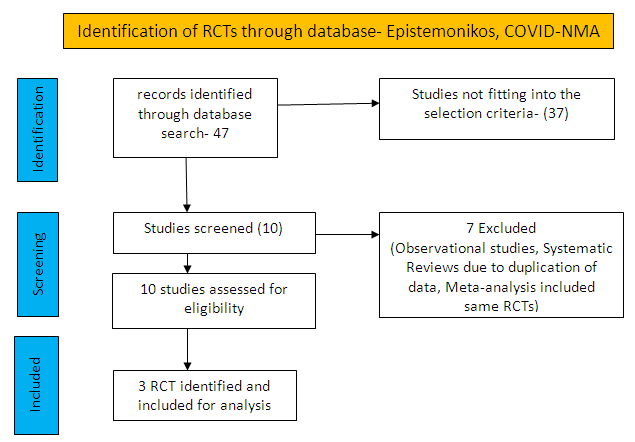
To identify all available systematic reviews pertaining to our PICO question, a systematic search of PubMed, covid-nma.org and the Epistemonikos databases was conducted. The search strategy was designed and validated by the Group’s information specialist. The search results were screened independently by two reviewers against the pre-defined criteria set out above.
There were total 47 studies available out of which 37 did not fulfil the inclusion criteria. The remaining 10 studies were screened and 3 RCTs were included.
For the risk of bias, Cochrane ROB2 tool was used. For all outcomes, one review author performed the assessment, and cross-checked it against the assessment by another review author. If there was a difference in more than one domain, it was assessed by a third independent author.
