Explanations:
a. Bernal,Bulter C, Caraco Yosef, Khoo, Thippabotta
b. Downgraded by one level for serious indirectness as the study population includes unvaccinated participants which does not reflect the current scenario
c. Downgraded by two level for serious imprecision as the lower bound of the 95% CI includes appreciable benefit and the upper limit includes harm
d. Aribas, Bernal, Bulter C, Caraco Yosef, Fischer
e. Downgraded by one level for serious imprecision as the upper CI limit represents harm and the event is very rare
f. Bernal, Bulter C, Fischer, Khoo, Thippabotta, Zou R
g. Downgraded by one level for inconsistency as we identified substantial heterogeneity (I2=63%)
h. Bernal
i. Downgraded by one level for serious imprecision: lower confidence interval, bound represents a mild benefit from Molnupiravir whereas the upper bound includes harm
j. Aribas, Bernal, Caraco Yosef, Fischer, Khoo, Thippabotta, Zou R
k. Downgraded by one level for serious imprecision as the lower bound of the 95% CI includes appreciable benefit and the upper limit includes harm
l. Aribas, Bernal, Buter C, Caraco Yosef, Fischer, Khoo, Kumarasamy, Thippabotta, Zou R
Molnupiravir was the first oral antiviral treatment developed specifically for COVID-19 developed by Merck, Sharp and Dohme and Ridgeback Therapeutics. Molnupiravir is a newer orally bioavailable prodrug of Emory Institute of Drug Development-2801 [EIDD-2801]/MK-4482), which is the synthetic ribonucleoside derivative N4-hydroxycytidine and ribonucleoside analog, with potential antiviral activity against a variety of RNA viruses recently been tested for COVID-19. The TP form of EIDD-1931 is incorporated into RNA and inhibits the action of viral RNA-dependent RNA polymerase. This results in the termination of RNA transcription, decreases viral RNA production, and viral RNA replication2. In November 2021, the UK authorised Molnupiravir for use in people who have mild to moderate COVID-19 and at least one risk factor (such as obesity, older age, diabetes mellitus or heart disease) for developing severe illness3. The FDA granted emergency use authorization for the treatment of mild COVID-19 infection in December 20214. With the advent of different covid variants, the monoclonal antibodies which were useful antivirals in mildly symptomatic or asymptomatic individuals who were at high risk of severe COVID-19 infection rapidly became ineffective5. Hence oral options like molnupiravir and Nirmatrelvir/ritonavir became attractive options. However, controversy remains regarding the utility of Molnupiravir with widespread vaccination globally thus reducing risk of severe disease in high risk patients. The drug though invented at Emory University was later acquired by Ridgeback therapeutics in partnership with Merck & Co, USA. Molnupiravir was initially developed as a possible treatment for influenza viruses, encephalitic alphaviruses like Venezuelan, Eastern and Western equine encephalitic viruses due to its significant inhibitory effect in cell cultures6. On December 23rd 2021 FDA gave emergency use authorization for the treatment of mild-to-moderate coronavirus disease (COVID-19) in adults (>18yrs) with who are at high risk for progression to severe COVID-19, including hospitalization or death, and for whom alternative COVID-19 treatment options were are not accessible or clinically appropriate. An in-vitro study by Zhou et al has suggested that the drug, though intended to disrupt only viral RNA, could also incorporate into and cause mutations in human DNA and potentially “contribute to the development of cancer”. Molnupiravir metabolite the ribonucleoside analog β-D-N4 -hydroxycytidine (NHC), is a potent mutagen.
However, even with the advent of new strains with immune escape, Molnupiravir retains its activity against the VOCs Alpha, GHB-03021/2020* and Omicron, making this a possible therapeutic option. This review aims to update the evidence from randomized clinical trials of Molnupiravir for treatment of COVID-19, for any duration, which could guide clinicians and researchers regarding the appropriate use of this drug.
We searched PubMed, Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language. We also reviewed reference lists of systematic reviews and included studies. We performed all searches up to 01st March 2023.
We searched the above databases and found 25 records. After removing duplicates and excluding studies that did not match our PICO question, 9 RCTs were included in the review.
