Itolizumab is an anti CD6 monoclonal antibody approved for use in India for the treatment of various cancers and psoriasis. Recently, the drug received ‘restricted emergency use’ approval for treatment of COVID 19 related “cytokine release syndrome” in India.
The data for Itolizumab in the setting of COVID 19 is from 1 very small randomized control trial with 30 patients. The impact of the drug in modifying the clinical course of severe COVID 19 cannot be estimated from the 1 small RCT of 30 patients assessing both clinical and laboratory parameters.
While the reported side effect profile of the drug in COVID 19 patients in published literature is small, 18 of the 22 patients in the study reported adverse effects. This was also echoed by clinicians who had personal experience using the drug. Some clinicians in the expert group reported infusion reactions and life-threatening anaphylaxis with the drug. In addition, the group also felt that the real-world efficacy of the drug was at best unpredictable. Hence, there was poor certainty regarding the clinical benefit or adverse event profile of the drug.
The expert group also commented that much has changed regarding the immunomodulatory landscape in COVID 19. Credible and largescale studies have proven the immunomodulatory efficacy of Tocilizumab and Baricitinib along with steroids and hence Itolizumab as an immunomodulatory drug when other better options are available is unwarranted.
In summary, there is too little evidence regarding the clinical efficacy or the side effect profile of the drug to support its use among patients with COVID 19 disease.
Date of latest search: 30th August 2021
Date of completion & presentation to Expert Working Group: 3rd September 2021
Date of planned review: 3rd February 2021
Evidence synthesis team: Priya N, Benjamin Earnest Williams, Sneha Varahala, Bhagteshwar Singh, Richard Kirubakaran and Priscilla Rupali.
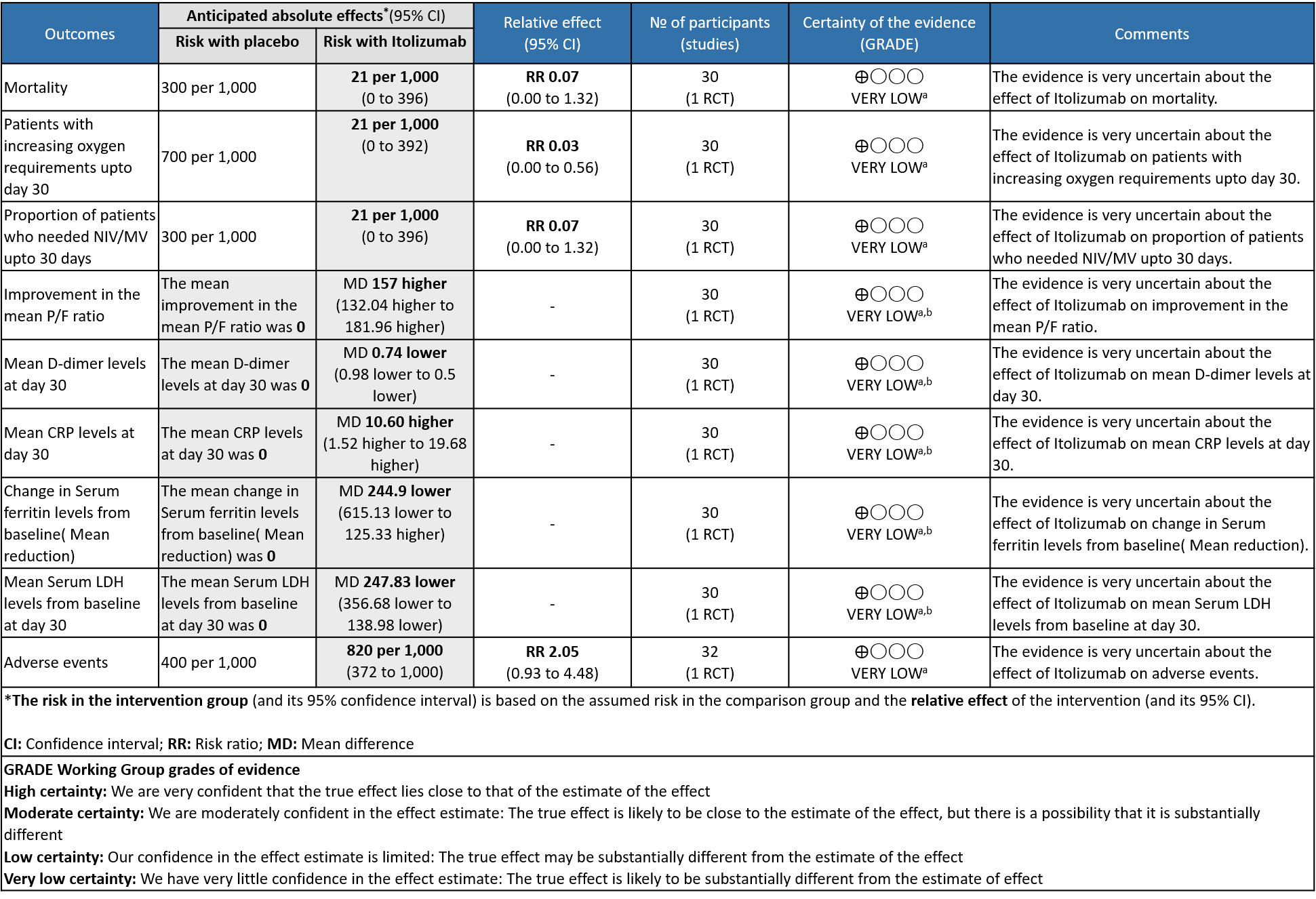
Itolizumab compared to standard of care for moderate to severe COVID-19
Explanations
a. Downgraded by two levels for serious imprecision due to very small sample size contributed by single study with low event rates , and further one level for the high risk of bias as it is a single trial with high risk of bias in one domain and some concerns for two other domains in RoB 2 tool.
b. Downgraded by two levels for serious imprecision due to very small sample size contributed by single study with wide 95% Confidence interval
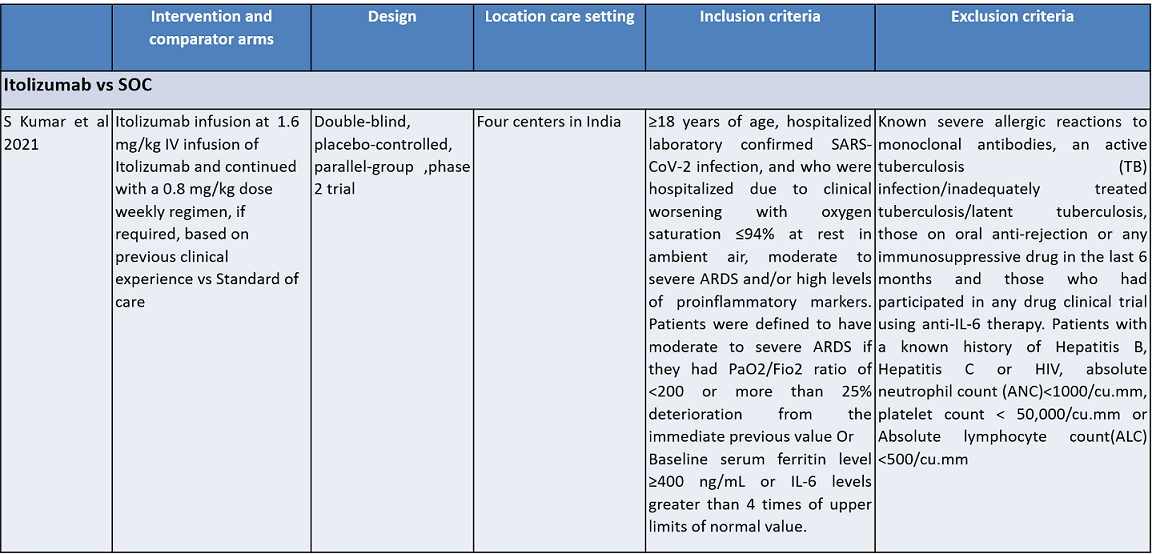
Itolizumab is an anti-CD6 humanized monoclonal antibody; it works by suppressing T-cell activation and downregulates the synthesis of pro-inflammatory cytokines and adhesion molecules. It was initially developed for various cancers. It was approved in India to treat moderate to severe chronic plaque psoriasis in 2013. Itolizumab is now being re-purposed for COVID-19. The potential utility of Itolizumab in COVID-19 was proposed first in Cuba with the approval of a single-arm clinical trial. It expanded access use is based on its unique mechanism of action in alleviating cytokine release syndrome. Subsequently, after receiving regulatory permission, a phase II, open-label, randomized, placebo-controlled trial was conducted in 30 COVID-19 patients in India. Based on its results, the Indian drug regulatory agency recently approved Itolizumab in July 2020 for 'restricted emergency use' to treat CRS in moderate to severe acute respiratory distress syndrome (ARDS) due to COVID-191.
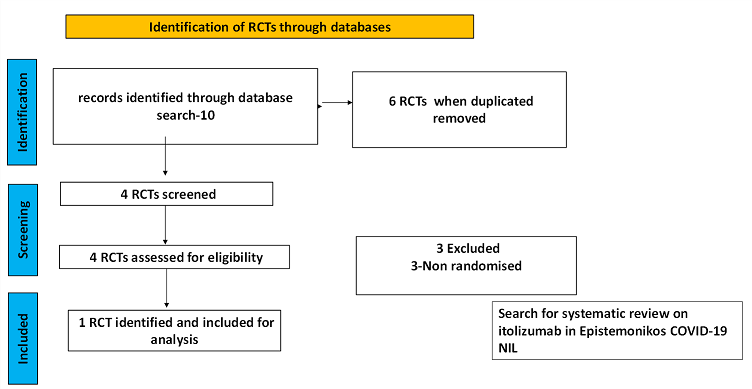
To identify all available systematic reviews pertaining to our PICO question, a systematic search of PubMed, covid-nma.org and the Epistemonikos databases was conducted. The search strategy was designed and validated by the Group’s information specialist. The search results were screened independently by two reviewers against the pre-defined criteria set out above, using Rayyan.
There was no systematic review available in the databases referred to. For randomized controlled trials, 6 were identified using databases, of which 2 were removed as they were duplicates. Of the 4 RCTs screened, 3 were non-randomised controlled trials and hence they were excluded. The remaining 1 RCT was assessed for eligibility and included for analysis.
For the risk of bias, Cochrane ROB2 tool was used. For all outcomes, one review author performed the assessment, and cross-checked it against the assessment by another review author. If there was a difference in more than one domain, it was assessed by a third independent author.
Data was entered into Review manager version 5.4 for meta-analysis. The results were entered into Grade ProGDT (online), to create the summary of findings (GRADE) table. We used risk ratios (RR) for dichotomous outcomes with 95% confidence intervals (CIs).
Population – Inpatients with moderate to severe Covid-19 infection
Intervention – Itolizumab
Control – standard of care
Following were the outcomes we wanted to extract data for:
Primary:
1. Overall mortality
2. Time to clinical recovery
Secondary:
1. Length of hospital stay
2. Need for NIV/invasive mechanical ventilation
3. Duration of invasive ventilation
4. Duration of stay in ICU care
Adverse events:
a) All
b) Serious
c) Infusion-related adverse events
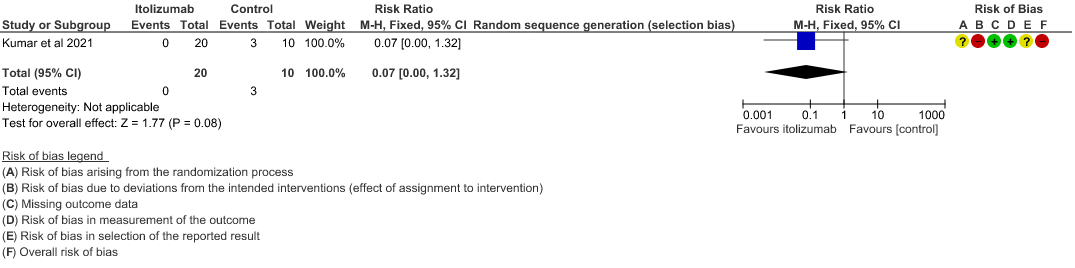
We found 1 randomized controlled trial that met our search criteria. However, this was a single trial with very low sample size of 30 and a high risk of bias as assessed by RoB 2 tool.
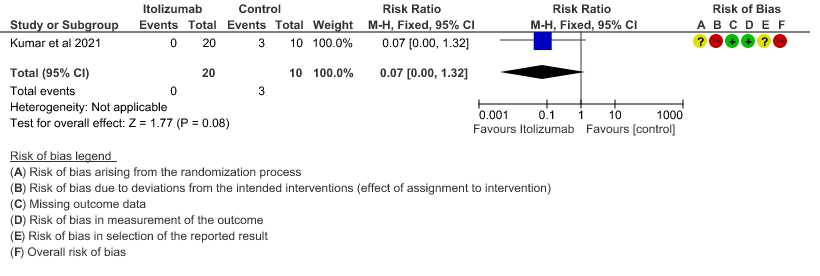
- All-cause mortality at 28 days: Very low certainty of evidence in 30 patients from 1 RCT2 including those with moderate to severe COVID-19 infection, showed that Itolizumab had a very uncertain effect on the mortality at day 28 RR 0.07 (95% CI 0.00 to 1.32).
- Patients with increasing oxygen requirements upto day 30: Very low certainty of evidence in 30 patients from 1 RCT2revealed a very uncertain effect of Itolizumab on increasing oxygen requirements RR 0.03
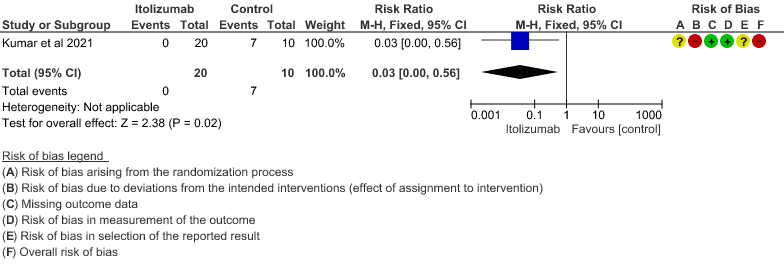
(95% CI 0.00 to 0.56). This suggested a very imprecise estimate that Itolizumab reduced the proportion of those needing increased oxygen requirements upto day 30 by 97% but this could vary from 44% to 100%. - Need for non- invasive/mechanical ventilation: Very low certainty of evidence in 30 patients from 1 RCT2revealed a very uncertain effect of Itolizumab on the need for NIV/mechanical ventilation upto 30 days RR 0.07(0.00 to 1.32). This suggested a very imprecise estimate of Itolizumab reducing the need for NIV/Mechanical ventilation by 93%( 95% CI 100% reduction or 32% increase).
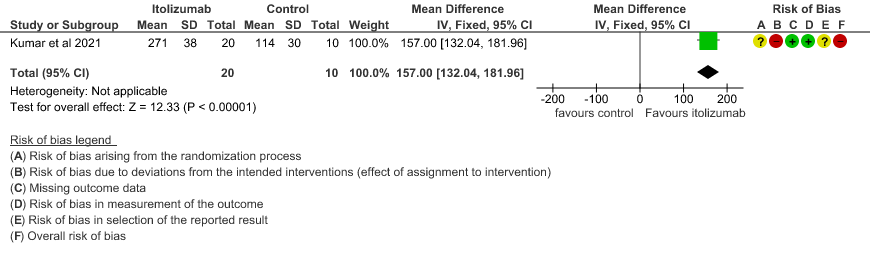
- Improvement in the PF ratio: Very low certainty of evidence in 30 patients from 1 RCT2revealed a very uncertain effect of Itolizumab on improvement in PF ratio with mean difference MD 157 higher(95% CI 132.04 to 181.96 higher) as compared to placebo.
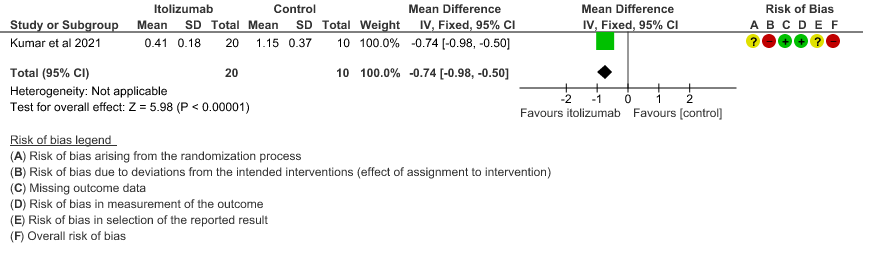
- Mean D-dimer levels at day 30: Very low certainty of evidence in 30 patients from 1 RCT2revealed a very uncertain effect of Itolizumab on mean D-dimer levels at day 30; MD was 0.74 lower (95% CI 0.5 - 0.98 lower). Baseline D-dimer level was higher in the control to begin with 5.15 (SD 7.85) μg/mL compared to Itolizumab arm 3.50 (SD 4.87) μg/mL. The mean D-dimer reduced to 2.83 (SD 5.46) μg/mL and 0.41 (SD 0.18) μg/mL on Days 14 and 30, respectively in the Itolizumab group. In control group, mean D-dimer was 0.86 (SD 0.71) μg/mL and 1.15 (SD 0.37) μg/mL on Days 14 and 30, respectively.
- Mean CRP levels at day 30: Very low certainty of evidence in 30 patients from 1 RCT2revealed a very uncertain effect of Itolizumab on the baseline CRP levels. Baseline C-reactive protein (CRP) was numerically higher in control group 103.88 (SD 87.89) mg/L vs 73.74 (SD 71.84) mg/L in the intervention group. In the Itolizumab group, mean CRP reduced to 6.45 (SD 4.14) mg/L and 13.69 (SD 20.45) mg/L on Days 14 and 30, respectively. In the control group, mean CRP reduced to 14.36 (9.29) mg/ L and 3.05 (2.62) mg/L on Days 14 and 30, respectively.
- Mean reduction in serum Ferritin levels from baseline: Very low certainty of evidence in 30 patients from 1 RCT2revealed a very uncertain effect of Itolizumab on reduction in Serum ferritin levels from baseline(Mean reduction) MD 244.9 lower(95% CI 615.13 lower to 125.33 higher). Baseline ferritin was high in Itolizumab group compared to control (943.34 ng/mL vs 577.95 ng/mL). In Itolizumab arm the mean ferritin reduced to 303.50 (SD 210.93) ng/dL and 189.22 (SD 129.96) ng/dL on Day 14 and 30, respectively. In control group, ferritin was 367.68 (SD 130.22) ng/dL and 285.25 (SD 157.76) ng/dL on Days 14 and 30, respectively. This suggested that a greater reduction from baseline was seen in serum ferritin levels in Itolizumab arm (−479.3 (620.95) ng/dL) in comparison to control (−234.4 (405.67) ng/dL) at day 30 (p-value = 0.078).
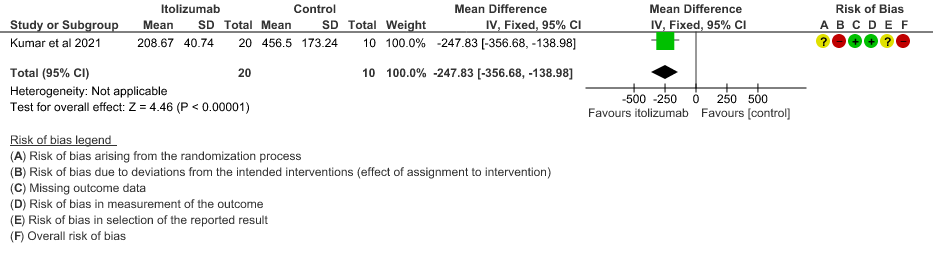
- Mean Serum LDH levels from baseline at day 30: Very low certainty of evidence in 30 patients from 1 RCT2revealed a very uncertain effect of Itolizumab on mean Serum LDH levels from baseline on day 30 ;MD 247.83 lower(95% CI 356.68 lower to 138.98 lower). Baseline lactate dehydrogenase (LDH) was comparable in both arms; 533.30 (SD 206.85) U/L in Arm-A and 645.30 (SD 292.79) U/L in Arm-B. In the Itolizumab arm, mean LDH reduced to 381.47 (SD 181.45) U/L and 208.67 (SD 40.72) U/L on Days 14 and 30, respectively. In the control arm, mean LDH was 330.20 (SD 91.63) U/L and 456.50 (SD 173.24) U/L on Days 14 and 30, respectively.
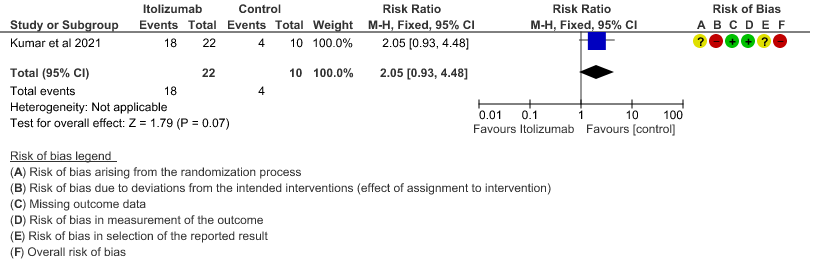
- All adverse events:Very low certainty of evidence in 30 patients from 1 RCT2revealed a very uncertain effect of Itolizumab on all adverse events. Based on the data, we anticipate that for 1000 patients with COVID-19 disease treated with placebo 400 adverse events are likely. We anticipate that there would be 420 higher (320 to 1000) adverse events per 1000 people if Itolizumab is administered for patients with COVID-19. This was due to the fact that 18 adverse events related to the drug occurred in the patients treated with Itolizumab(N=22) among those 30 patients studied in our trial. Two patients had infusion reactions with Itolizumab infusion and hence the drug was not given. Considering that was a single trial conducted among 30 patients, of which 20 patients received Itolizumab with high risk of bias in RoB2 tool, the evidence is very uncertain about the effect of Itolizumab on adverse events.
ITOLIZUMAB COMPARED TO SOC IN PATIENTS WITH MODERATE TO SEVERE COVID 19.
1. Mortality at day 28
2. Patients with increasing oxygen requirements at day 30
3. Need for NIV/Mechanical ventilation at day 30
4. Improvement in the PF ratio
5. Mean D-dimer levels at day 30
6. Mean CRP levels at day 30
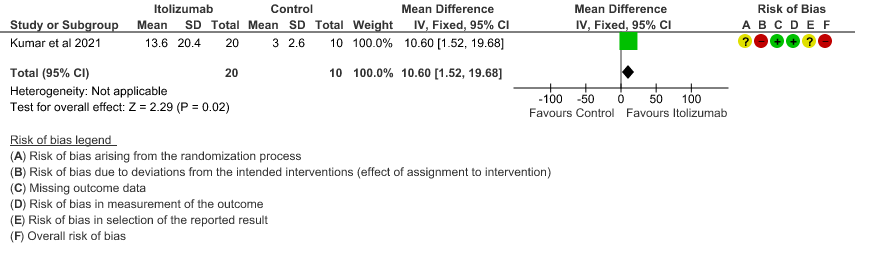
7. Mean reduction in serum Ferritin levels from baseline
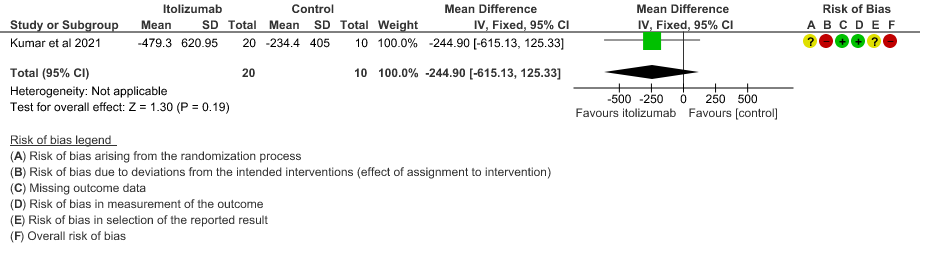
8. Mean Serum LDH levels from baseline at day 30
9. All adverse events
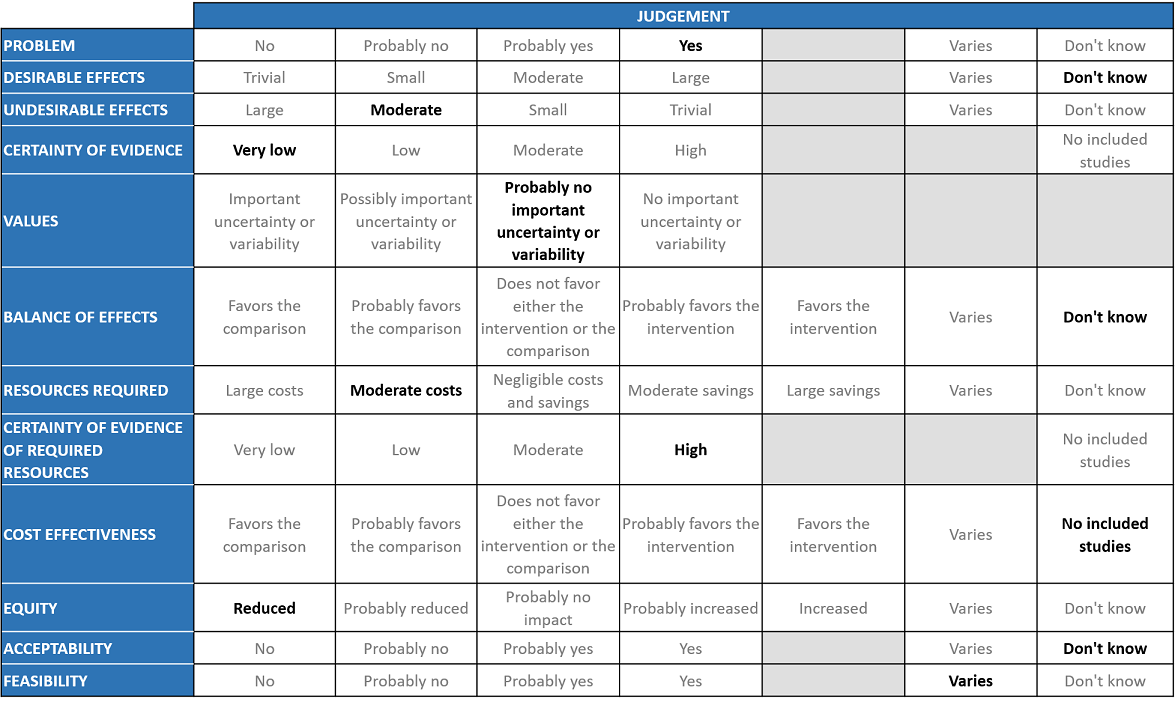
The Antibody and Anti-inflammatory Expert Working Group met on 3rd September 2021 to consider Itolizumab as a treatment for COVID-19. Conflict of interest declarations were reviewed by the Steering Committee; none were found to be relevant to Itolizumab.
A summary and then more detailed explanations of the Expert Working Group's judgements follow.