Data was entered into Review manager version 5.4 for meta-analysis. The results were entered into Grade ProGDT (online), to create the summary of findings (GRADE) table. We used risk ratios (RR) for dichotomous outcomes with 95% confidence intervals (CIs). Our PICO question is given below
Population – In-patients with severe Covid-19 infection
Intervention – Sarilumab
Control – standard of care/placebo
Following were the outcomes we wanted to extract data for:
1. Primary Outcomes:
a. Time to clinical improvement (WHO ordinal scale)
b. Proportion of patients progressing to severe respiratory failure, ICU admission or death
2. Secondary Outcomes:
a. Duration of ventilatory support
b. Time to non-invasive ventilation (NIV), invasive mechanical ventilation (IMV)
c. Length of ICU and hospital stay
d. All-cause mortality
e. Incidence of adverse reaction
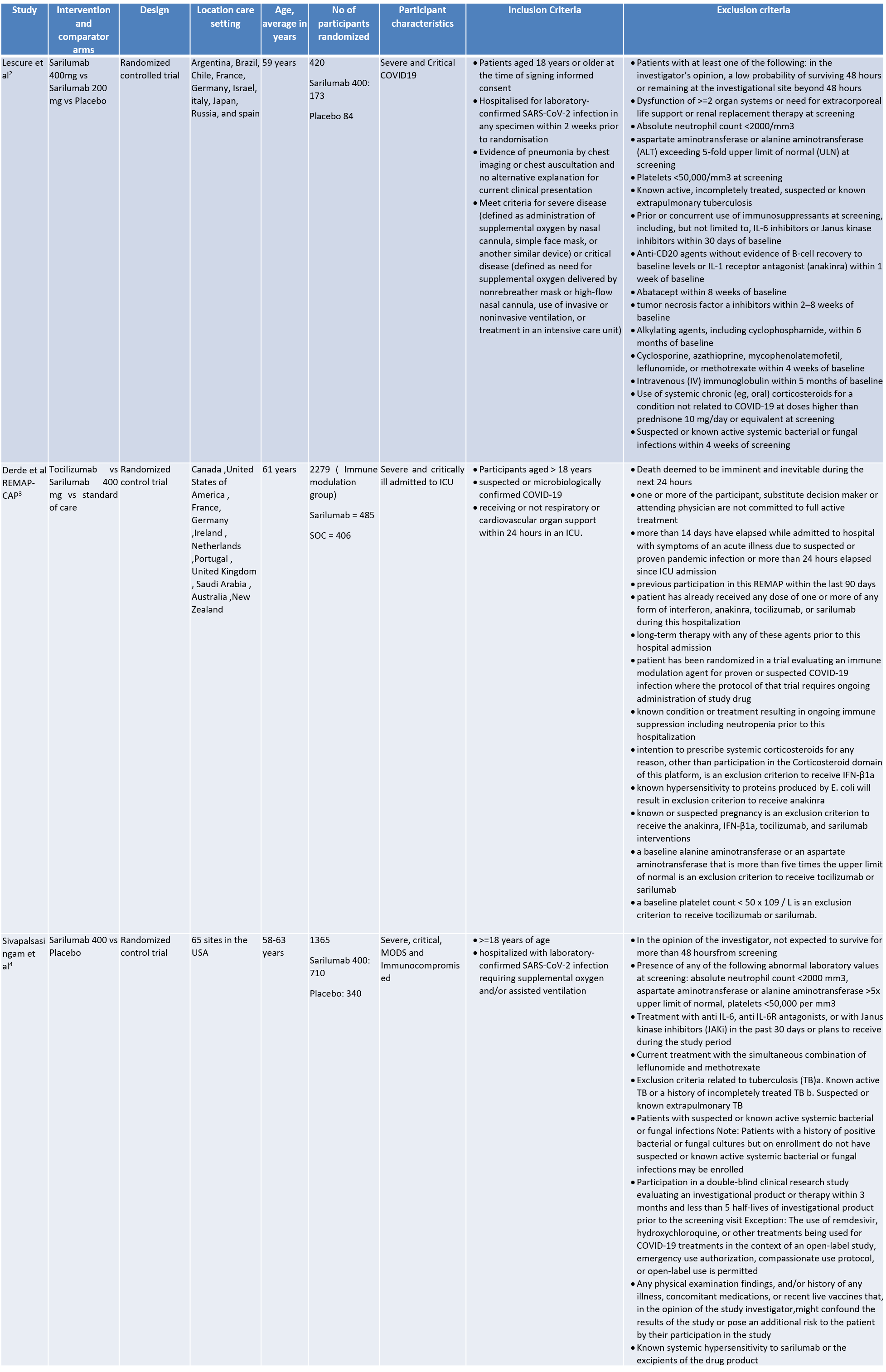
We found 3 randomised controlled trials that met our search criteria
SARILUMAB COMPARED TO STANDARD OF CARE FOR COVID-19
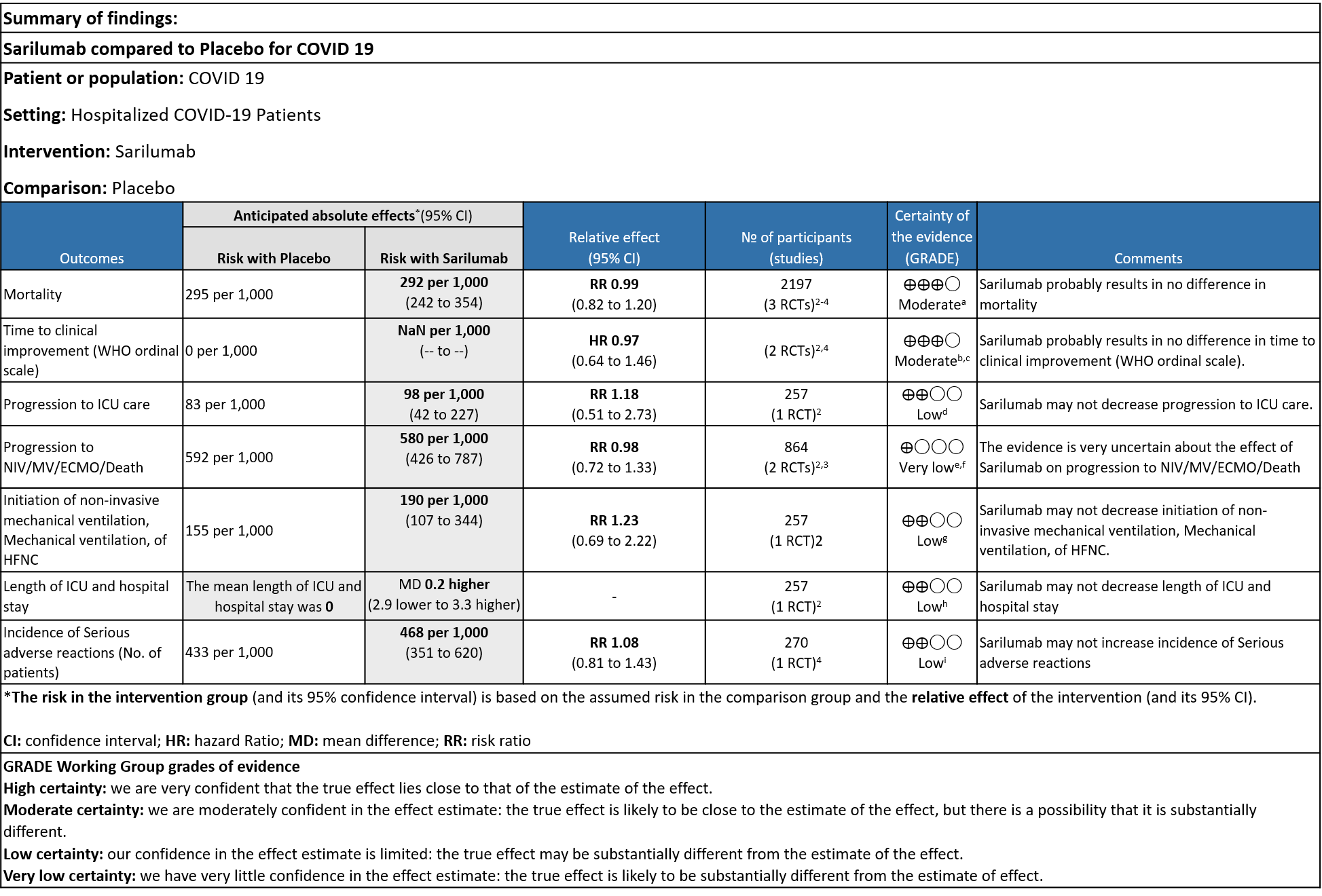