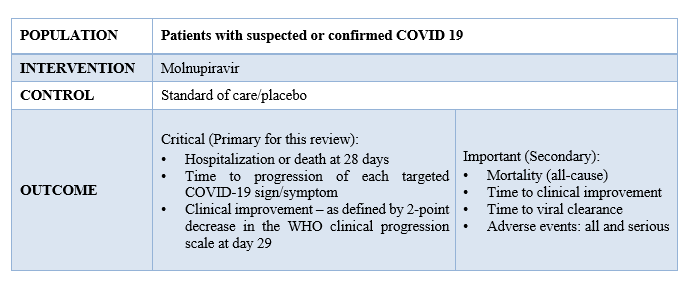
We extracted data for the following outcomes, pre-defined by the Expert Working Group:
PICO:
Two reviewers independently assessed eligibility of studies from the search results using the online Rayyan tool. Data extraction was performed by one reviewer, and checked by another, using a piloted data extraction tool. Risk of bias (RoB) was assessed using the Cochrane RoB v2.0 tool, with review by a second reviewer and compared with covid.nma database. If there was a difference in more than one domain it was assessed by a third independent author. We used risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). We performed a meta-analysis if outcomes were measured and reported in a similar way for the populations in each trial using random-effects models with the assumption of substantial clinical heterogeneity and the I2 test to calculate residual statistical heterogeneity, using Review Manager (RevMan) v5.4. Evidence would then be synthesized and reviewed by the Methodology Committee. We considered use of fixed effects model if we found that adequate weightage was not given to bigger trials on advice from the methodology committee. We used GRADE methodology to prepare summary of findings tables on GRADEPro GDT.
We found 9 RCTs that matched the PICO question as pre-defined by the expert working group.
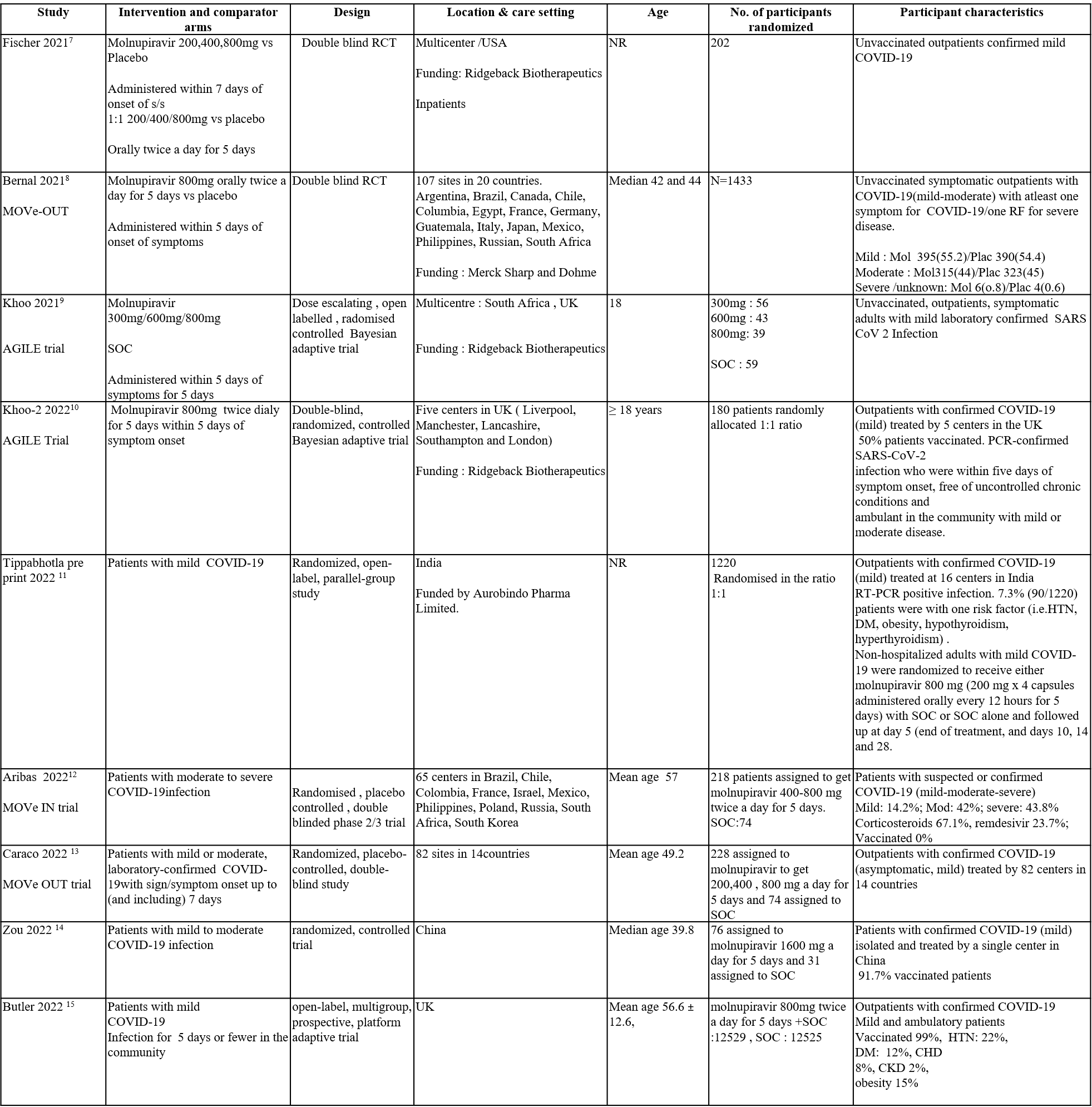
