Overall
Explanations
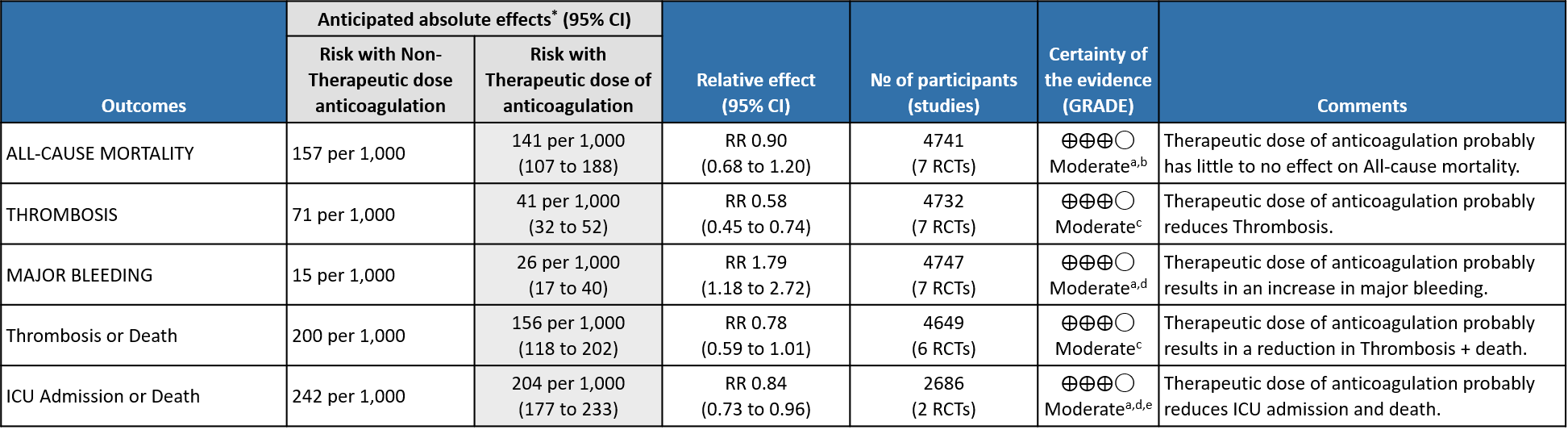
a. Not downgraded since risk of bias (RoB) assessment with RoB 2.0 tool scored 'some concerns' in only 1 domain of each of the studies, for this outcome. In Marcos et al1. Domain 1 was marked down for some concerns due to inadequacy in allocation concealments. In the MPRCT4,5, Domain 2 was marked down for 'some concerns' in view of significant deviations in intended interventions in trial, which probably did not affect outcomes. In HESACOVID 3 trial, Domain 5 was marked down for 'some concerns' in view of not enough information being provided to completely rule out risk of bias in selection of the reported result.
b. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from appreciable benefit to harm.
c. Downgraded by 1 level for serious risk of bias; RoB assessment with RoB 2.0 tool scored 'some concerns' . In Marcos et al1 Domain 1 was marked down for some concerns due to inadequacy in allocation concealments. In 2 domains of the mpRCT studies4,5 'some concerns' were identified. Regarding Domain 2 see explanation (a). Domain 4 of RoB 2.0 tool was assessed to have some concerns because of the open-labelled nature of the trials which may have impacted aspects of assessment. This would not have a bearing on 'harder' outcomes like mortality, OSFD or major bleeding.
d. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from a clinically unimportant harm to appreciable harm.
e. Not downgraded for indirectness in these outcomes (Thrombosis/Bleeding), as the direct anti-thrombotic effect of therapeutic anticoagulation form both Rivaroxaban and Heparins could be considered comparable.
1. Outcomes in the WHO mild category of patients
Explanations
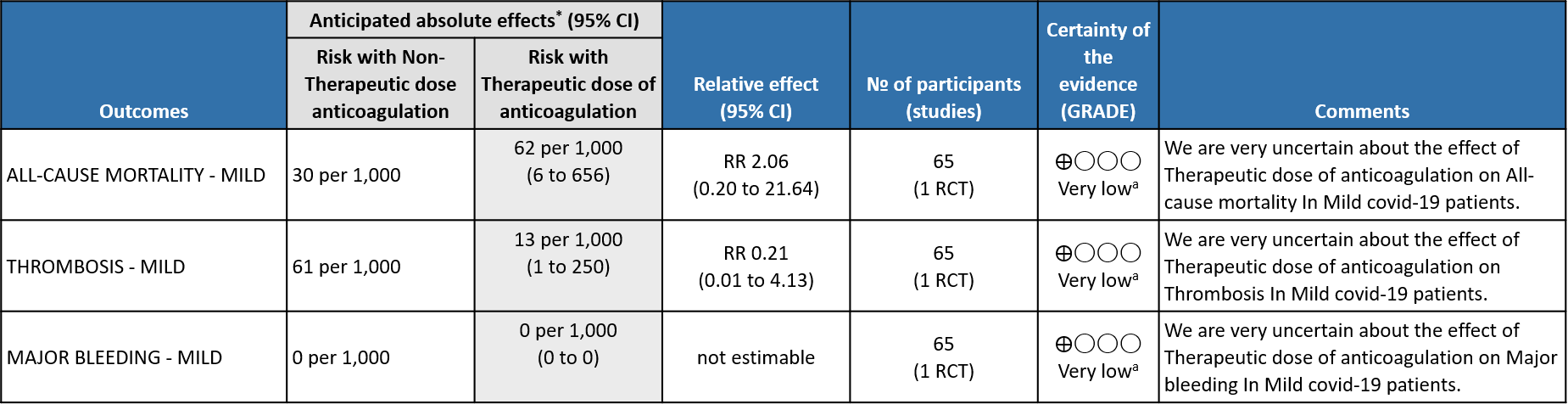
a. Not downgraded since risk of bias (RoB) assessment with RoB 2.0 tool scored 'some concerns' in only 1 domain of each of the studies, for this outcome. In the MPRCT4,5, Domain 2 was marked down for 'some concerns' in view of significant deviations in intended interventions in trial, which probably did not affect outcomes.
b. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from appreciable benefit to harm.
c. Downgraded by 1 level for serious risk of bias; RoB assessment with RoB 2.0 tool scored 'some concerns’. in 2 domains of the mpRCT4,5 Re Domain 2 see explanation (a). Domain 4 of RoB 2.0 tool was assessed to have some concerns because of the open-labelled nature of the trials which may have impacted aspects of assessment. This would not have a bearing on 'harder' outcomes like mortality, OSFD or major bleeding.
d. Not downgraded for indirectness in these outcomes (Thrombosis/Bleeding), as the direct anti-thrombotic effect of therapeutic anticoagulation form both Rivaroxaban and Heparins could be considered comparable.
e. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from clinically low harmful effect to appreciable harm.
f. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from clinically insignificant benefit to appreciable benefit.