Critical (primary) Outcomes: As presented in the Summary of findings table, the evidence is of low or very low certainty about the effect of Sarilumab on progression to ICU Care, non-invasive ventilation, invasive mechanical ventilation, length of ICU stay and adverse events. Initiation of NIV / MV / HFNC also had low evidence of certainty. Whereas time to clinical improvement and mortality had a moderate certainty of evidence.
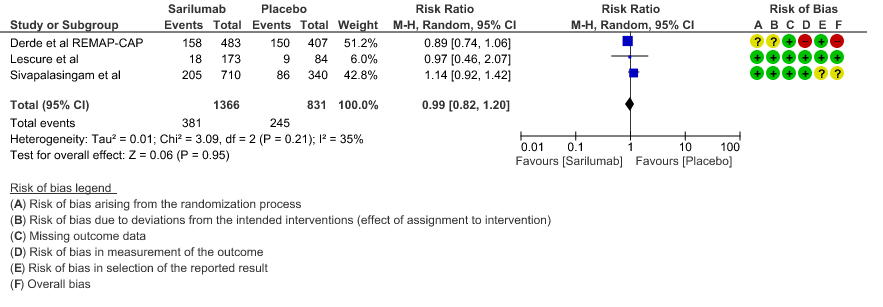
a. All-Cause mortality: Moderate certainty of evidence in 2197 patients from 3 RCTs2-4 found that there is probably no difference between Sarilumab and standard of care RR 0.99 (95 % CI 0.82 to 1.20)
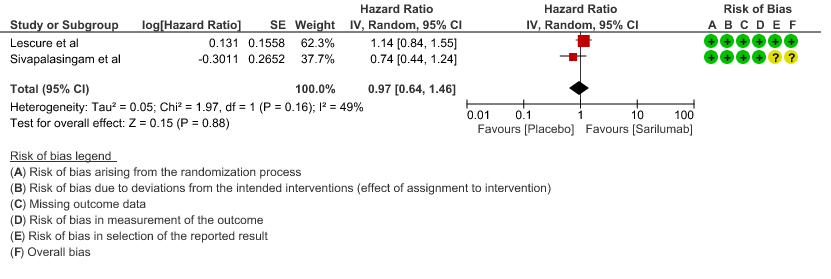
b. Time to clinical improvement: Moderate certainty of evidence in 2 RCTs2,4 found that there was probably no difference in time to clinical improvement (WHO Ordinal Scale) between Sarilumab and Standard of care HR 0.97 (95% CI 0.64 to 1.46).