Apart from the 3 trials we included in the previous recommendation, we have included 6 more trials for the evidence update. The trials included a total of 29725 participants, all of whom were adults. Three trials were a multicenter trials involving many countries like Brazil, Chile, Colombia, France, Israel, Mexico, Philippines, Poland, Russia, South Africa, and South Korea. Two trials reported from UK, one from India and one from china. Four trials included unvaccinated patients and three trials had 50-60% of population, vaccinated. One trial had 99% of its population vaccinated. Four trials compared Molnupiravir with placebo whereas the other five trials compared Molnupiravir with standard of care.
Each trial and its results are described below, characteristics of the trials are summarized in the Summary of characteristics of included trials table. Risk of bias for each outcome as per domain in each trial is displayed alongside the forest plots below. The following comparisons were investigated in the trials (we compared outcomes for arms randomized to Molnupiravir vs. standard of care or placebo).
- Six trials compared Molnupiravir with placebo (2606 participants).
- Three trials compared Molnupiravir with standard of care vs Standard of care alone (27119 participants).
Our expert working group classified the following outcomes as critical - hospitalization or death in 28 days, time to progression of each targeted COVID-19 sign/symptom, clinical improvement –as defined by 2-point decrease in the WHO clinical progression and mortality (all-cause), time to clinical improvement, time to viral clearance, Adverse events: all and serious as important outcomes.
Outcomes
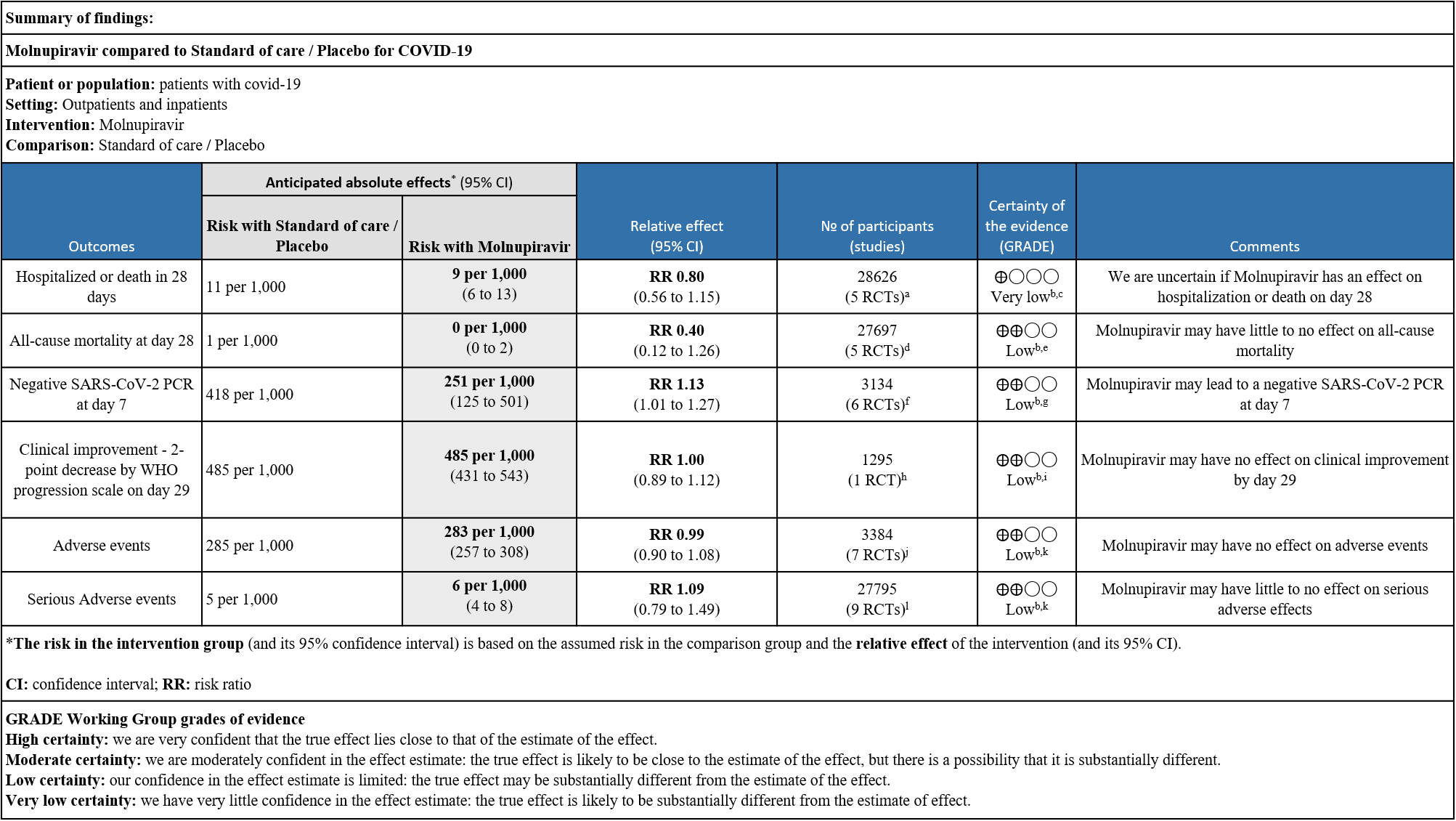
As presented in the ‘Summary of Findings’ table, the effect of Molnupiravir on mortality, negative SARS-CoV-2 PCR at day 7, clinical improvement, adverse events and serious adverse events is of low certainty. In addition, the effect of Molnupiravir on hospitalized or death at 28 days was found to be very low quality evidence.
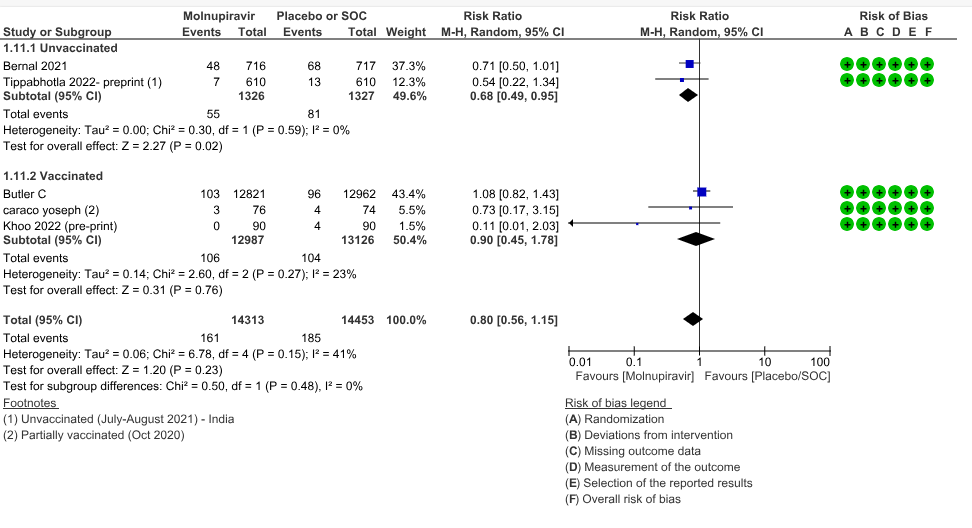
a. Hospitalized or death in 28 days: Very low certainty evidence in 5 trials with 28766 patients revealed that Molnupiravir has uncertain effect on hospitalization or death in 28 days compared to standard of care or placebo. However, there was a wide confidence interval, ranging from important benefit to important harm (RR 0.80; 95% CI 0.56 to 1.15, I2=41%).
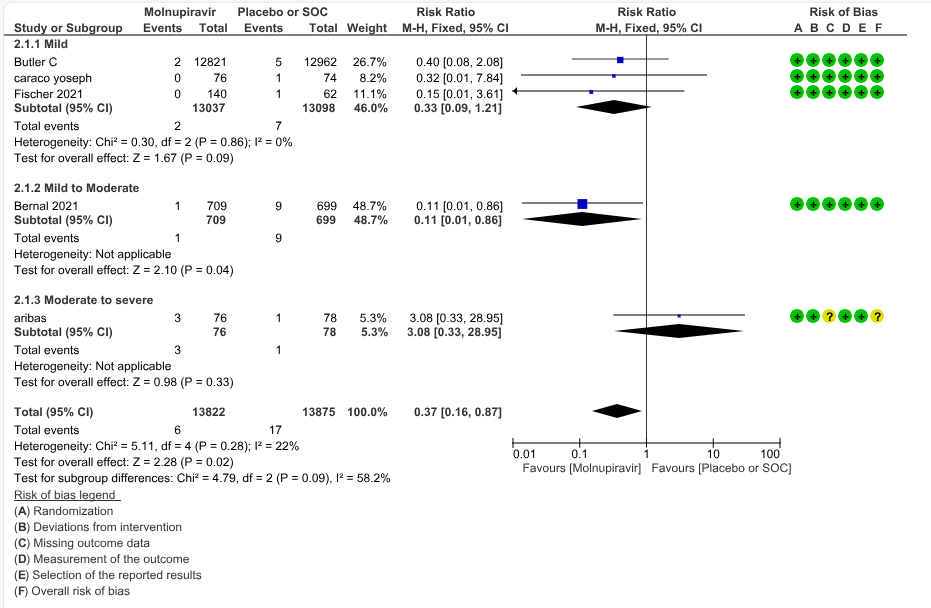
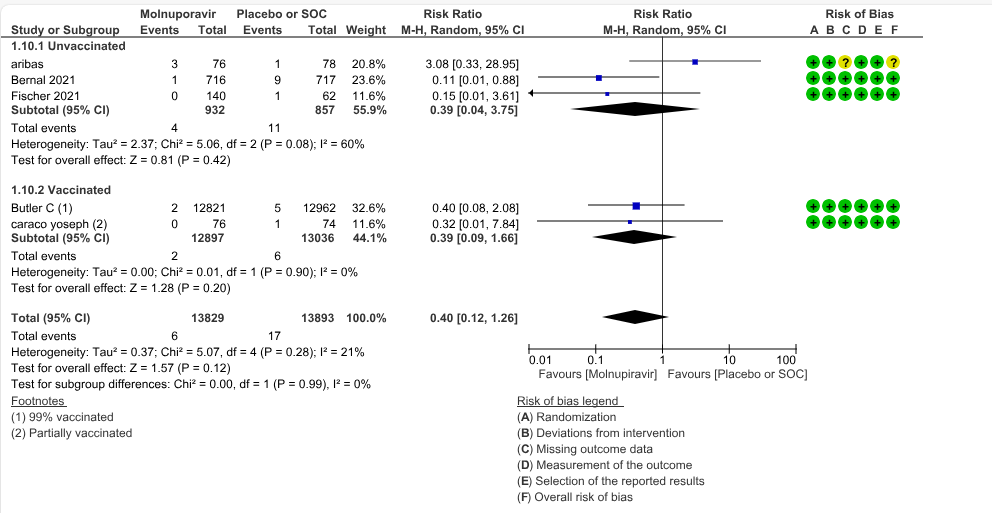
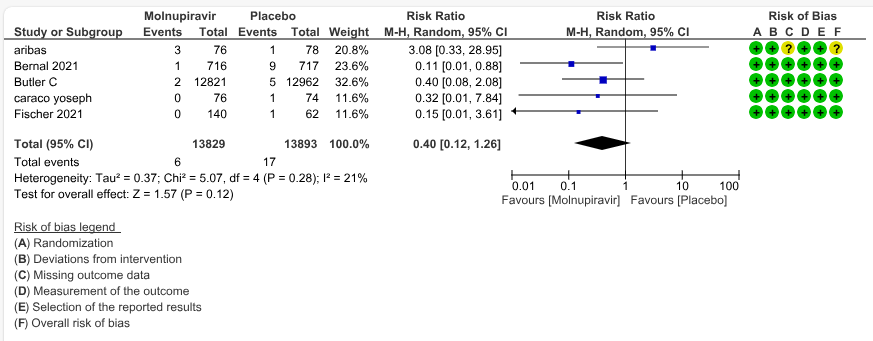
b. All-cause mortality: Low certainty evidence in 5 trials with 27697 patients suggested that Molnupiravir may have little to no effect on all-cause mortality compared to placebo or standard of care. We used a fixed effect model for this meta-analysis to give appropriate weightage to the larger trials suggesting benefit (RR 0.40; 95% CI 0.12 to 1.26, I2=21%)