2. Outcomes in the WHO moderate (on oxygen) and severe category of patients
Explanations
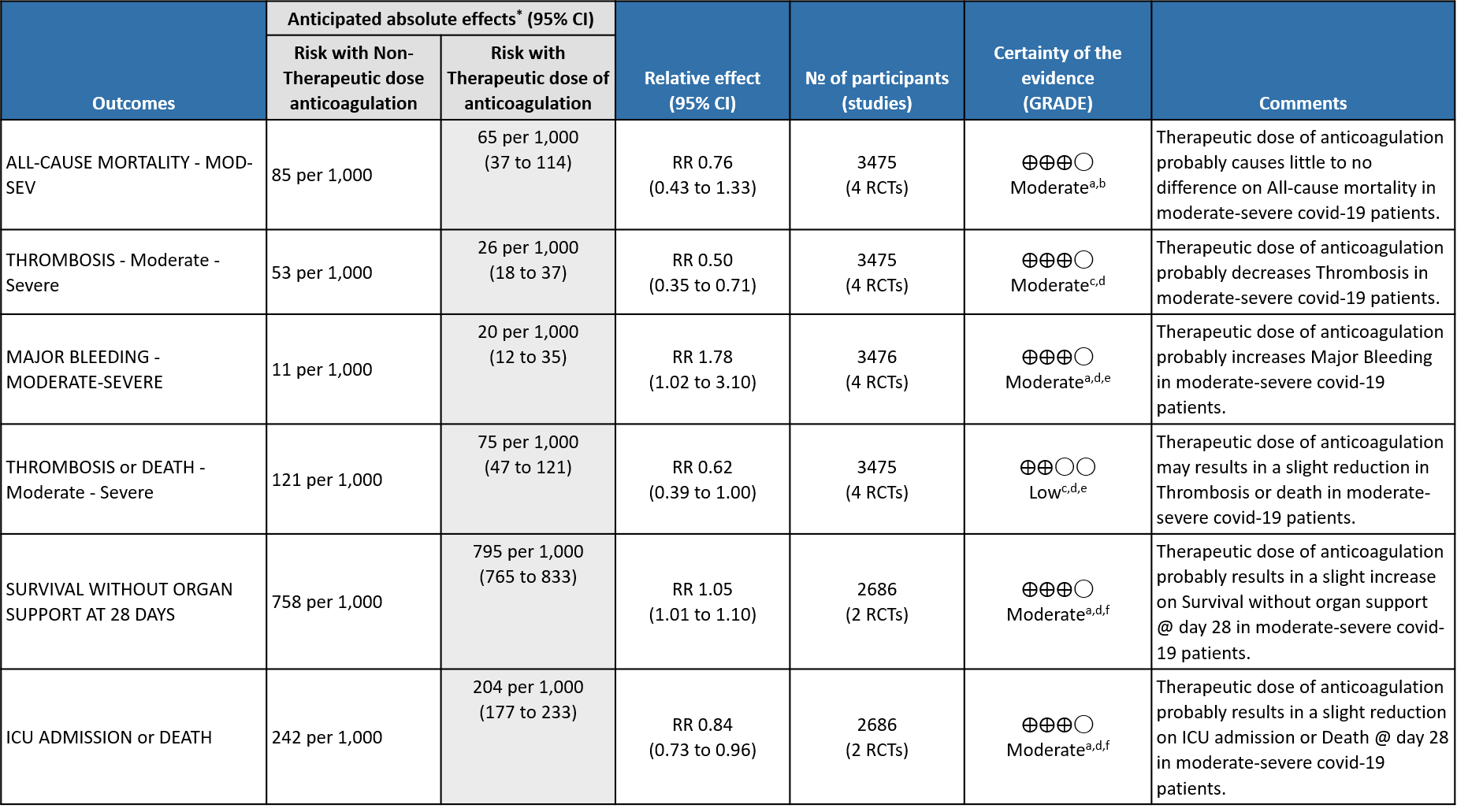
a. Not downgraded since risk of bias (RoB) assessment with RoB 2.0 tool scored 'some concerns' in only 1 domain of each of the studies, for this outcome. In the MPRCT4,5, Domain 2 was marked down for 'some concerns' in view of significant deviations in intended interventions in trial, which probably did not affect outcomes.
b. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from appreciable benefit to harm.
c. Downgraded by 1 level for serious risk of bias; RoB assessment with RoB 2.0 tool scored 'some concerns’. in 2 domains of the mpRCT4,5 Re Domain 2 see explanation (a). Domain 4 of RoB 2.0 tool was assessed to have some concerns because of the open-labelled nature of the trials which may have impacted aspects of assessment. This would not have a bearing on 'harder' outcomes like mortality, OSFD or major bleeding.
d. Not downgraded for indirectness in these outcomes (Thrombosis/Bleeding), as the direct anti-thrombotic effect of therapeutic anticoagulation form both Rivaroxaban and Heparins could be considered comparable.
e. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from clinically low harmful effect to appreciable harm.
f. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from clinically insignificant benefit to appreciable benefit.
