Explanations
a. Downgraded by one level for serious indirectness as the study population is unvaccinated which does not reflect the current scenario
b. Downgraded by one level for serious imprecision as the lower bound of the 95% CI includes no difference and the number of events and participants do not satisfy the requirements of the optimal information size.
c. Downgraded by one level for serious imprecision as the 95% CI ranges from 0.02-.068. Sample size did not meet the OIS criteria
d. Downgraded by one level for high risk of bias in selected studies (Fischer)
e. Downgraded by one level for serious imprecision as the 95% CI crosses 1(0.94-1.38)
f. Downgraded by one level for serious imprecision as the 95% CI crosses 1(0.75-1.26)
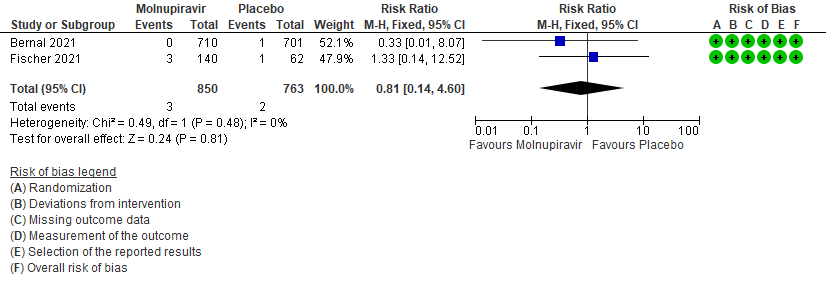
g. Downgraded by one level for serious imprecision as the 95% CI is wide (0.14-4.60)
Molnupiravir is a newer orally bioavailable prodrug of Emory Institute of Drug Development-2801 [EIDD-2801]/MK-4482), which is the synthetic ribonucleoside derivative N4-hydroxycytidine and ribonucleoside analog, with potential antiviral activity against a variety of RNA viruses that has been recently been tested in COVID-19. The TP form of EIDD-1931 is incorporated into RNA and inhibits the action of viral RNA-dependent RNA polymerase. This results in the termination of RNA transcription decreases viral RNA production, and viral RNA replication1. The drug was invented at Emory university for viral diseases of concern and was later was acquired by Ridgeback therapeutics in partnership with Merck & Co, USA. Molnupiravir was initially developed as a possible treatment for influenza viruses, encephalitic alphaviruses like Venezuelan, Eastern and Western equine encephalitic viruses due to its significant inhibitory effect in cell cultures5. On December 23rd 2021 FDA gave emergency use authorization for the treatment of mild-to-moderate coronavirus disease (COVID-19) in adults (>18yrs) with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death, and for whom alternative COVID-19 treatment options authorized by the FDA are not accessible or clinically appropriate. The drug is indicated to be initiated within 5 days of symptom onset6.On December 2021 DCGI gave emergency use authorization for the antiviral in India for the treatment of adult patients with Covid-19 who are at a high risk of progression to severe disease.Molnupiravir’s mechanism against Covid-19 has some experts concerned about its mutagenic potential in human cells. An in vitro study by Zhou et al has suggested that the drug, though intended to disrupt only viral RNA, could also incorporate into and cause mutations in human DNA.The study authorsconclude that ‘’mutations in host DNA could potentially “contribute to the development of cancerand that the Molnupiravir metabolite the ribonucleoside analog β-D-N4 -hydroxycytidine (NHC), was a far more potent mutagen than favipiravir (FAV), which is a drug that has widely known issues related to teratogenicity and links to birth defects7.A recent article (preprint) also showed that invitro, Molnupiravir retains its activity against the VOCs Alpha, GHB-03021/2020* and Omicron. This is in accordance with the observation that the target proteins of these antivirals are highly conserved8.
This review aims to provide a summary of the available evidence from randomized clinical trials of Molnupiravir for treatment of COVID-19, for any duration, which could guide clinicians and researchers regarding the appropriate use of this drug in the future.
We searched PubMed, Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language. We also reviewed reference lists of systematic reviews and included studies. We performed all searches up to 02/01/ 2022.
We searched the above databases and found 25 records. After removing duplicates and excluding that did not match our PICO question, 3 RCTs were included for meta-analysis.
