Explanations:
a. Downgraded by 1 level for serious indirectness as the study population is unvaccinated which does not reflect the current scenario
b. Downgraded by 1 level for serious imprecision as optimal information size was not met
c. Downgraded by two levels for serious imprecision as the 95% CI includes appreciable benefit and harm and optimal information size was not met
d. Downgraded by 1 level for serious imprecision as optimal information size was not met
Remdesivir is a monophosphoramidate adenosine analogue prodrug which is metabolized to an active triphosphate form that inhibits viral RNA synthesis via RNA dependent RNA polymerase (2). It was developed as a therapeutic agent for treating RNA-based viruses such as Ebola virus, MERS, SARS-CoV-1 and other zoonotic corona viruses (5). It has demonstrated in vitro and in vivo antiviral activity against SARS-CoV-2 (6). Remdesivir is widely used across the world and a number of guidelines have recommended its use in severe COVID-19 patients (7,8). The WHO living guideline provides a conditional recommendation against the usage of Remdesivir in COVID-19 irrespective of the severity of the disease (9). A number of therapeutic options are available currently for patients with mild covid-19 at high risk for progression to severe disease.
On January 21, 2022, FDA approved a supplemental application for Remdesivir use in adults and children >12 years of age in out-patients with mild to moderate COVID-19 illness at high risk of progression to severe COVID-19[10]. Remdesivir, as a short duration treatment has recently been studied in outpatients with mild covid-19 based on the hypothesis that early treatment for other viral infections have shown to improve clinical outcomes and reduce mortality (12,13,14).
This review aims to provide a summary of the available evidence from randomized clinical trials of Remdesivir for treatment of outpatients with mild COVID-19 for any duration.
We searched PubMed, Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language. We also reviewed reference lists of systematic reviews and included studies. We performed all searches up to 01/03/ 2022.
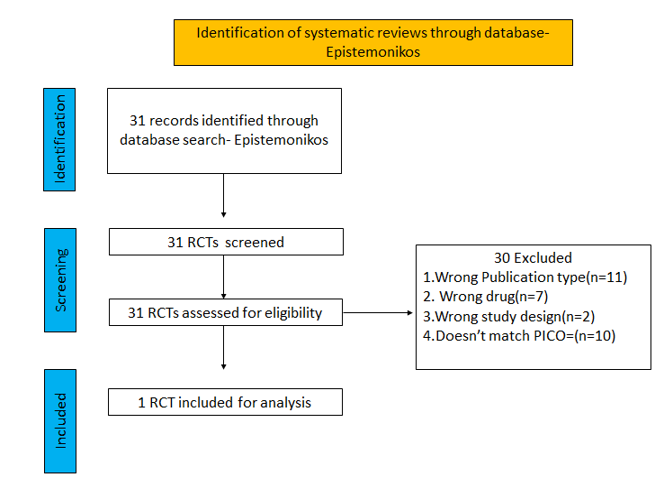
We searched the above databases and found 31 records. After removing duplicates and excluding that did not match our PICO question, 1 RCTs were included for meta-analysis.
We extracted data for the following outcomes, pre-defined by the Expert Working Group:
- Critical (primary for this review):
- Admission into hospital (covid-19 related)
- Covid-19 related medical visits
- Progression to severe disease- requiring O2, mechanical ventilation or ICU care
- Important (secondary):
- Duration of hospitalisation
- Mortality all cause(28-30 days)
- Time to clinical recovery
- Time to resolution of covid-19 signs/symptoms
- Time to viral clearance(negative PCR)
- Complications of covid-19
- Thrombotic events
- Pulmonary function/ Fibrosis
- Secondary Infections
- Adverse events: All and Serious
Two reviewers independently assessed eligibility of search results using the online Rayyan tool. Data extraction was performed by one reviewer, and checked by another, using a piloted data extraction tool on Microsoft Word. Risk of bias (RoB) was assessed using the Cochrane RoB v2.0 tool, with review by a second reviewer and compared with covid-nma database. If there was a difference in more than one domain it was assessed by a third independent author. We planned to use risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). We planned to meta-analyze if appropriate, i.e., if outcomes were measured and reported in a similar way for similar populations in each trial, using random-effects models with the assumption of substantial clinical heterogeneity (the appropriateness of which would be checked by the Methodology Committee), and the I2 test to calculate residual statistical heterogeneity, using Review Manager (RevMan) v5.4. We used GRADE methodology to make summary of findings tables on GRADEPro GDT.
We found 1 RCT that matched the PICO question as pre-defined by the expert working group.