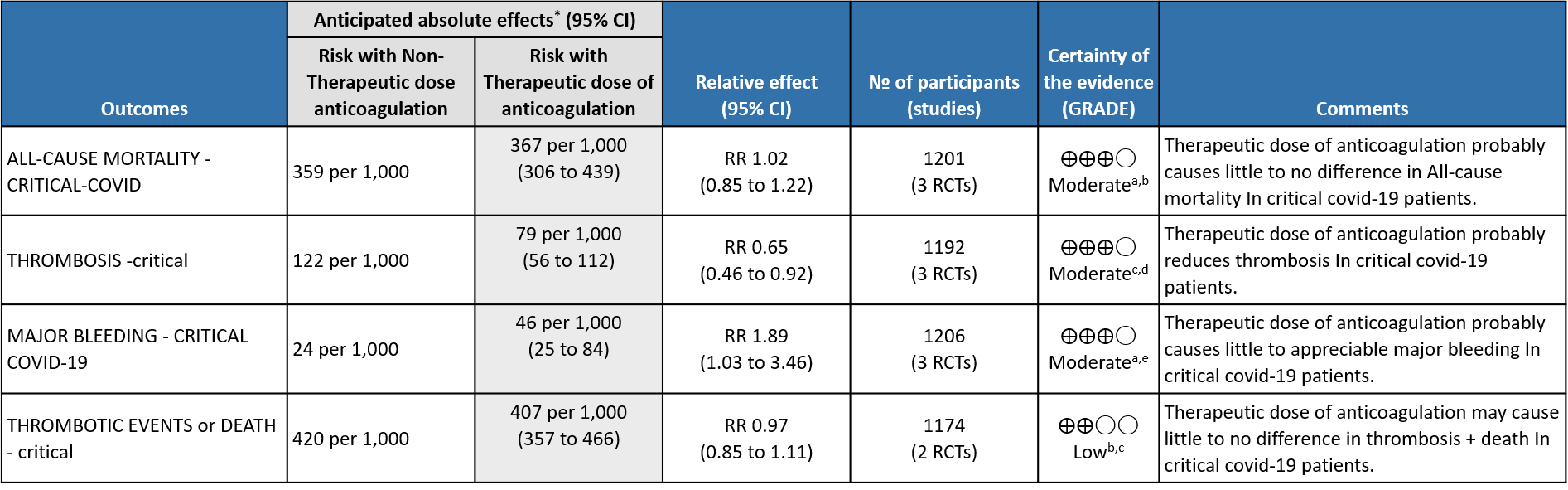
3. Outcomes in critical category of patients
Explanations
a. Not downgraded since risk of bias (RoB) assessment with RoB 2.0 tool scored 'some concerns' in only 1 domain of each of the studies, In the MPRCT4,5, Domain 2 was marked down for 'some concerns' in view of significant deviations in intended interventions in trial, which probably did not affect outcomes. In HESACOVID3 trial, Domain 5 was marked down for 'some concerns' in view of not enough information being provided to completely rule out risk of bias in selection of the reported result.
b. Downgraded by 1 level for serious imprecision; 95% CI ranges from appreciable benefit to harm.
c. Downgraded by 1 level for serious risk of bias; RoB assessment with RoB 2.0 tool scored 'some concerns' in 2 domains for mpRCT (Zarychanski et al4.), and in one domain for other trials, for measurement of this outcome. In the mpRCT (Zarychanski et al.)4, Domain 2 was marked down for 'some concerns' - see explanation a. Domain 4 was assessed to have 'some concerns' because of the open-labelled nature of the trials which may have impacted aspects of assessment of this outcome. In HESACOVID trial3, Domain 5 was marked down for 'some concerns' in view of not enough information being provided to completely rule out risk of bias in selection of the reported result.
d. Not downgraded for imprecision as, even at the upper 95% CI, the benefit was considered clinically significant.
e. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from clinically low harmful effect to appreciable harm.
Since December 2019, a worldwide pandemic labelled COVID-19, caused by SARS-CoV-2 virus has adversely impacted humanity in diverse ways. Clinical studies of hospitalized patients with SARS-CoV-2 initially showed flu-like symptoms, most commonly cough, sore throat, fever, myalgia, and fatigue at onset of COVID-19 illness, which could then develop into a viral pneumonia with varying severity 8. Abnormal coagulation profiles and thrombotic complications, both venous and arterial, are common among the hospitalized severe and critically ill patients 9, with pulmonary embolism being the most common site 10. Multiple autopsy reports show unprecedented pulmonary microvascular thrombosis and endothelial damage 11 which could be related to the direct viral cytopathic effect on the endothelial cells due to shared receptors with the alveolar cells 12. Other etiopathogenetic mechanisms include immune/cytokine mediated dysregulation of pro-coagulant & anti-fibrinolytic pathways.
Though hypercoagulability in COVID-19 has now been well-recognized, uncertainty still exists as to how best to manage clotting risk in these patients. In addition, an increased risk of hyper-coagulability leading to thrombotic events have been reported and recognized extensively in the media and among peers. There is a prevailing assumption that the delta variant in India may be contributing to an increased number of thrombotic events, however this needs to be systematically studied and documented. Over the past 2 year several guidance documents have recommended the use of anticoagulation in hospitalized patients with COVID-19 13–15. Most of these guidelines recommend the use of unfractionated heparin (UFH) or low molecular weight heparin (LMWH) but whether to use at a therapeutic or a non-therapeutic dose remains unclear.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, MEDLINE, Epistemonikos (COVID Living Overview of Evidence platform), and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language published from March 2020 up to 14th October 2021. We also reviewed reference lists of systematic reviews and included studies.
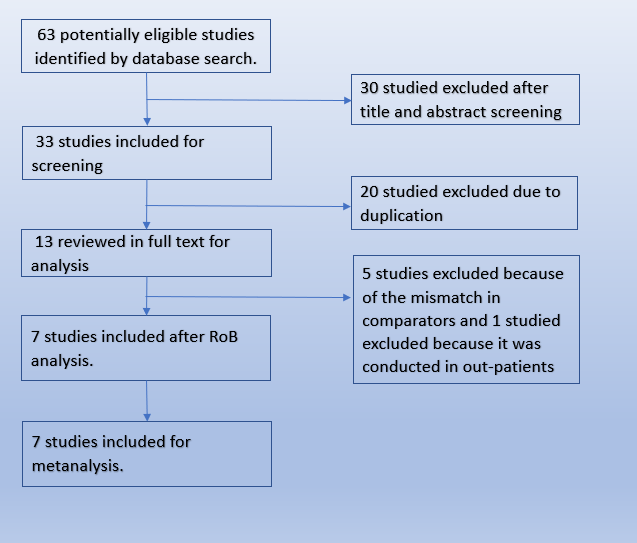
We searched the Pubmed database and found 63 potentially eligible records. After removing duplicates, and excluding studies that were not RCTs or did not involve the intended intervention, we found 7 trials that matched our PICO.
We extracted data for the following outcomes, pre-defined by the Expert Working Group:
- Critical (primary for this review):
- Mortality (all-cause) – at 21-30 days, or in-hospital
- Thrombotic events
- Important (secondary):
- Time to clinical improvement
- Organ support free days (OSFD): organ support defined as ventilator or dialysis or inotropic requirements.
- Survival without organ support at day 28
- Duration of hospitalization
- Bleeding events
Two reviewers (SS & JSJ) independently assessed eligibility of search results. One reviewer extracted data from each included study, and both assessed risk of bias using the Cochrane Risk of bias (RoB) v2.0 tool.
We used RevMan 5.4 to perform meta‐analysis using fixed & random‐effect models for outcomes where pooling of effect estimates was appropriate. We used risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). Since the guidelines were going to be specific to each severity category, we grouped studies as per their inclusion criteria into their severity categories and combined outcomes to provide pooled estimates. We used the I2 statistic to measure residual heterogeneity. We used GRADE methodology to assess the certainty in the evidence and documented this in a ‘Summary of findings’ table using GradeProGDT.
Varied dosing strategies are used for anticoagulation depending on indication, organ dysfunction, BMI and adverse drug reactions, based on available literature and package insert recommendations. For purposes of our guidelines in the expert working group meeting it was decided to broadly specify doses - low & intermediate doses of non-therapeutic anticoagulation as 1mg/kg IV once daily AND therapeutic dose anticoagulation as 1mg/kg twice daily.
| Product | Thromboprophylaxis dose | Therapeutic dose | |
| Low dose | Intermediate dose | ||
| Low Molecular Weight Heparin (LMWH) - Enoxaparin | 40 mg q24h
[BMI>40/Weight >120 Kg – dose increase to 40 mg q12h] |
1 mg/kg q24h | 1mg/kg q12h |
| Unfractionated Heparin (UFH) | 5000U q12h | 5000 U q8h
OR 7500 U q12h |
80 U/kg bolus followed by 18 U/kg/hour infusion
Targeting APTT of 55-75 seconds |
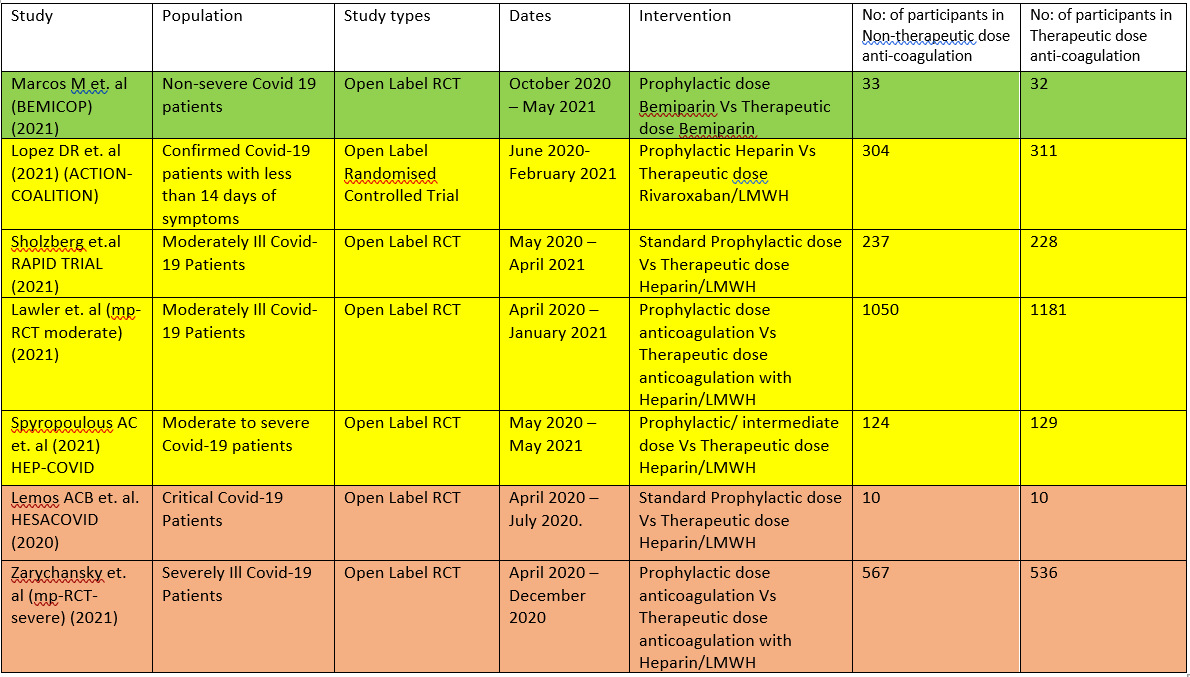
The 7 RCTS compare Therapeutic dose anticoagulation with non-therapeutic doses:
- HESACOVID 3:- compared prophylactic vs therapeutic anti-coagulation (20 participants).
- mpRCT(Critically ill) 4: Zarychanski et al. is a collaboration of 3 RCT platforms, ACTIV-4a, REMAP-CAP and ATTACC, looking specifically at critically ill patients (total of 1074 participants) – comparing therapeutic vs non-therapeutic anticoagulation.
- mpRCT(Non-critically ill) 5:- Lawler et al. is a collaboration of above mentioned 3 RCT platforms, looking specifically at non-critically ill hospitalized patients (total of 2291 participants) – comparing therapeutic vs non-therapeutic dose of anticoagulation.
- COALITION- ACTION 2: Looks specifically at the moderate-severe category of patients. In this trial, severe patients were administered LMWH, while moderately ill patients were administered Rivaroxaban, which is a directly acting Factor Xa inhibitor.
- RAPID Trial 6:- It compared prophylactic dose of anticoagulation Vs Therapeutic dose of anticoagulation in moderate to severe ill patients
- HEP-COVID Trial7 :- Therapeutic Vs. non-therapeutic dose of anticoagulation in moderate to critically ill patients.
- BEMICOP Trial 1:- Prophylactic Vs. Therapeutic dose of Bemiparin is compared in mild Covid-19 patients.
The critical outcomes that were available and extracted for analysis from these studies included
- All-cause mortality (21-30 days),
- Thrombosis,
- Organ support free days [i.e., surviving to hospital discharge with reduced need for ICU-level organ support, including invasive and non-invasive mechanical ventilation]
- Survival without organ support at 28 days
- Major bleeding events.
Though we are using the term non-therapeutic anticoagulation, the intent in all these trials was prophylaxis. Two different doses are being compared in each of these trials, therapeutic dose anticoagulation vs nontherapeutic dose (prophylactic or intermediate doses).
We included 7 trials1–7 with 4741 patients of which 1 trial did not contribute much data 3. The patients were compared across different pre specified COVID-19 severity groups, for the different outcomes as mentioned above (See summary of characteristics tables). The duration of administration of anticoagulation varied from a minimum of 96 hours to 14 days or till the patient got better.
We excluded 2 trials INSPIRATION 16 and Perepu17 et. al from our analysis as they are comparing prophylactic dose of anticoagulation with intermediate dose of anticoagulation with the intent here being “prophylaxis” rather than treatment.
The group of patients studied in the mpRCT (Critically ill) study, correlated directly with WHO severity criteria of critical category. The group of patients studied in the mpRCT (non-Critically ill) study, correlated with WHO severe category also overlapping with a few in the WHO moderate category. The population in the Hep-covid study 7 included “moderate to critical” category of patients. Hence, when performed a sub-group analysis we stratified data obtained from authors and analysed moderate to severe patients separately and critical patients separately.
Overall analysis: Using GRADE methodology, certainty of evidence is shown in Summary of Findings tables.
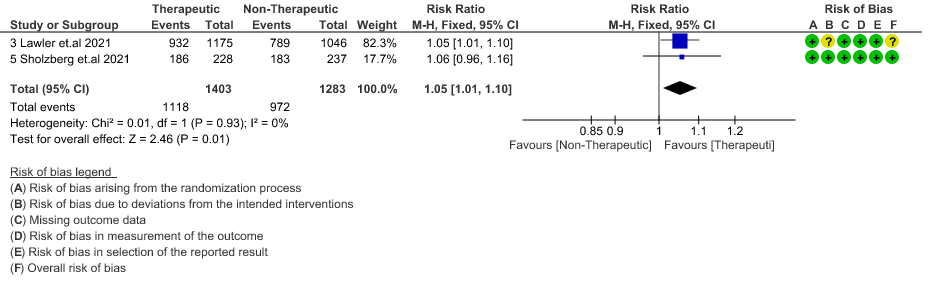
- All-cause Mortality- Moderate certainty of evidence in 7 RCTs1–7 with 4741 patients, revealed that therapeutic anticoagulation possibly results in little to no difference in all-cause mortality, compared with non-therapeutic anticoagulation (RR 0.90, 95 % CI=0.68 to 1.20).
- Thrombosis- Moderate certainty of evidence in 7 RCTs1–7 with 4732 patients, revealed that therapeutic anticoagulation probably reduces thrombosis, when compared with non-therapeutic anticoagulation (RR= 58, 95 % CI = 0.45 to 0.74).
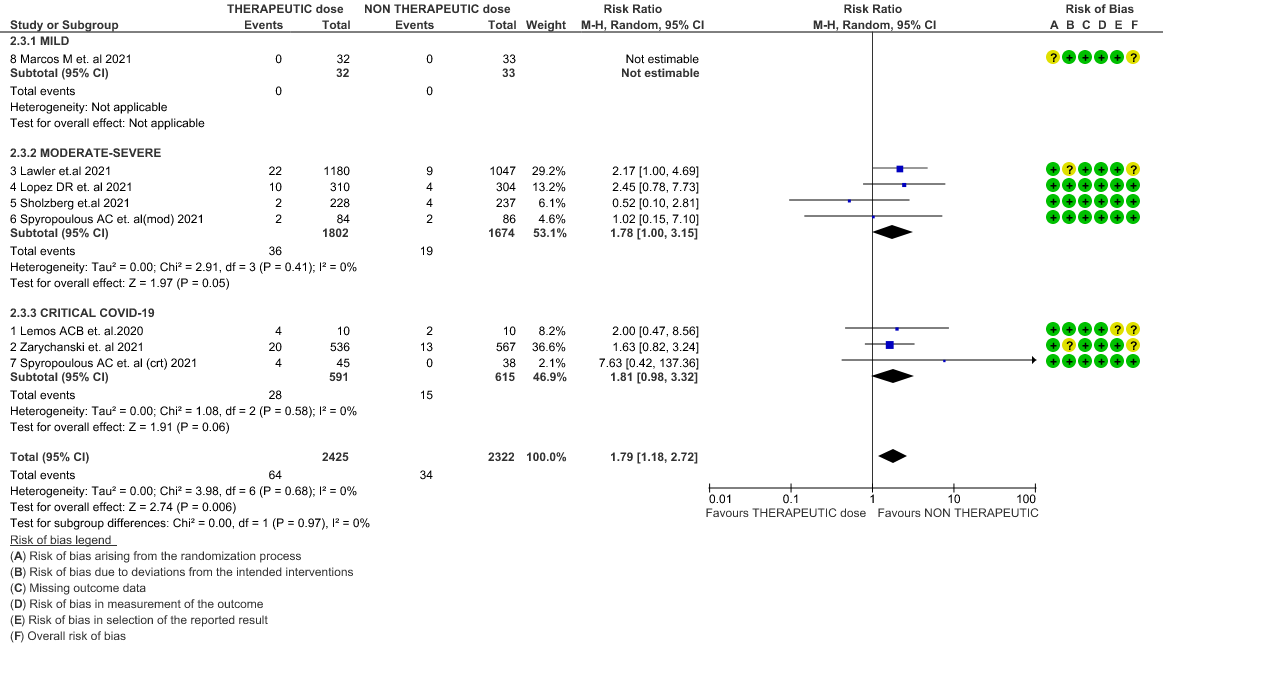
- Major bleeding- Moderate certainty of evidence in 7 RCTs 1–7with 4747 patients, revealed that therapeutic anticoagulation probably results in an increase in major bleeding, compared with non-therapeutic anticoagulation (RR 1.79, 95% CI = 1.18 to 2.72).
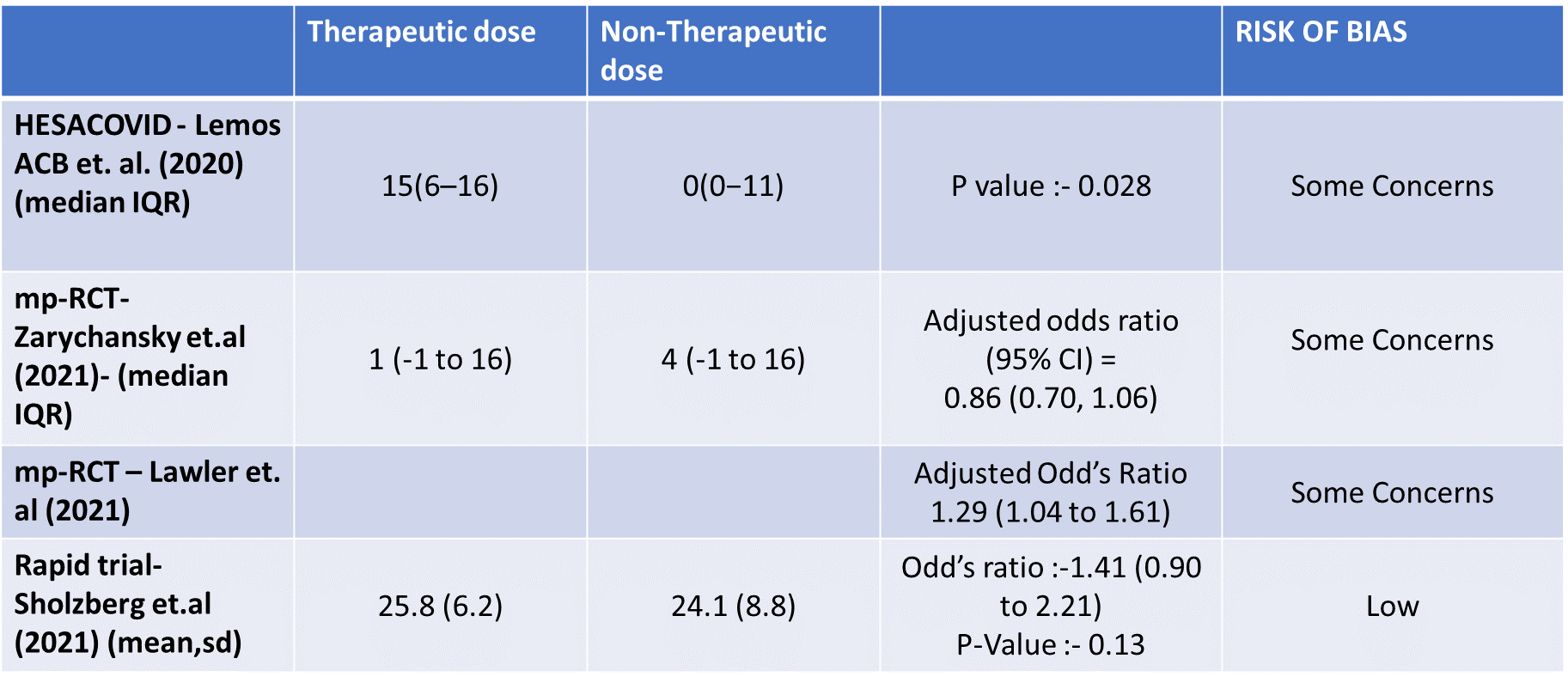
- Organ support free days:- Evidence for OSFD data was compiled from 4 studies 3–6 and this outcome was variably expressed as mean ±SD, median with IQR, Odds ratio or adjusted Odd’s ratio. As the data cannot be pooled, we have represented the data in a table and no meta-analysis was done for OSFD. 2 studies5,6 that included moderate-severely ill patients showed that therapeutic anticoagulation gives more organ support free days than non-therapeutic anti-coagulation. Critically ill category of patients had data available from 2 trial 1 trial with 1103 patients 4 with critical category of patients showed no difference in OSFD with therapeutic or non-therapeutic anti-coagulation and 1 trial with only 20 participants 3 with critically ill patients showed significant benefit in OSFD, but had only 20 participants.
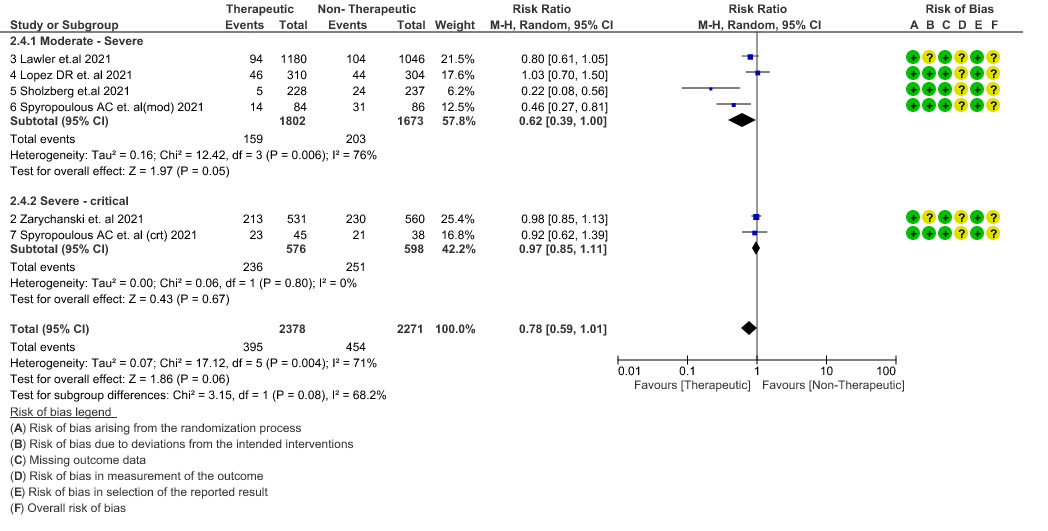
- Thrombosis or Death (composite outcome) :- Moderate certainty of evidence in 6 RCTs 2–7 with 4649 patients suggested that therapeutic anticoagulation probably reduces thrombosis or death in COVID-19 patients [RR : 0.78, 95% CI : 0.59 to 1.01].
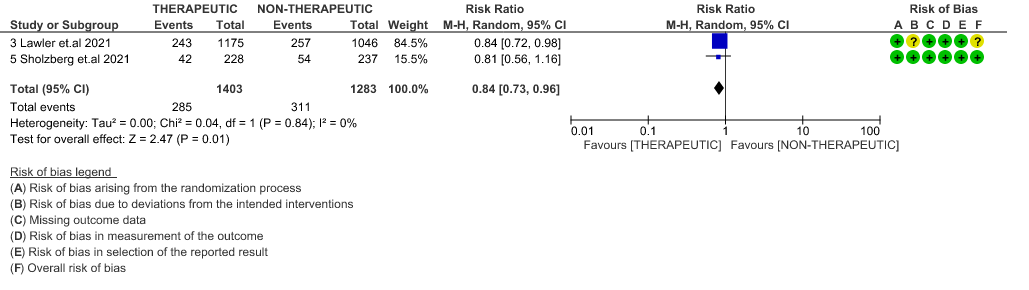
- ICU admission or death: - Moderate certainty of evidence in 2 RCTs5,6 with 2686 patients suggested that the therapeutic anti-coagulation probably reduces [RR : 0.84, 95% CI : 0.73 to 0.96] the need of ICU admission or death in moderate to severely ill patients.
Since guidelines are to provide recommendations for each severity strata, disaggregated data was also assessed to provide analysis to help formulate recommendations specific to each severity strata.