We extracted data for the following outcomes, pre-defined by the Expert Working Group:
- Critical (primary for this review):
- Hospitalized or death in 28 days
- Time to progression of each targeted COVID-19 sign/symptom
- Clinical improvement –as defined by 2-point decrease in the WHO clinical progression scale
- Important (secondary):
- Mortality (all-cause)
- Time to clinical improvement
- Time to viral clearance
- Adverse events: all and serious
Two reviewers independently assessed eligibility of search results using the online Rayyan tool. Data extraction was performed by one reviewer, and checked by another, using a piloted data extraction tool on Microsoft Word. Risk of bias (RoB) was assessed using the Cochrane RoB v2.0 tool, with review by a second reviewer and compared with covid.nma database. If there was a difference in more than one domain it was assessed by a third independent author. We planned to use risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). We planned to meta-analyze if appropriate, i.e., if outcomes were measured and reported in a similar way for similar populations in each trial, using random-effects models with the assumption of substantial clinical heterogeneity (the appropriateness of which would be checked by the Methodology Committee), and the I2 test to calculate residual statistical heterogeneity, using Review Manager (RevMan) v5.4. We used GRADE methodology to make summary of findings tables on GRADEPro GDT.
Results
We found 3RCTsthat matched the PICO question as pre-defined by the expert working group.
We found 3 RCTs that matched the PICO question as pre-defined by the expert working group.
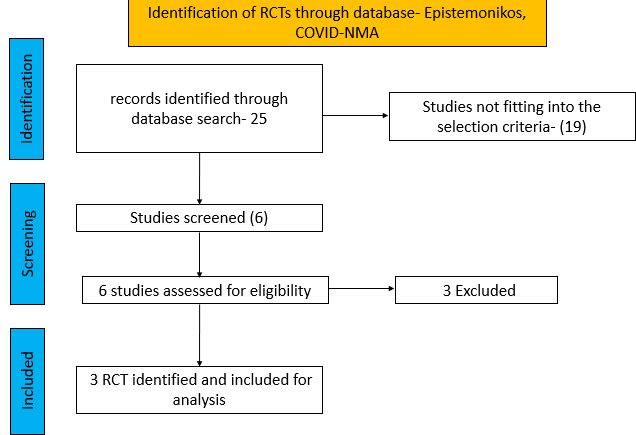
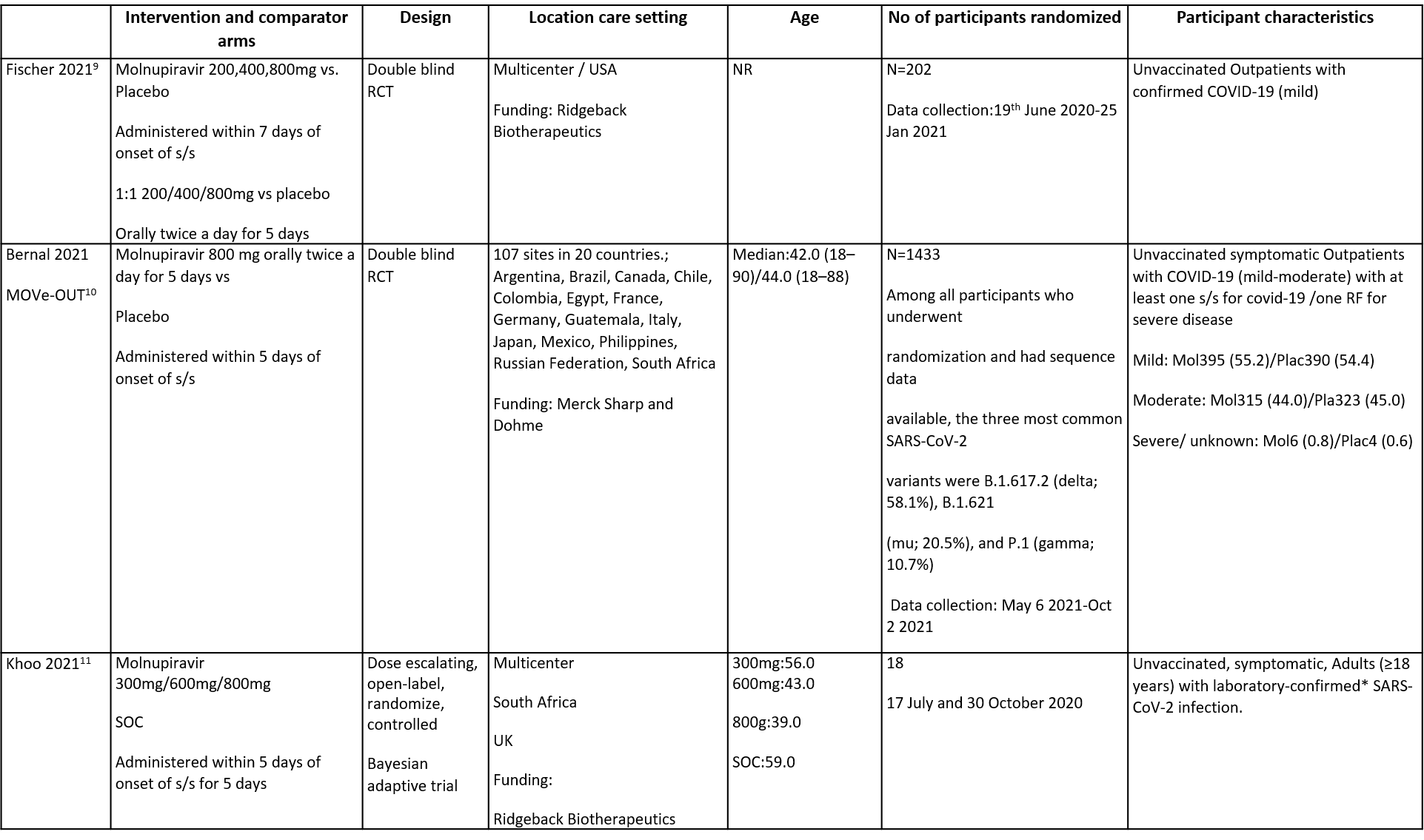
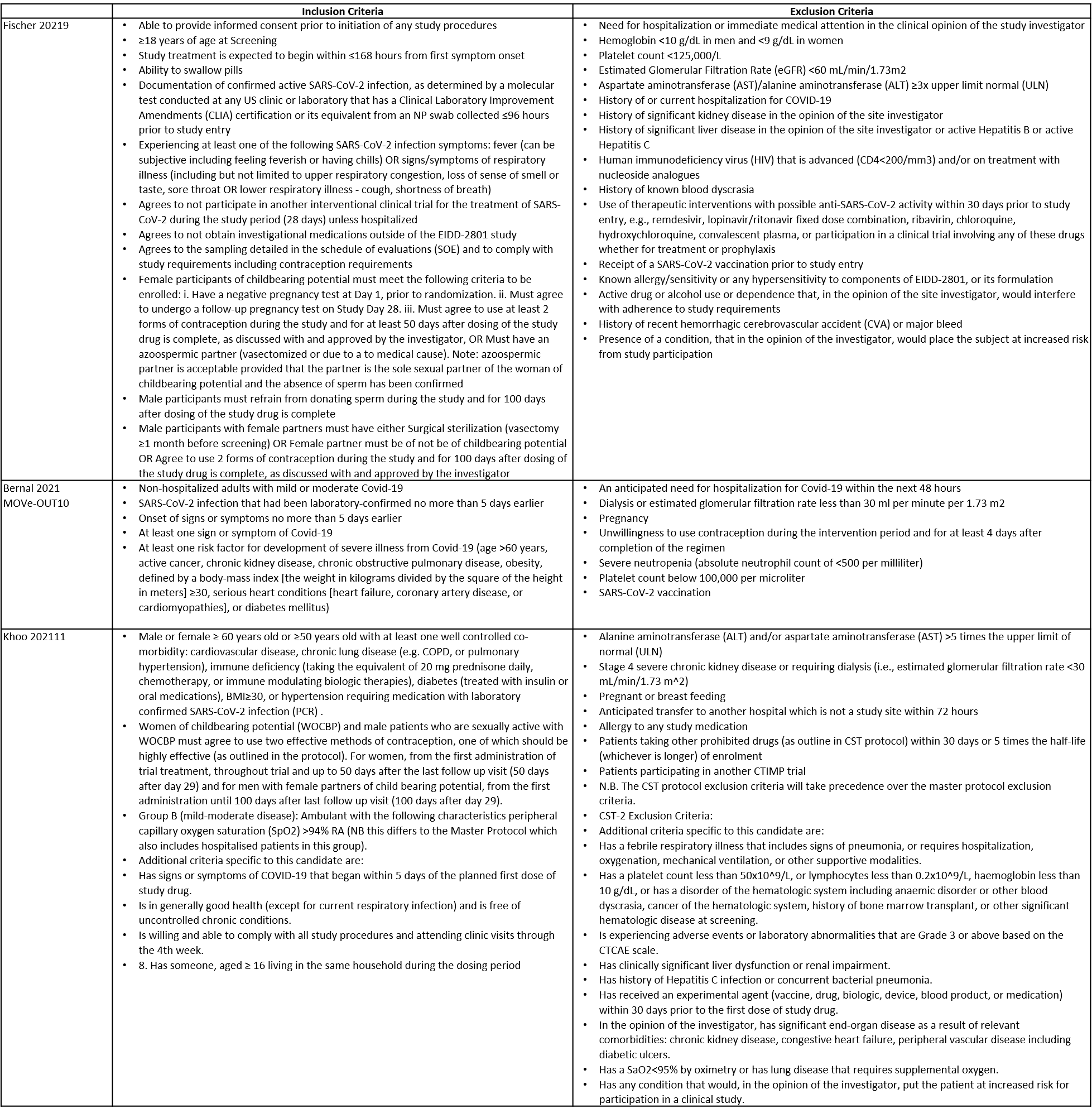
The trials included a total of 1653 participants, all of whom were adults9,10,11. One trial reported from United States of America9, other two are multicenter studies, one involving 107 sites in 20 countries10 and the other trial from UK and south Africa11. The first two trials9,10 compared Molnupiravir with Placebo whereas the third compared Molnupiravir with standard of care11. The trials recruited patients who were unvaccinated with mild or mild to moderate disease. Molnupiravir was administered within 5 days of symptom onset in two trials10,11 whereas in the other it was within 7 days9.
Each trial and its results are described below; characteristics of the trials are summarised in the Summary of characteristics of included trials table. Risk of bias for each domain per trial is displayed alongside the forest plots below.
The following comparisons were investigated in the trials (we compared outcomes for arms randomized to Favipiravir vs. standard of care or placebo).
● Two trials compared Molnupiravir with placebo (1635 participants).9,10
● One trial compared Molnupiravir with standard of care (18 participants).11
Our expert working group classified hospitalised or death in 28 days, time to progression of each targeted COVID-19 sign/symptom, clinical improvement –as defined by 2-point decrease in the WHO clinical progression scale as critical outcomes whereas mortality (all-cause), time to clinical improvement, time to viral clearance, Adverse events: all and serious as important outcomes.
Critical (primary) Outcomes
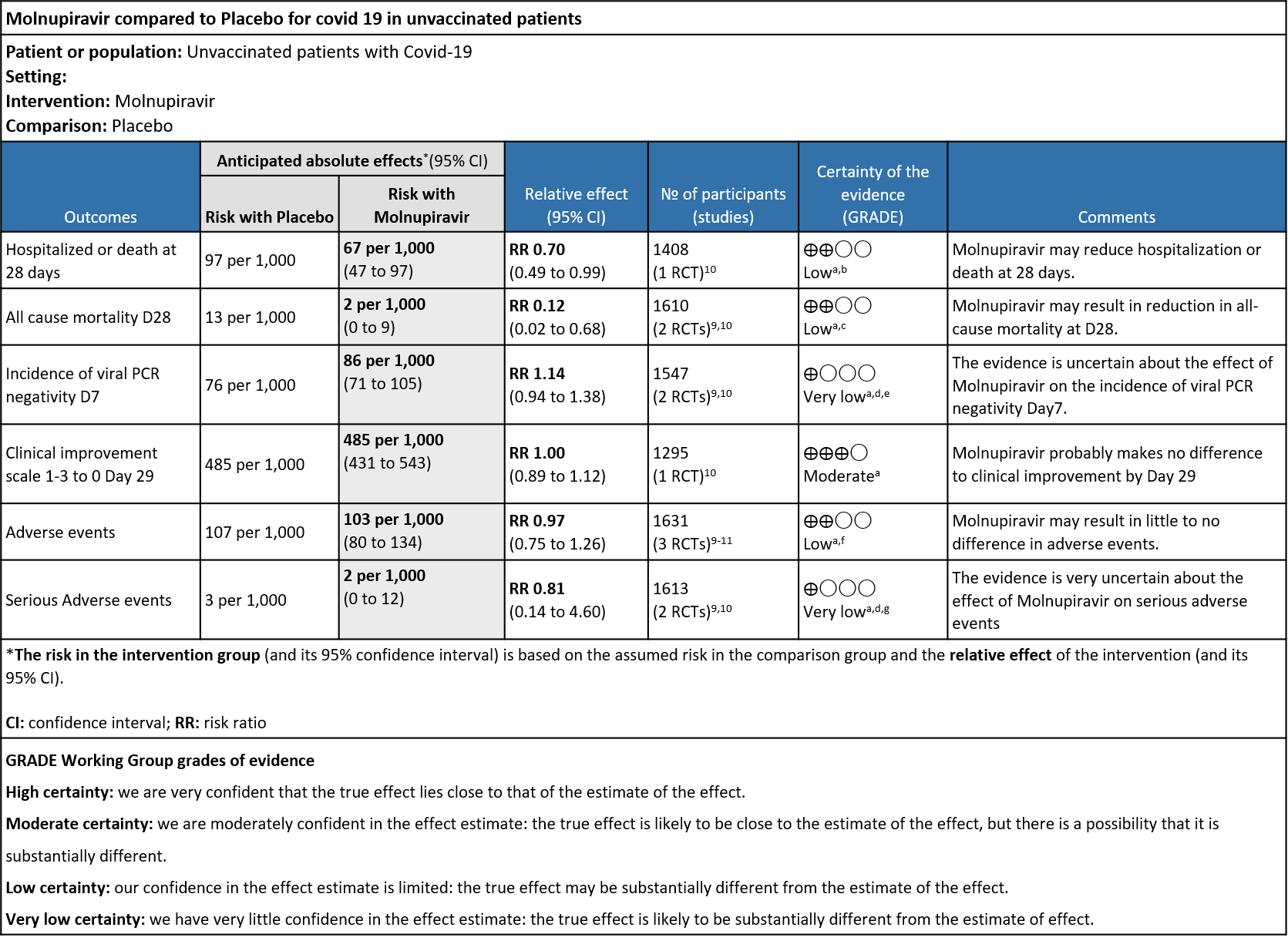
As presented in the ‘Summary of findings’ table, there is low or very low certainty evidence for effect of Molnupiravir on hospitalization or death at 28 days, all-cause mortality, incidence of viral PCR negativity D7, adverse events and serious adverse events. Clinical improvement scale 1-3(mild) to 0 (no symptoms) at Day 29 was found to have moderate quality evidence.
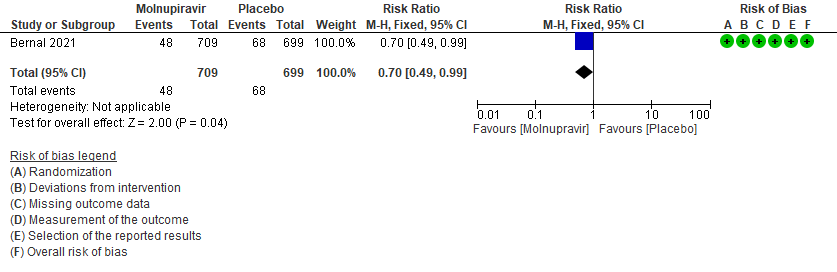
- Hospitalized or death in 28 days: Low certainty of evidence in 1408 patients in one RCT10 found that Molnupiravir may reduce hospitalization or death in 28 days compared to placebo (RR: 0.69; 95%CI: 0.49 to 0.99).
- All-cause mortality D28: Low certainty of evidence in 1610 patients in two RCTs9,10 found that Molnupiravir may reduce all-cause mortality vs. placebo (RR 0.12; 95% CI 0.02-0.68). However the event rate in this trial was very low in each of the groups.
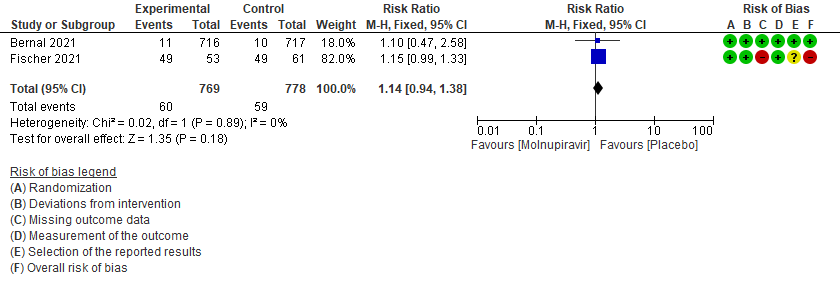
- Incidence of viral PCR negativity by D7: Very low certainty of evidence in 1547 patients in two RCTs9,10 found that the evidence is very uncertain about the effect of Molnupiravir on the incidence of viral PCR negativity by Day7 (RR 1.14; 95% CI 0.94-1.38).
- Clinical improvement by WHO progression scale 1-3 to 0: Moderate certainty of evidence in 1295 patients in one RCT10 found that Molnupiravir probably makes no difference to clinical improvement by Day 29(RR 1; 95% CI 0.89-1.12).
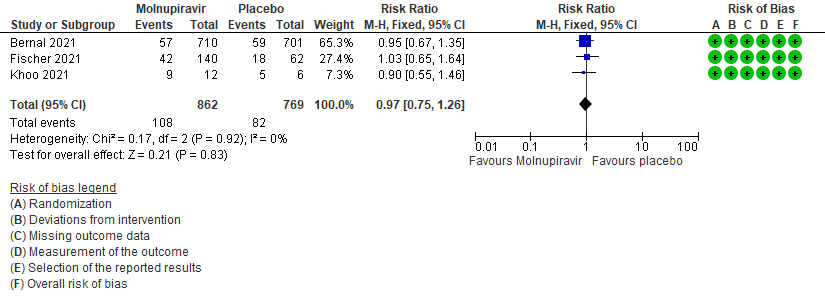
- Adverse events: Low certainty of evidence in 1631 patients in three RCTs9-11 found that Molnupiravir may result in little to no difference in overall adverse events (RR 0.97; 95% CI 0.75-1.26).
- Serious Adverse events: Very low certainty of evidence in 1613 patients in two RCTs9,10 found that the evidence is very uncertain about the effect of Molnupiravir on serious adverse events (RR 0.81; 95% CI 0.14-4.60).
A subgroup analysis was attempted as suggested by the Methodology group on a few outcomes based on the observations made by the experts.
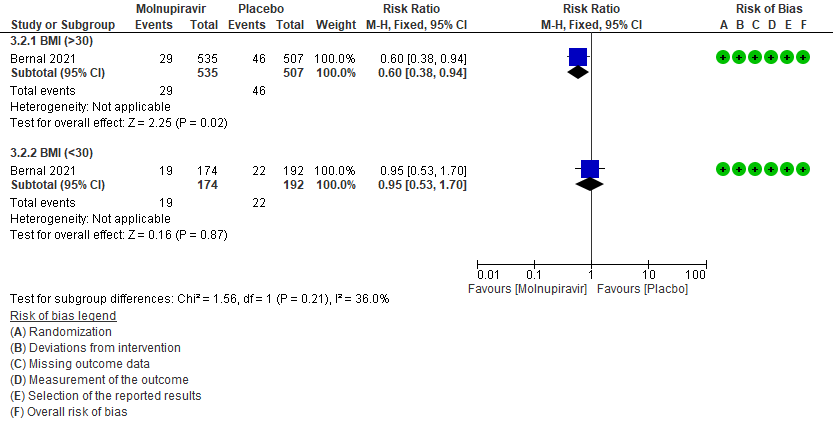
a. Incidence of hospitalization or death at day 29 by subgroup BMI>30
In the Bernal 2021/MOVe-OUT trial10 75% of the patient population were obese. And since obesity is considered as a risk factor for progression to severe disease, a subgroup analysis was attempted in patients with BMI >30 and in those with BMI 30 by 40% (RR:0.60;95% CI 0.38-0.9).
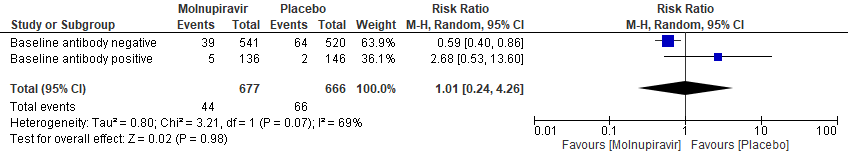
b. Incidence of hospitalization or death at day 29 (MITT) Subgroup analysis by baseline SARS-CoV2 nucleocapsid antibody status(suggesting previous infection)
In the Bernal 2021/MOVe-OUT trial10, Molnupiravir seemed to have a better response in those who were antibody negative at baseline. However, this was an observation made from available data in very few patients (RR: 0.59; 95% CI 0.40-0.86).
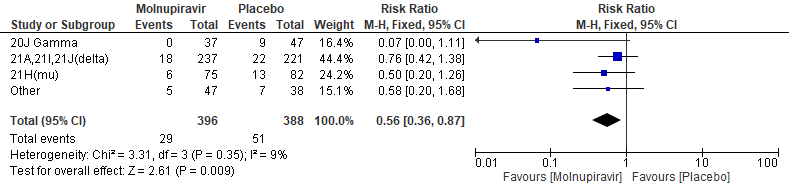
c. Incidence of hospitalization or death at day 29(mitt) baseline clade variant
In the Bernal 2021/MOVe-OUT trial10, among all participants who underwent randomization and had sequence data available, the three most common SARS-CoV-2 variants were B.1.617.2 (delta; 58.1%), B.1.621(mu; 20.5%), and P.1 (gamma; 10.7%). Molnupiravir did not show any differential benefit in any of the variants included in the subgroup analysis.The final effect estimate for this outcome is unknown, pending completion of all baseline sequencing; the current effect size may be an over or under-estimate. “Other” includes the following clades: 19B, 20A, 20B, 20C, 20D, and unknown clades or those that could not be classified.
Primary:
1. Hospitalization or death at 28 days10
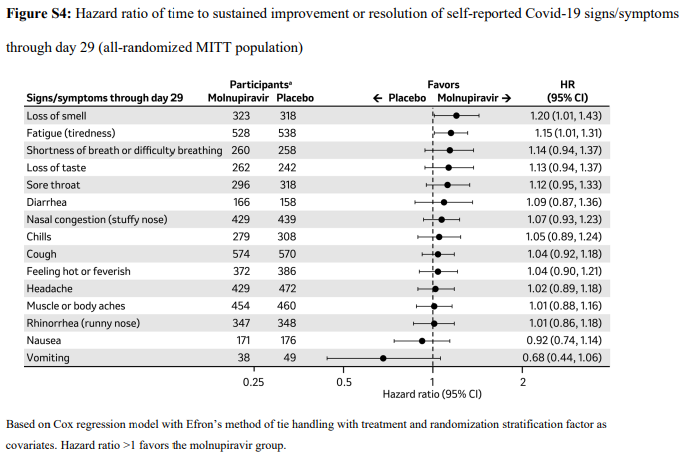
2. Time to progression of each targeted COVID-19 sign/symptom- Bernal 202110
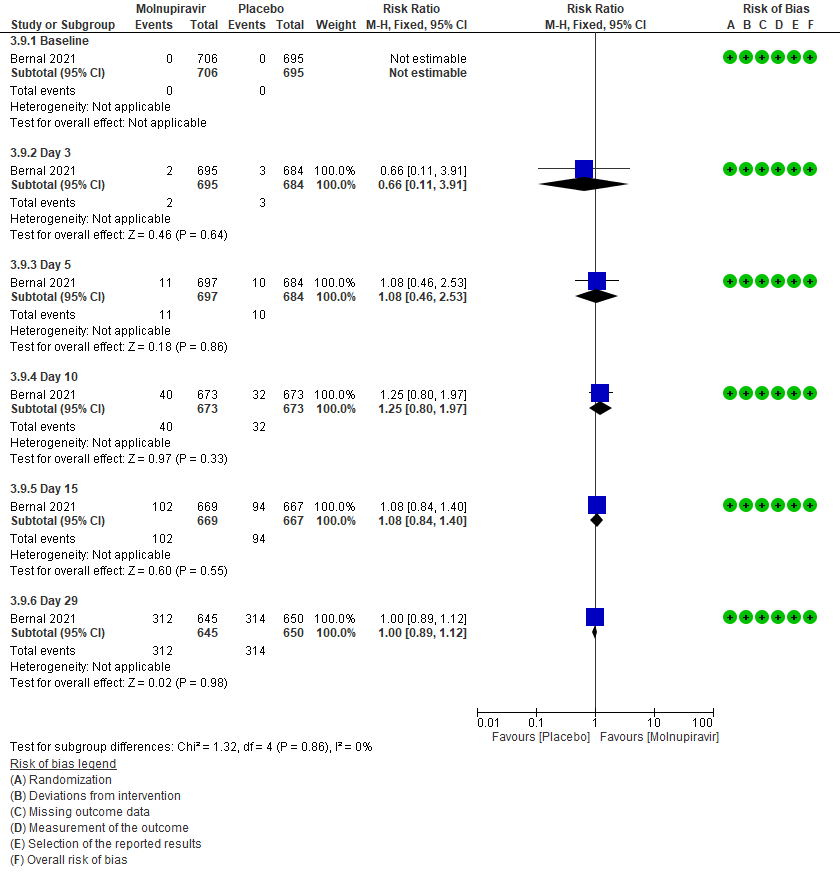
3. Clinical improvement –as defined by 2-point decrease in the WHO clinical progression scale10. Since most of the patients were classified as mild to moderate, at baseline they were at scale 1-3 (mild symptoms). For clinical improvement we have tracked those who moved from WHO progression scale 1-3 to 0.