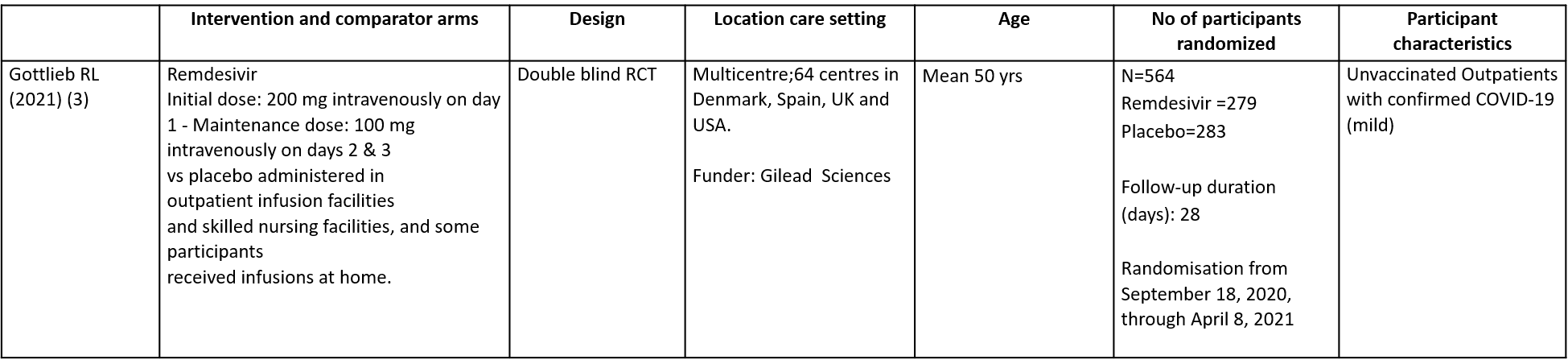
This double blind randomized controlled trial included a total of 562 participants, of which 30.2% were ≥60 years and 1.4% was below the age of 18 (3). The trial was done across 64 centres in Denmark, Spain, UK and USA from September 18, 2020, through April 8, 2021 and was funded by Gilead Sciences. It compared Remdesivir with placebo in unvaccinated patients with mild Covid-19 and at least one pre-existing risk factor for progression to severe Covid-19 or were 60 years of age or older, regardless of whether they had other risk factor. Remdesivir was administered for a period of 3 days.
The included trial and its results are described below; characteristics of the trials are summarised in the Summary of characteristics of included trials table. Risk of bias for each domain per trial is displayed alongside the forest plots below.
The following comparisons were investigated in the trial (we compared outcomes for arms randomized to Remdesivir vs. standard of care or placebo).
Our expert working group classified admission into hospital (Covid-19 related), Covid-19 related medical visits, progression to severe disease- requiring O2, mechanical ventilation or ICU care as critical outcomes. Duration of hospitalisation, all cause mortality at 28-30 days, time to clinical recovery, time to resolution of