Analysis according to severity strata
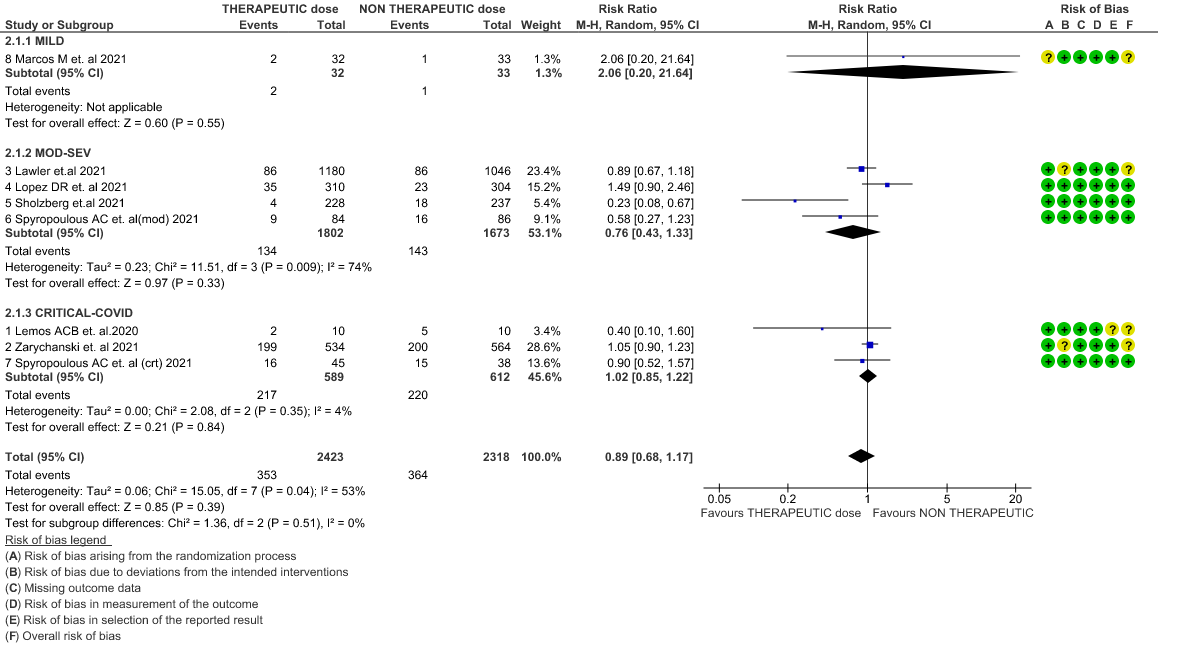
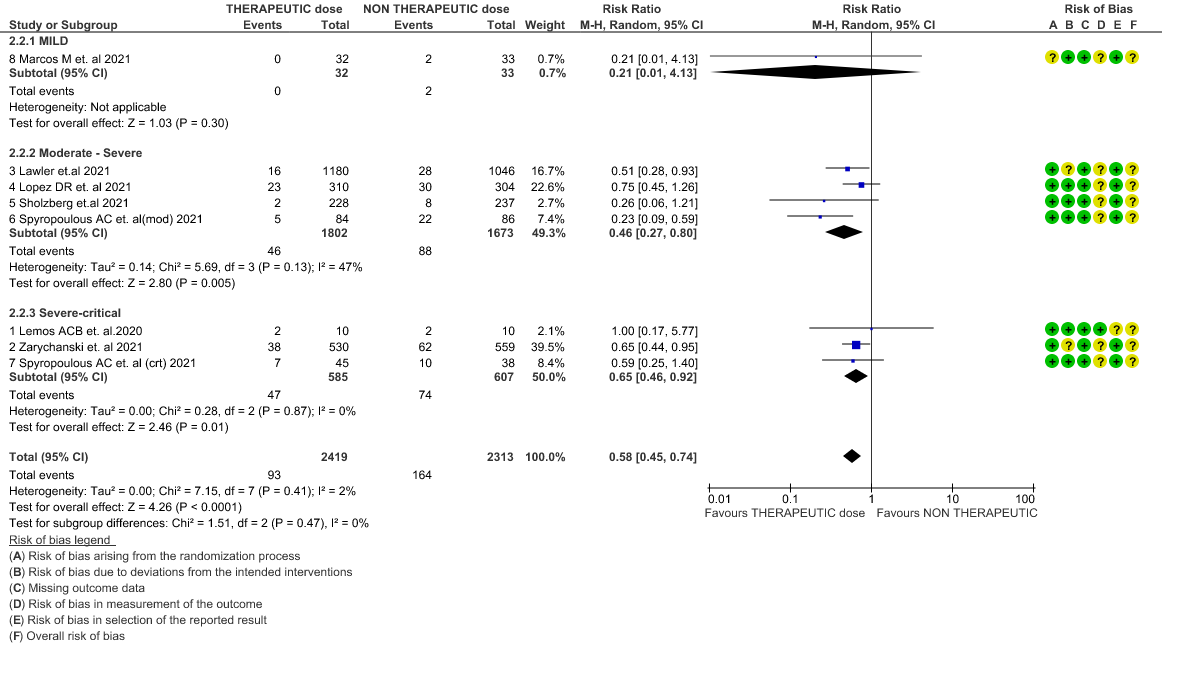
WHO Critical group: Moderate certainty evidence from 3 RCTs3,4,7 in 1201 patients showed that therapeutic anticoagulation results in little to no difference to all-cause mortality in critical COVID-19 illness (RR-1.02, 95 % CI = 0.85 to 1.22). However, moderate certainty evidence did show decreased thrombosis in patients (RR 0.65, 95 % CI = 0.46 to 0.92) but with an increase in bleeding (RR 1.89, 95 % CI = 1.03 to 3.46). In addition, evidence from 1 trial 4, with a larger group (1103) of patients, showed that therapeutic anticoagulation did not improve days free from organ support compared to prophylactic anticoagulation [Odds Ratio (OR) 0.86, 95% CI 0.70 to 1.06].
WHO Moderate/Severe group: Moderate certainty evidence from 4 RCTs2,5–7 in 3475 patients suggested that therapeutic dose of anticoagulation provided little to no mortality benefit [RR 0.76 (95% CI 0.43 to 1.33)] but did prevent thrombosis by 50% (RR 0.50, 95 % CI 0.35 to 0.71) with an increased risk of major bleeding (RR 1.78, 95 % CI 1.02 to 3.10).