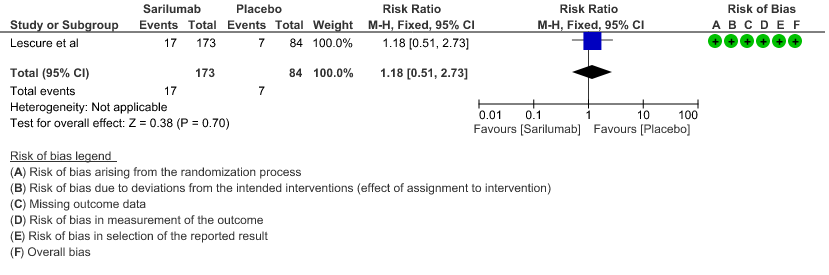
c. Progression to ICU Care: Low certainty of evidence in 257 patients from 1 RCT2 found that Sarilumab may not decrease progression to ICU when compared to standard of care RR 1.18 (95% CI 0.51 to 2.73).
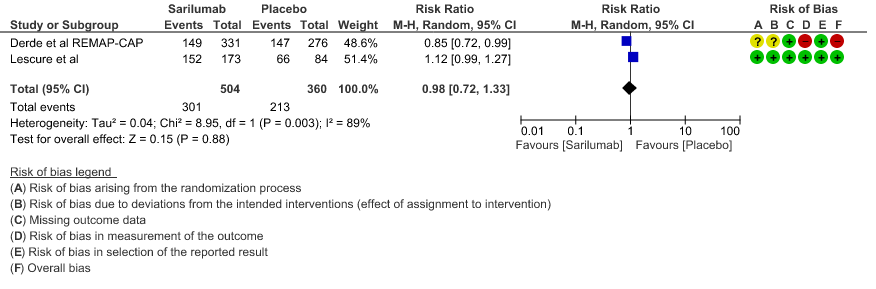
d. Progression to NIV/MV/ECMO/Death: Very low evidence of certainty in 864 patients from 2RCTs2,3 revealed that the effect of Sarilumab on progression to NIV/ MV / ECMO or death was very uncertain RR 0.98 (95% CI 0.72 to 1.33).
e. Initiation of NIV, IMV or HFNC (high flow nasal cannula): Low certainty of evidence in 257 patients from 1 RCT2 found that Sarilumab may not decrease initiation of non-invasive ventilation, invasive mechanical ventilation, or HFNC RR 1.23 (95% CI 0.69 to 2.22).
f. Length of ICU Stay: Low certainty of evidence in 257 patients from 1 RCT2 found that Sarilumab may not decrease the length of ICU and hospital stay with a mean difference MD 0.20 (95% CI 2.90 lower to 3.30 higher).
g. Incidence of Serious adverse reactions (No. of patients): Low certainty of evidence from in 270 patients from 1 RCT4 found that Sarilumab may not increase the incidence of serious adverse reactions RR 1.08 (95% CI 0.81 to 1.43)
1. All Cause Mortality
2. Time to clinical improvement
3. Progression to ICU care
4. Progression to NIV/MV/ECMO/Death
5. Initiation of NIV, IMV or HFNC
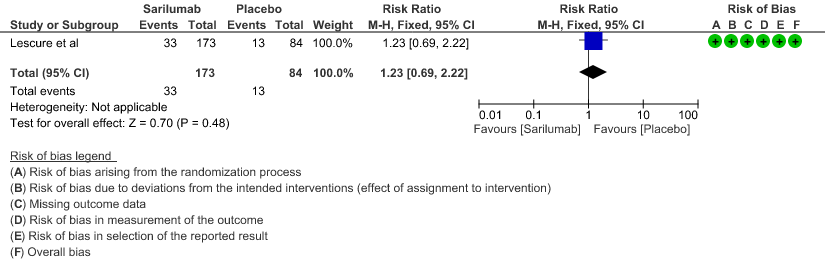
6. Length of ICU stay
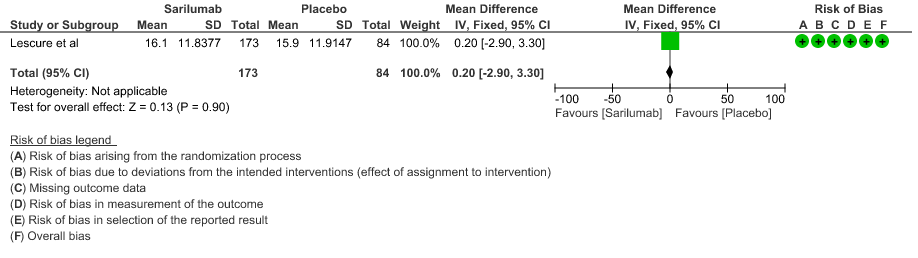
7. Adverse events: