Explanations
a. “Continuous infusion of sedative/analgesic drugs was administered to 20 patients (37%) in the helmet group and in 10 patients (18%) in the high-flow nasal oxygen group. Over the initial 48 hours of treatment, the mean (SD) Fio2 used in the helmet and high-flow nasal oxygen groups were 0.54 (0.12) and 0.58 (0.9), respectively. As per clinical decision, 32 patients (60%) in the high-flow nasal oxygen group vs 0 in the helmet group underwent prone position.” This would make the RoB2 high risk for domain 2, and therefore overall.
b. Downgraded by 2 levels for very serious indirectness; The data were from 1 trial in 1 high income country, Median severity of ARDS was higher than the usual severity at which NIV would be started, Helmets were used for NIV and the pressure settings were higher than usual maintenance settings used in most ICUs in India. Weaning off NIV in the first arm involved using HFNO, which is not followed in many ICUs in India.
c. Downgraded by 2 levels for imprecision due to few events and CI including important benefit and important harm.
d. Downgraded by 1 level for imprecision as CI crosses the clinical decision threshold between recommending and not recommending treatment.
e. Downgraded by 1 level for imprecision due to CI including no difference and important benefit.
f. Downgraded by 1 level for imprecision due to CI including important benefit and important harm with NIV.
The COVID-19 pandemic in India has caused considerable strain on the oxygen supply chain and ventilator manufacturers. Several treatment modalities have been tried for the management of respiratory failure and respiratory distress. Since the mortality rate was found to be higher in those who require invasive ventilation, appropriate management with non-invasive ventilation in the early phase of ARDS is of utmost importance (2). Continuous Positive Airway Pressure therapy (CPAP), Bilevel Positive Airway Pressure therapy (BPAP), Non-invasive ventilation mode on the ventilator and High Flow Nasal Cannula/Oxygen (HFNC/HFNO) are common non-invasive Respiratory Support (NRS), devices and are being used in various centres in India and abroad (3). Various interfaces for these ventilators have also been tried, such as helmet, oronasal mask and face mask(4).
The NIH guidelines (December 2020) and the Surviving Sepsis guidelines (March 2020) recommend the use of HFNO over NIV in cases of persistent respiratory failure despite conventional oxygen therapy (5,6). Their conclusion was based on the following two non-COVID data studies. In non-COVID acute hypoxemic respiratory failure, Jean-Pierre Frat JP et al. found that patients on HFNO had more ventilator-free days, lower 90-day mortality, and lower intubation rates than patients on NIV (7). A meta-analysis by Yue-Nan Ni et al. looked at the effectiveness of different oxygenation methods before intubation. They found that HFNO had a reduced rate of intubation and ICU mortality compared to NIV in patients with acute respiratory failure (8). Most other guidelines, including WHO, ERS, and the Australian National Guidelines, recommend either NIV or HFNO based on availability. Hence an equipoise exists as to whether NIV or HFNO would be the preferred initial ventilatory strategy in COVID19 ARDS. Each of these strategies have implications with regard to costs, specialised devices or equipment and optimum utilisation of oxygen as well as trained personnel to administer these modalities.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com for studies on this topic published up to 25 June 2021. Very few studies were found on this topic. Only 1 randomized control trial was available but we found many observational studies and a review article. Hence, we also included the latest Cochrane systematic review on Non-COVID acute hypoxic respiratory failure and the two studies (1 RCT and 1 Systematic review) based on which NIH guidelines were made.
We extracted data for the following outcomes, predefined by the Expert Working Group:
- Critical (primary for this review):
- All-cause mortality
- Need for intubation
- Important (secondary):
- Time to clinical improvement (WHO ordinal scale or other definitions)
- Length of stay in hospital
- Length of stay in critical care
- Duration of days free from invasive ventilation
- Adverse events:
- All
- Serious
- Nosocomial infections
- Pneumothorax/pneumomediastinum
- Discontinuation due to inability to tolerate therapy
Two reviewers independently assessed the eligibility of search results. One reviewer extracted data from the RCT study and assessed the risk of bias using the Cochrane Risk of bias (RoB) v2.0 tool. The two Non-COVID meta-analyses were evaluated with AMSTAR2. Later the whole team reviewed the ROB and AMSTAR and corrected discrepancies if any.