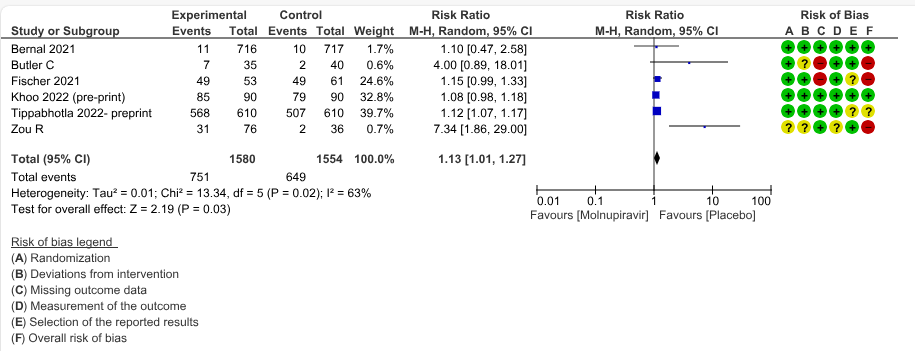
c. Negative SARS-CoV-2 PCR at day 7: Low certainty evidence in 6 small trials with 3134 patients revealed that Molnupiravir may lead to a negative SARS-CoV-2 PCR at day 7 (RR 1.13; 95% CI 1.01 to1.27, I2=63%).
d. Clinical improvement – 2-point decrease by WHO progression scale by day 29: Low certainty evidence in one trial with 1295 patients suggested that Molnupiravir has no effect on clinical improvement by day 29 (RR 1.00; 95% CI 0.89 to 1.12, I2=0%).
e. Adverse events: Low certainty evidence in 7 trials with 3384 patients suggested that Molnupiravir has no effect on adverse events compared to those receiving placebo or standard of care. (RR 0.99; 95% CI 0.90 to 1.08, I2=0%).