Covid-19 signs/symptoms, time to viral clearance (negative PCR), complications of Covid-19: thrombotic events, pulmonary fibrosis, secondary Infections and adverse events: all and serious were designated as important outcomes.
Critical (primary) Outcomes
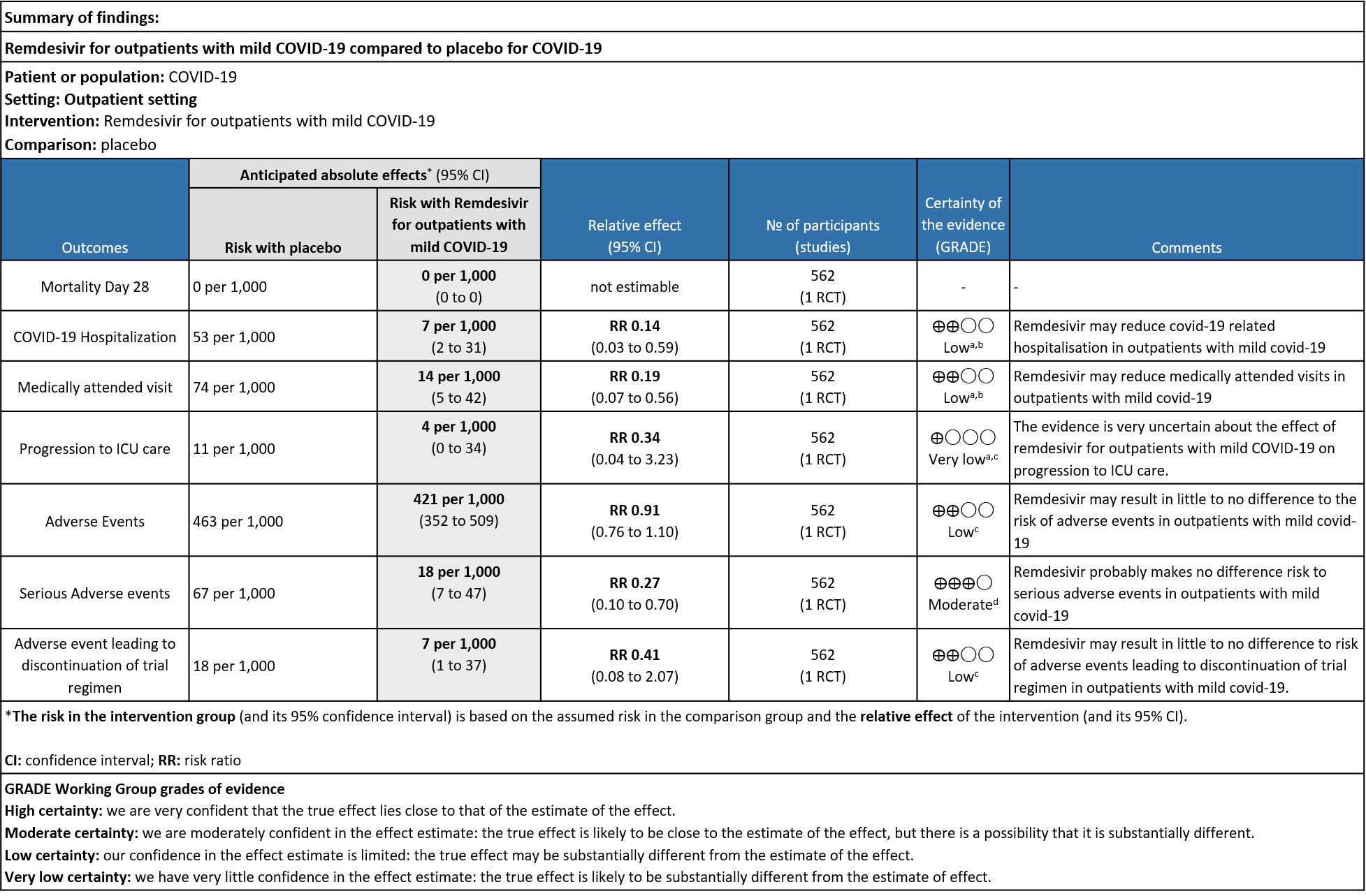
As presented in the ‘Summary of findings’ table, the evidence regarding effect of Remdesivir on covid-19 related hospitalisation, medically attended visits, progression to ICU care and adverse events leading to drug discontinuation is of low certainty but it is moderate certainty for adverse events.
a. All-cause mortality D28: No events reported.
b. Covid-19 related hospitalisation by D28: Low certainty of evidence in 562 patients in one RCT [3] found that the Remdesivir may reduce hospitalisation by Day 28 in outpatients with mild covid-19 [RR 0.14 (95% CI 0.03 to 0.59)]
c. Medically attended visit : Low certainty of evidence in 562 patients in one RCT [3] found that the Remdesivir may reduce medically attended visits day 28 in outpatients with mild covid-19 [RR 0.19 (95% CI 0.07 to 0.56)]
d. Progression to ICU care: Very low certainty of evidence in 562 patients in one RCT [3] found that the evidence is very uncertain about the effect of remdesivir for outpatients with mild COVID-19 on progression to ICU care [RR 0.34 (95% CI 0.04 to 3.23)]