We used RevMan 5.4 to perform meta‐analysis using a random‐effects model for outcomes where pooling of effect estimates was appropriate. We used risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). We used GRADE methodology to assess the certainty in the evidence and documented this in a 'Summary of findings table using GradeProGDT.
On advice from the methodology committee, we also summarised data from the non COVID-19 systematic reviews and non-randomized observational studies.
We looked at one RCT, 3 systematic reviews and 7 obeservational studies. (7–16) The RCT was a comparison between Helmet NIV vs HFNO. A conventional face mask NIV should be better than Helmet NIV due to less leak and better delivery of the pressure support. The RCT had a high risk of bias due to deviation from the intended interventions. More people in the HFNO group received proning and the need for IV sedation/analgesia was more in the NIV group. We reviwed information from three non-COVID ARDS related meta-analysis due to lack of data from COVID patients.
Out expert working group classified all cause mortality and need for intubation as critical outcomes. Time to clinical improvement, length of stay in hospital and ICU, duration of days free from invasive ventilation, nosocomial infections and barotrauma were other important outcomes considered. However we had only one RCT addressing most of the above outcomes.
The HENIVOT Trial
The HENIVOT Trial was the only RCT on this topic published so far. (8) It was a multicentre RCT done in 4 ICUs in Italy. The study compared Helmet NIV with high pressures of PS 10-12 cm of H20 and PEEP of 10-12 cm of H2O against HFNO 60L/min for at least two days. The patients in both arms were comparable and had no risk of bias at randomization. The NIV arm patients received HFNO after two days once they met predefined criteria for improvement. There was deviation of protocol on account of sedation/analgesia infusion (more in NIV) and proning (more in HFNO) compared to the other arm. Proning is an independent confounding factor for improvement and may tilt the evidence in favour of HFNO.
Critical outcomes
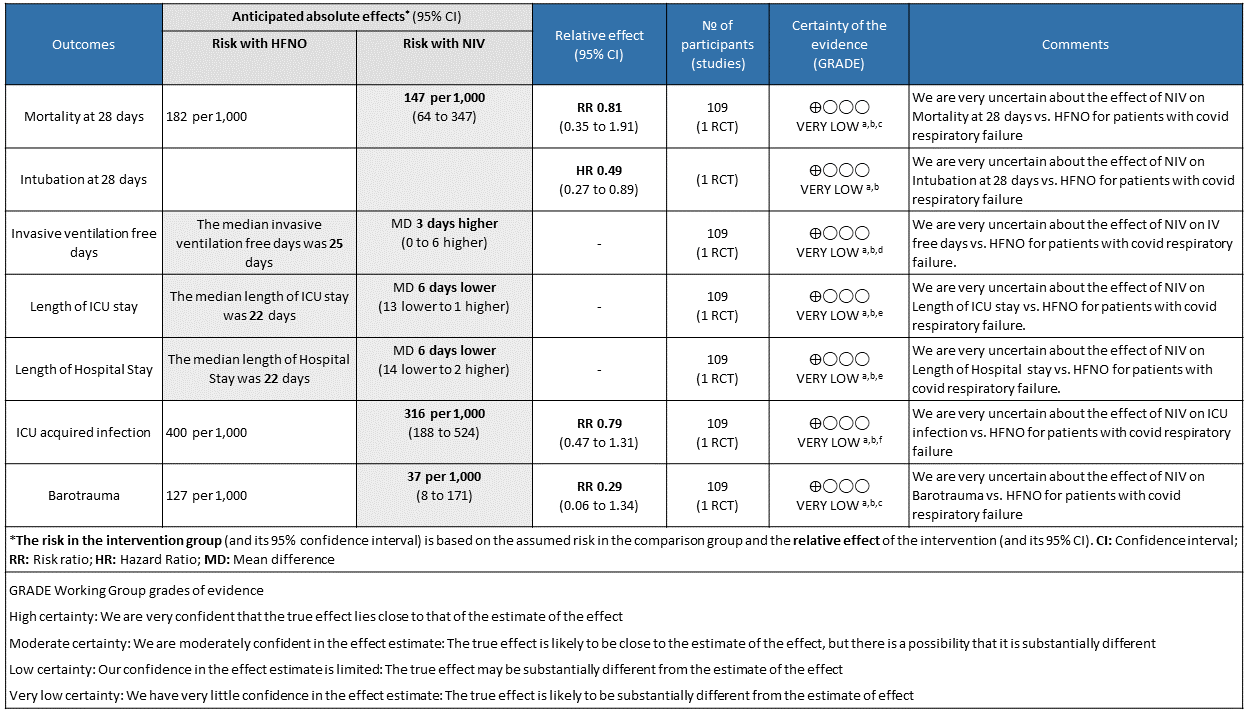
a. All-cause mortality at 28 days
Very low certainty of evidence revealed that there was an uncertain effect of NIV over HFNO for the outcome of all-cause mortality at day 28; RR=0.81 (95% CI 0.35-1.81). The in-hospital mortality was 13 (24%) in the NIV arm compared to 14 (25%) in the HFNO arm, with an absolute difference of 1 death. This was not considered statistically or clinically different.
b. Need for intubation.
Very low certainty evidence revealed that intubations were lower (intubation at 28 days) in the Helmet NIV group (28%) compared to HFNO (51%), HR = 0.49 (95% CI, 0.27-0.89 and this was statistically significant with a p value = 0.02).
Important Outcomes
a. Length of stay in hospital
Very low certainty of evidence revealed that the length of stay in hospital was lower in the NIV group with a mean difference of 6 days (14 lower to 2 higher). In addition, median (IQR) was 21 (14 to 30) in the NIV group as compared to the HFNO group 22 (13 to 44) and seemed that the median was not statistically or clinically significant.
b. Length of stay in critical care
Very low certainty of evidence revealed a very uncertain effect of NIV as compared to HFNO with regard to ICU stay but the mean difference was 6 days (1-13 days). When expressed as median (IQR), the length of stay in ICU was lower in the NIV group 9 (4 to 17) [compared to the HFNO group 10 (5 to 23), but was not statistically or clinically significant.
c. Duration of days free from invasive ventilation
Very low certainty evidence revealed an uncertain effect of NIV vs HFNO with regard to invasive ventilation free days mean difference = 3 days (0-6 days). Invasive ventilation free days at 28 days was higher in the NIV group 28 days (13 to 28) [median (IQR), d] compared to the HFNO group 25 days (4 to 28). This mean difference of 3 days was both clinically and statistically significant (p = 0.04)
d. Nosocomial infections
Very low certainty of evidence revealed an uncertain effect of NIV vs HFNO in patients with COVID-19 ARDS; RR = 0.79 (0.47-1.31). Numerically 22 (40%) patients in the HFNO group acquired nosocomial infections compared to the NIV group 17 (31%), but this was not statistically significant.
e. Pneumothorax/pneumomediastinum
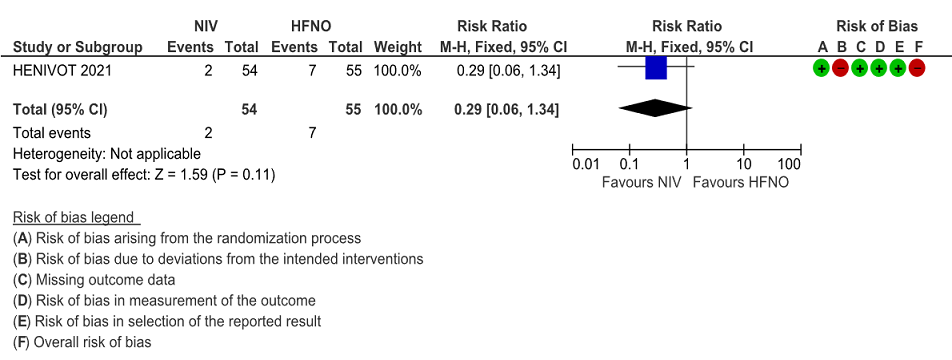
Very low certainty of evidence revealed that barotrauma was not significantly different between NIV (4%) and HFNO (13%) with RR=0.29(95% CI 0.06-1.34). When subcutaneous emphysema was evaluated, it was slightly more in the HFNO group [0% NIV vs 9% HFNO, p= 0.06].
This suggested that NIV via helmet with high pressure (PS) or Positive end expiratory pressure (PEEP) was better than HFNO at 60L/min in reducing intubation rate and ventilator-free days in moderate to severe ARDS.
In conclusion, NIV was found comparable to HFNO in terms of mortality, hospital stay and adverse events. Though intubations and invasive ventilation free days were secondary outcomes in the HENIVOT trial, they were found to be adequately powered on retrospective calculations. This suggested that NIV via helmet with high pressure (PS) or Positive end expiratory pressure (PEEP) was superior to HFNO at 60L/min in reducing intubation rate and ventilator-free days in moderate to severe ARDS. However, the major limitation with this trial was that patients in the NIV arm received NIV alone for only 48 hours; after which they also received HFNO in addition with the outcomes being recorded at 28 days. Hence it was difficult to assess whether the outcomes in terms of benefits/harm reported in the NIV arm were related to NIV or the NIV followed by additional HFNO.
1. Non-COVID Systematic reviews
Two Non-COVID-19 systematic reviews were considered due to the shortage of literature comparing these two modalities in the COVID-19 era(2,10). Lewis SR et al. included RCTs up to April 2020 that looked at NIV vs HFNO in non-COVID-19 ARDS (10). This Cochrane review also included patients who were initiated on NIV/HFNO after being extubated from invasive mechanical ventilation. They found no significant difference between NIV and HFNO in terms of escalation of therapy, intubation and in-hospital mortality. Respiratory infection was also no different in each of type of respiratory support group or HFNO groups RR 0.51 (0.17 to 1.52). There was a subgroup analysis for treatment failure in patients who had no prior intubations vs those previously intubated, which also showed no difference between high flow nasal oxygen vs non-invasive respiratory support which included non-invasive positive pressure ventilation (NIPPV), continuous positive airway pressure (CPAP) and bilevel positive airway pressure (biPAP) in both the groups. Ferreyro BL et al. performed a network meta-analysis, that compared conventional oxygen therapy, facemask NIV, helmet NIV and HFNO(11). This non-COVID-19 ARDS study showed no difference between Face mask NIV vs HFNO in terms of all-cause mortality and intubation.
2. Non-randomized observational studies.
Crimi C et al. analyzed observational studies on COVID and Non-invasive respiratory support published till April 2020. The pooled statistics showed that the overall failure rate of NRS varied between 52% to 92%. The mean utilization rate was 31% for HFNO and 30% for NIV across the studies. There were 39 observational studies identified in our literature search, of which 14 had data for our predefined outcomes as per our PICO. Six of these studies compared HFNO and NIV to some extent in certain aspects, but they were non-randomized trials. The outcomes of interest from these studies are tabulated as follows. A direct comparison is not possible as the NIV/HFNO protocols used in these centres may vary widely. We were also unable to determine which interface was used to deliver cPAP or NIV in most studies. Some centres which lack experienced manpower in running NIVs preferred using HFNO. Timing and criteria of intubation also varied between various centres. On closer observation of the table, despite all the above mentioned drawbacks, NIV and CPAP are very much comparable to each other in terms of intubation and mortality.
Table 1 Non-randomized observational Studies
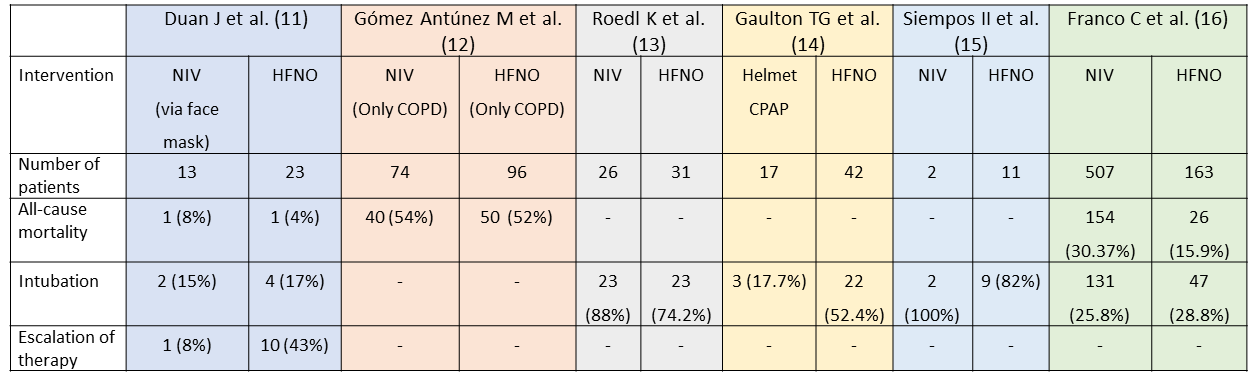
Figure 1: Mortality at 28 days
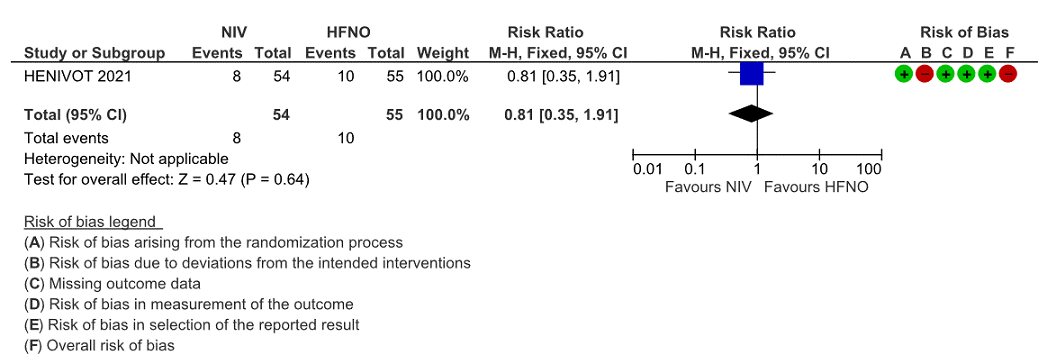
Figure 2: Intubation at 28 days
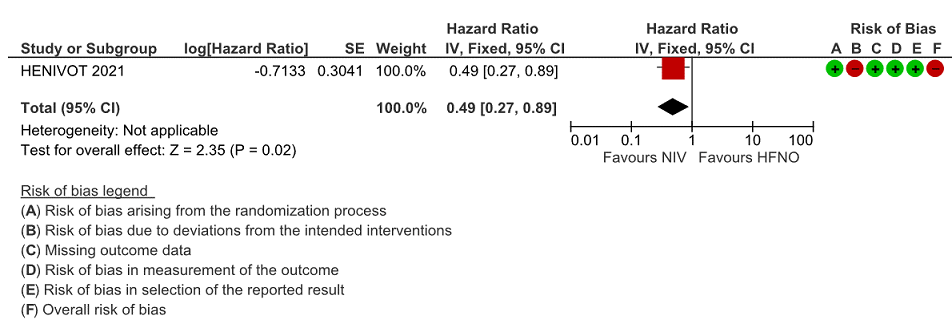
Figure 3: Intubation at 4, 8 and 28 days
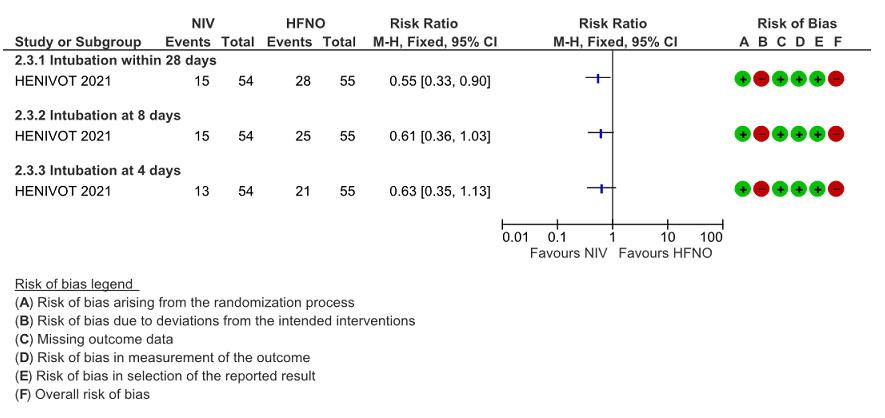
Figure 4: Invasive ventilation free days
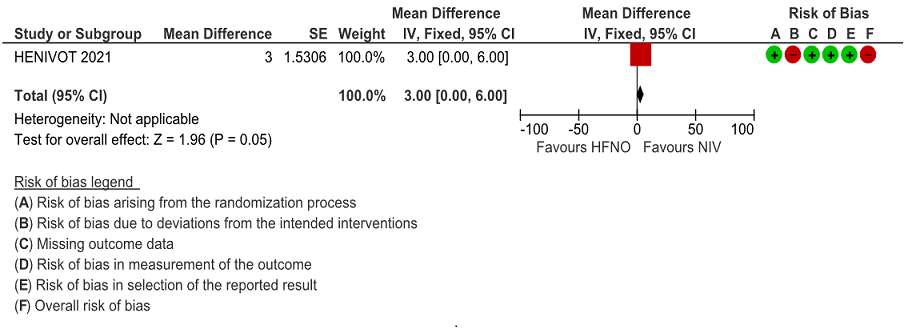
Figure 5: Length of ICU stay
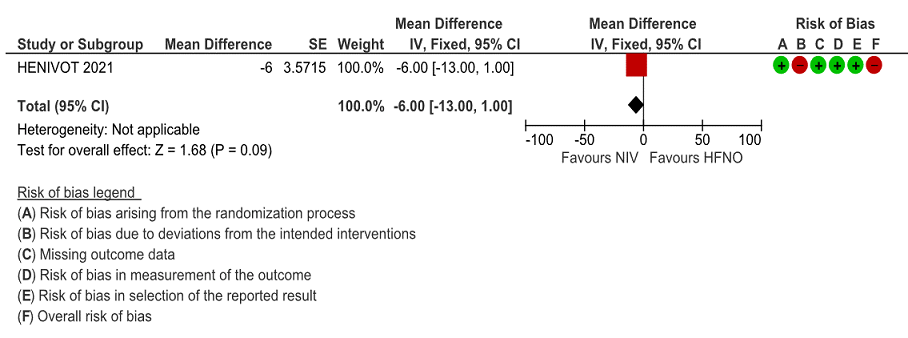
Figure 6: Length of Hospital Stay
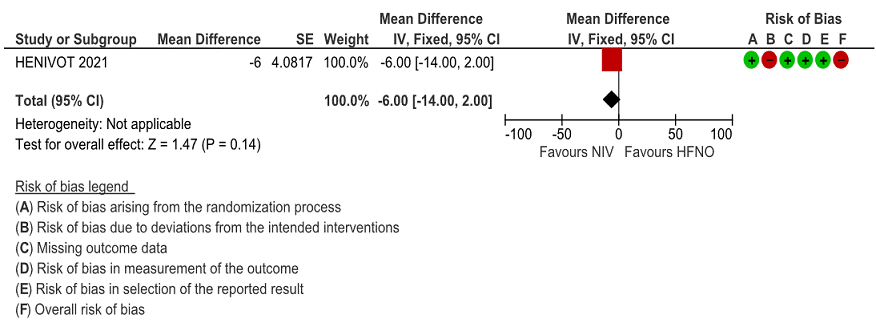
Figure 7: ICU Acquired infection
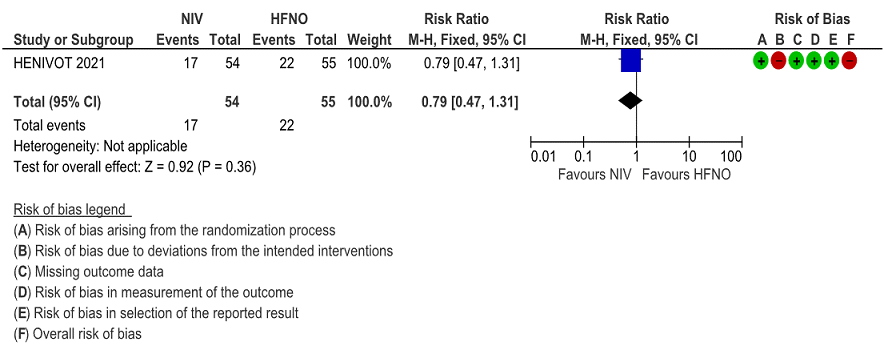
Figure 8: Barotrauma