3 (a): Clinical improvement: Those who moved from WHO progression scale 1-3 to 0.
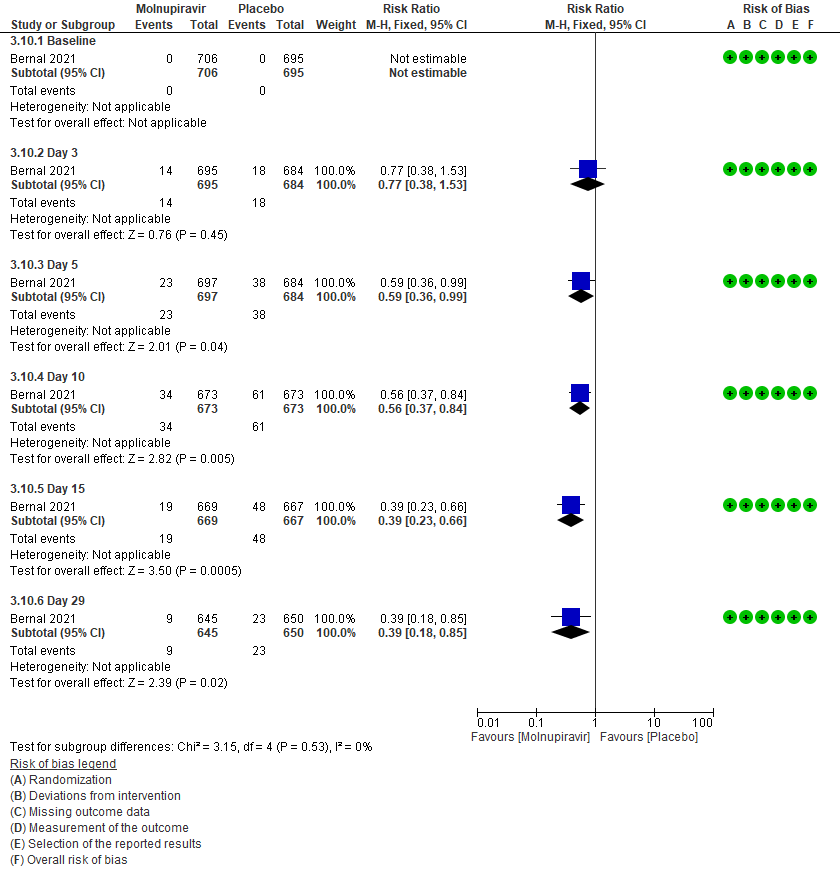
3 (b) Clinical worsening: Those who moved WHO progression scale 1-3 at baseline to > 3 at different time points
Secondary outcomes:
4. Mortality (all-cause)9,10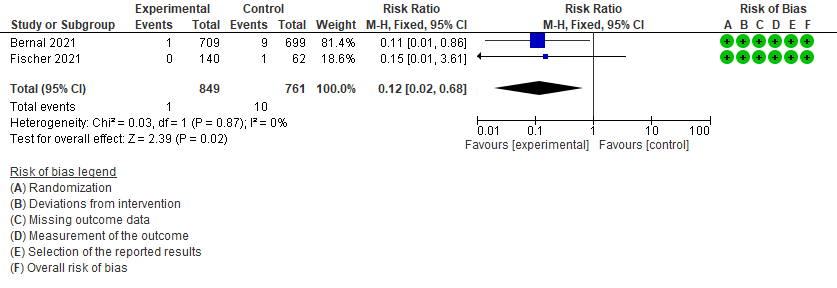
5. Incidence of viral PCR negativity at D79,10
6. Adverse events: All9,10,11
7. Adverse events: serious9,10