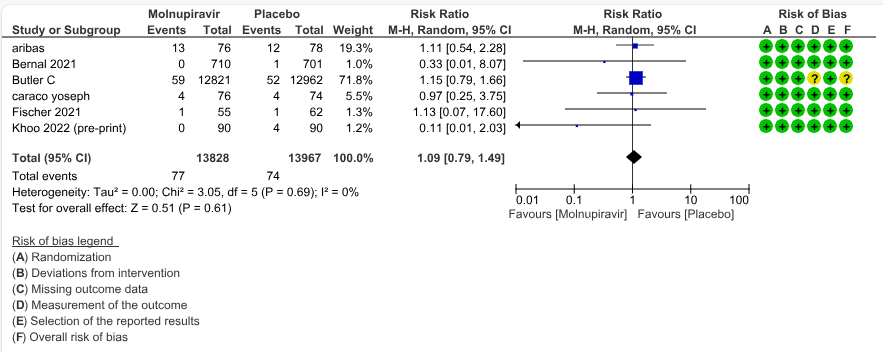
f. Serious Adverse events: Low certainty evidence in 9 trials with 27795 patients revealed that Molnupiravir may cause little to no effect on serious adverse effects in those receiving placebo or standard of care (RR 1.09; 95% CI 0.79 to 1.49, I2=0%).
1. Hospitalization or death at 28 days
Based on Severity
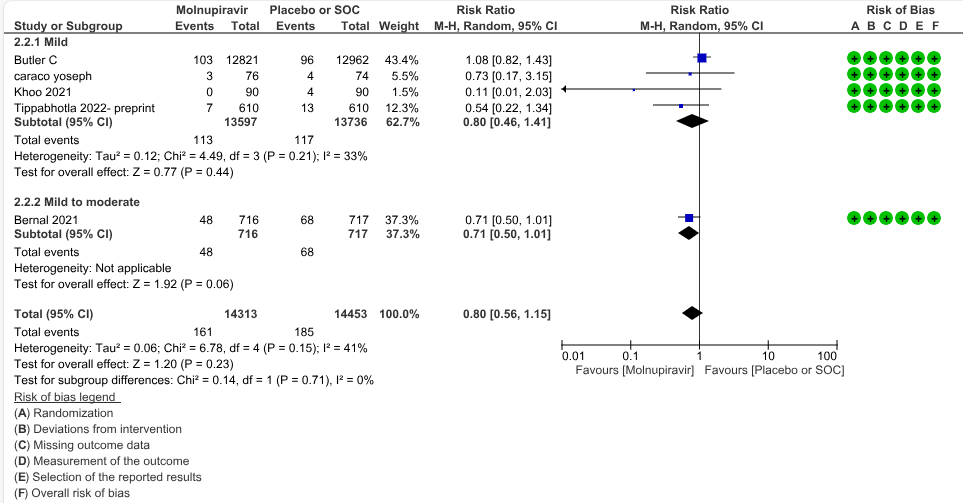
Based on vaccination status
2. All-cause mortality at day 28