WHO mild group : Very low certainty of evidence from 1 RCT1 suggests that the effect of therapeutic anticoagulation was uncertain on all-cause mortality, thrombosis and major bleeding, in mild Covid-19 patients. One trial Activ-4b was excluded from the analysis as the study was conducted in an out-patient setting, which is not consistent with the definition laid out in our PICO. 18
Therapeutic vs Non-Therapeutic Dose of Anti-Coagulation
1. All-cause mortality (21-30 days)
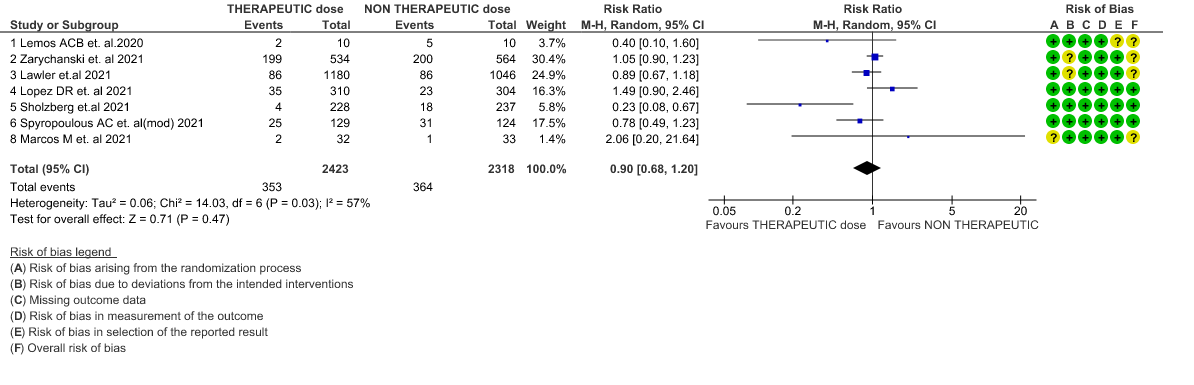
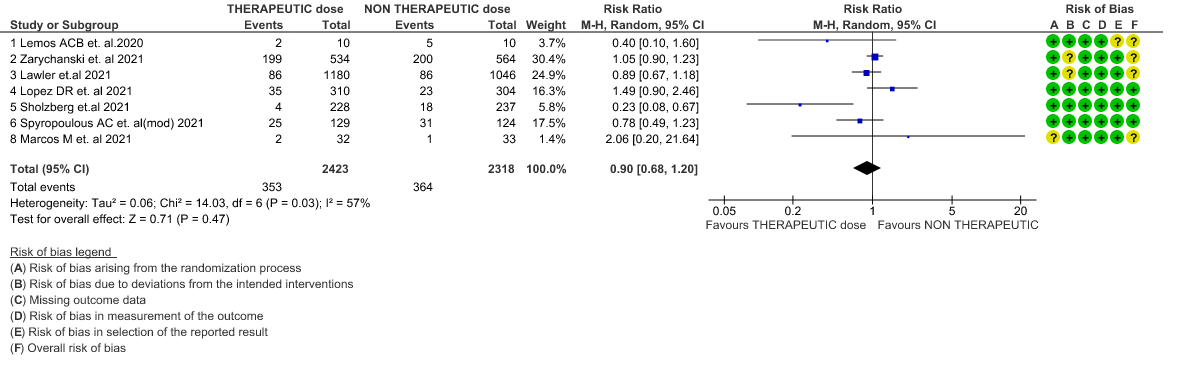
2. All-cause mortality (21-30 days) (stratified according to the severity of disease)
3. Thrombosis
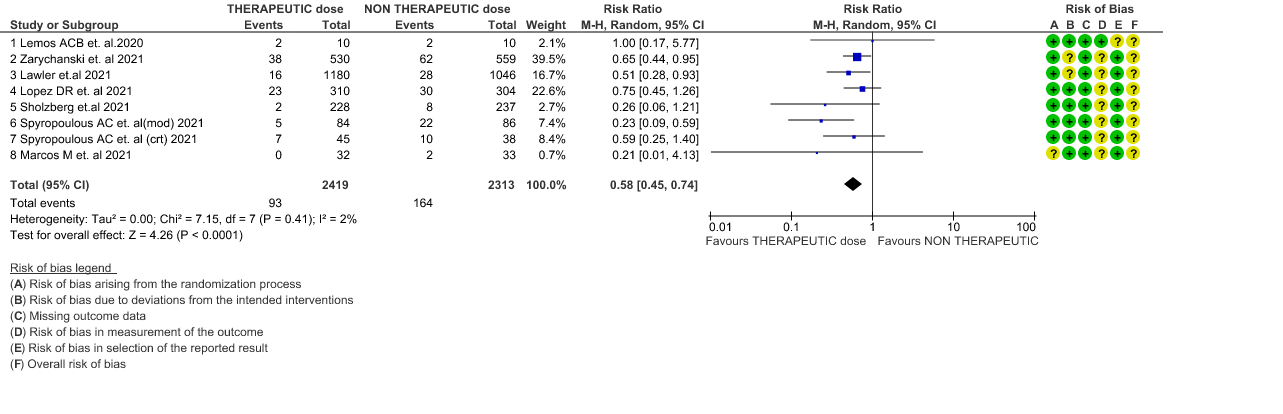
4. Thrombosis (stratified according to the severity of the disease)
5. Major Bleeding
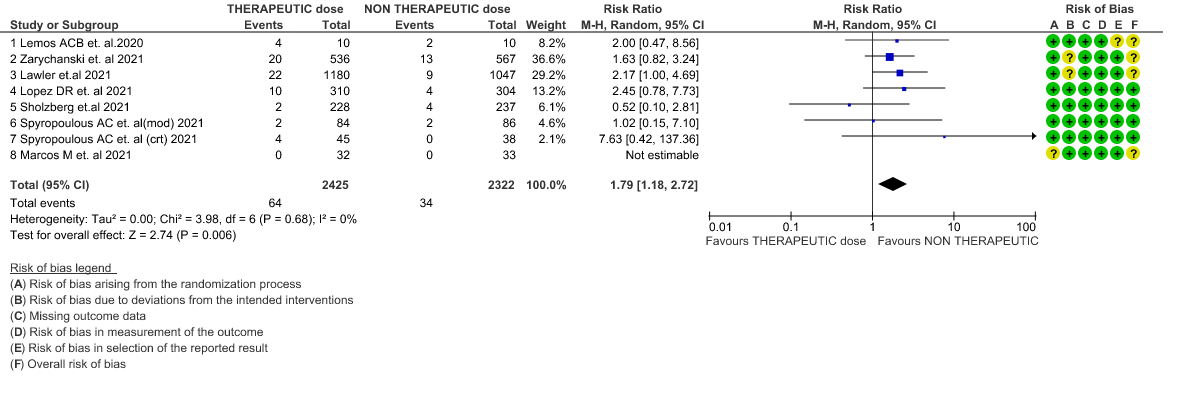
6. Major Bleeding (stratified according to the severity of the disease)
7. Composite outcome of Thrombosis or Death
8. Organ Support free days in median (IQR)
9. Composite outcome of ICU admission or Death
10. Survival Without Organ Support at Day 28