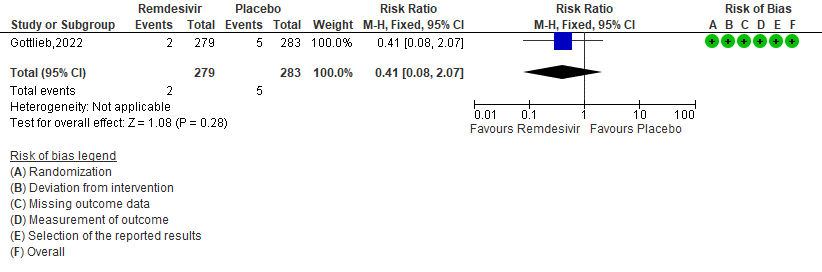
e. Adverse events: Low certainty of evidence in 562 patients in one RCT [3] found that the evidence suggests Remdesivir may result in little to no difference to the risk of adverse events in outpatients with mild covid-19 [RR 0.91 (95% CI 0.76 to 1.10)]
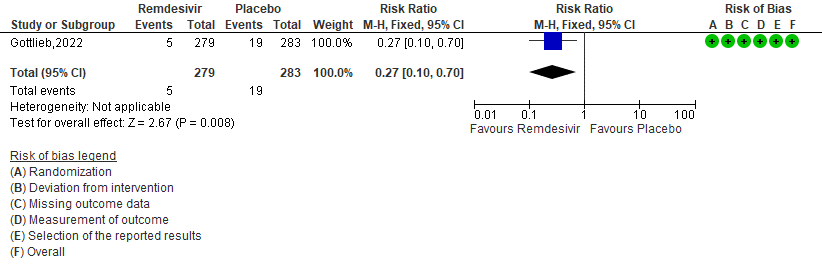
f. Serious Adverse events: Moderate certainty of evidence in 562 patients in one RCT [3] found that Remdesivir probably makes no difference risk to serious adverse events in outpatients with mild covid-19 [RR 0.27 (95% CI 0.10 to 0.70)]
g. Adverse event leading to discontinuation of trial regimen: Low certainty of evidence in 562 patients in one RCT [3] found that the Remdesivir may result in little to no effect on progression to ICU care in outpatients with mild covid-19 [RR 0.41 (95% CI 0.08 to 2.07)]

Primary:
1. Mortality all cause by day 28[3]
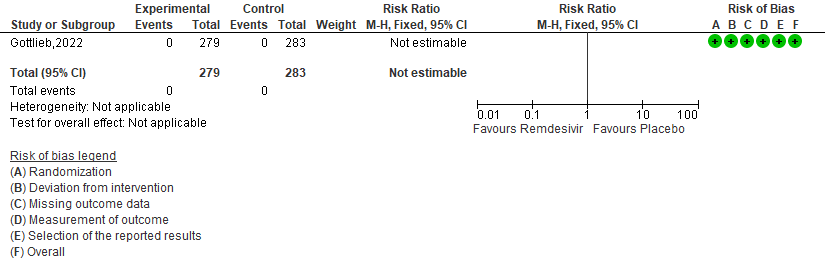
2. (COVID-19) Related Hospitalization (Defined as at Least 24 Hours of Acute Care) [3].
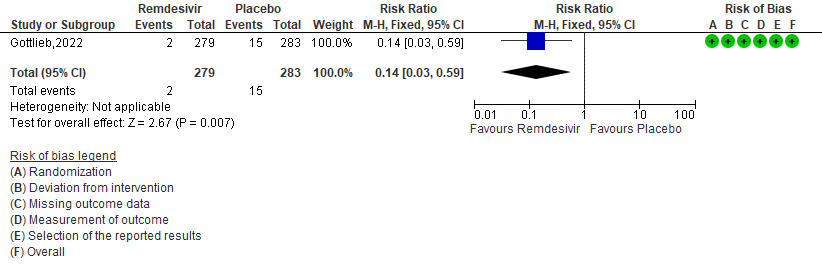
3. Medically attended visits[3]
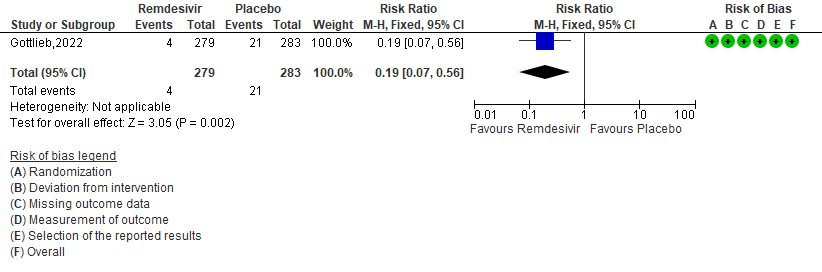
4. Progression to ICU care [3]
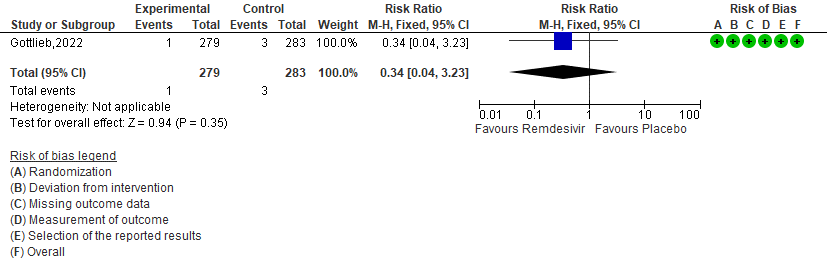
Secondary:
5. Adverse events [3]
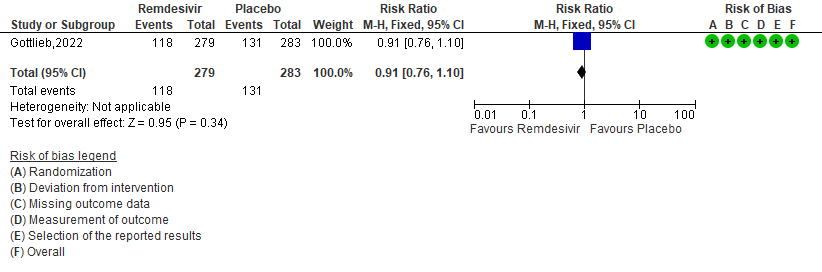
6. Serious Adverse events [3]
7. Adverse events leading to drug interruption [3]