Based on Severity
Based on Vaccination Status
1.Hospitalization or death by day 28
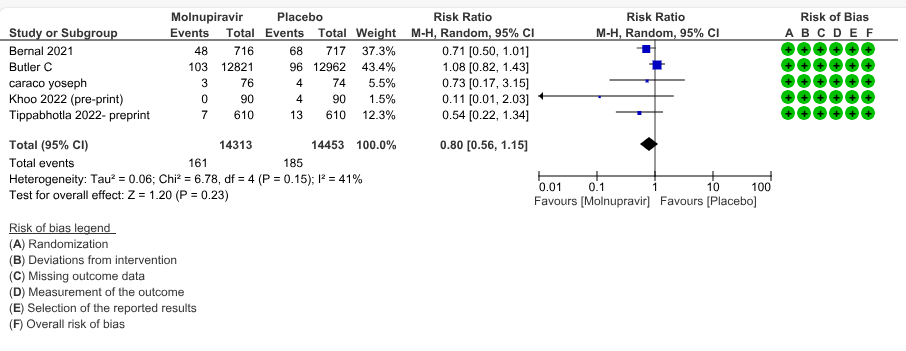
2. All-cause mortality D28
Sensitivity - Fixed Effect Analysis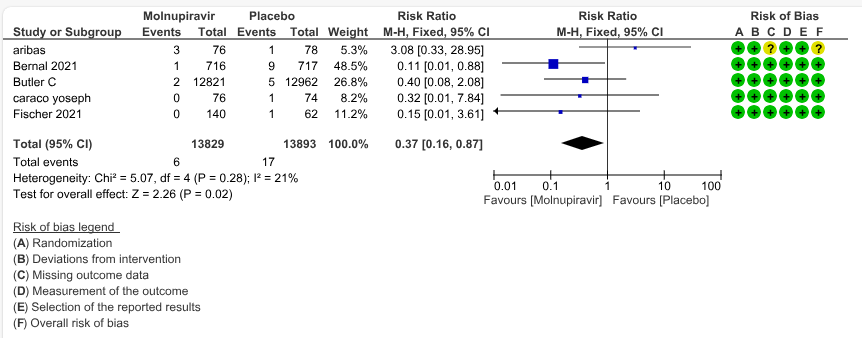
3. Negative SARS-CoV-2 PCR at Day 7
4.Clinical improvement scale 1-3 to 0 at Day 29
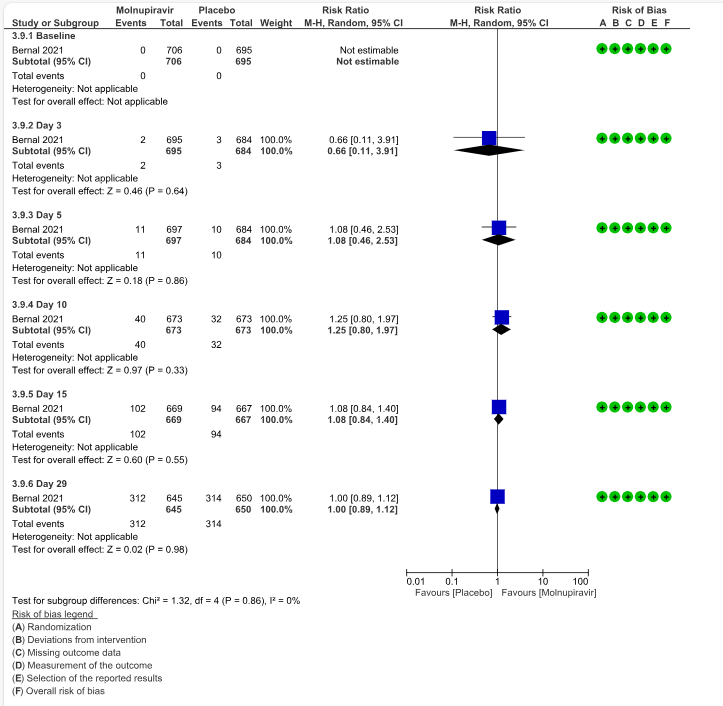
5. Adverse events
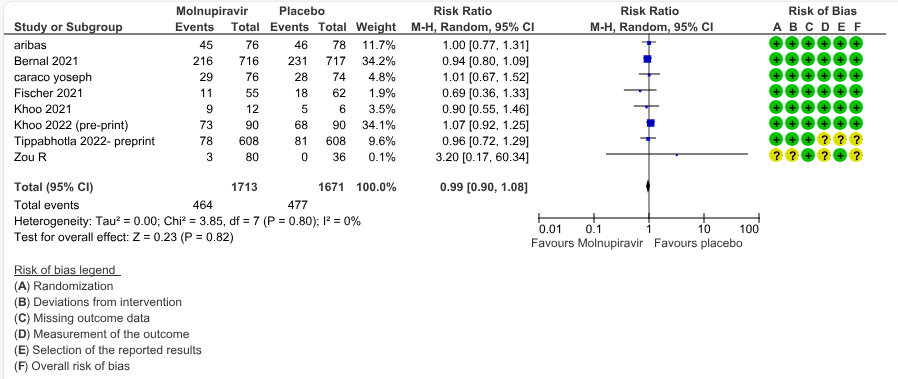
6.Serious adverse events