Problem
The COVID-19 pandemic in India with more than 30 million cases and over 0.39 million deaths has significantly impacted and stressed the health structure of the country. With a shortage of intensive care unit beds, oxygen and trained personnel the country is facing a major health crisis. Itolizumab was used as an anti-inflammatory in severe COVID-19 in the absence of other immunomodulators during the second wave of COVID19 Infection. It received EUA by DCGI in July 2020 and this EUA has not since been revised since. The picture now as compared to July 2020 is different and we do have evidence available now that drugs like Tocilizumab and Baricitinib are efficacious. However, in case of Itolizumab, there is not enough data. It is a priority thus to review data available to make a recommendation on Itolizumab as it is being used in the country as a re purposed immunomodulatory drug for COVID-19 infection. It was re purposed and approved by DCGI under restricted emergency use authorization for treatment of cytokine storm syndrome. CDSCO also provided feedback on how the phase 4 study should be conducted. These considerations were based on biological plausibility however it should be kept in mind that the mechanisms of action are different. This is a cell surface anti CD6 molecule.
Desirable effects
The group agreed that the evidence suggests that Itolizumab does not have a clinically significant effect on mortality, progression to oxygen, progression to NIV/IMV, improvement in the mean P/F ratio, mean D-dimer level, mean CRP level, change in serum ferritin levels and mean serum LDH. All the critical and important outcomes were of very low certainty evidence in a small, drug-company sponsored, methodologically flawed trial in 30 patients.
Undesirable effects
The pooled data suggested that the evidence is very uncertain about the effect of Itolizumab on adverse events. So hence even though the evidence we found was uncertain, numerically in the study 18/22 patients had adverse events which was in keeping with the clinical experience of the group. However clinicians who have used it among the expert group revealed that this drug had many side effects -infusion reactions with clinicians reporting anaphylaxis leading to death as well. In addition the group felt that the efficacy in real world practice when used was unpredictable. Other experts in the group reported that autoimmune flare ups are reported with this drug when used for other indications along with infusion reactions. This in fact prompted them to stop using the drug.
Certainty of evidence
Using GRADE methodology, the evidence synthesis team rated certainty of evidence as very low certainty of evidence for all outcomes including mortality, progression to oxygen requirement, progression to NIV or IMV, improvement in mean P/F ratio, mean D-dimer levels, mean CRP levels, changes in serum ferritin levels, mean serum LDH levels and adverse events. The group agreed with these judgements and rated the overall certainty of evidence as very low.
Values
The outcomes studied in this study were time to clinical improvement, progression to respiratory failure and assessment of biomarkers at baseline and day 14, 30 to assess utility of this drug in the cytokine storm. The group felt that there was probably no important uncertainty or variability in what outcomes were chosen in the trial. Discussion was also held about how to measure the efficacy of this drug, however unlike Tocilizumab where IL-6 is a common marker used, here anti-CD6 activity can only be measured by flow cytometry.
Balance of effects
The expert working group felt that the evidence in this small methodologically flawed study with 30 patients sponsored by the pharma company did not provide enough information for us to make a clear decision in favor or against the drug. However, the overwhelming opinion of the group was this drug should not be recommended at this time for the treatment of COVID-19 infection as it had side effects recorded in the study as well the personal experience in the group did not favor the use of this drug.
Resources required
The group felt that this drug is expensive and in the absence of clear-cut evidence of efficacy does result in moderate costs. The approved drugs in dealing with cytokine storm are Baricitinib which is cheaper as it is generically produced and varies in cost from Rs 420 per course to Rs 22,000/-. Tocilizumab is also expensive and has a defined subset of population where it is likely to work. The group discussed and concluded that the requirement of resource was of moderate costs. The group includes clinicians in different types of Indian hospitals who have a good idea of drug and hospitalization costs.
Certainty of evidence of required resources
The group discussed that the costs are known (the drug is available for use in indications other than COVID-19). It has been approved prior for use in chronic psoriasis and commonly used dose is 100 – 200 mg and hence felt that there was high certainty of evidence for required resources to implement this intervention.
Cost effectiveness
The group discussed that there are no studies looking at this. Though in other previous beneficial interventions which impact mortality or progression to invasive mechanical ventilation the groups made a judgement call based on cost effectiveness, market costs and the benefit it is likely to provide. The group felt that the same would not be necessary here.
Equity
At this point in time this intervention would reduce impact on equity as this is an expensive intervention which is not available freely.
Acceptability
The group felt that this intervention has no formal data available. Clinicians feel they would not use this drug with the present data available. 4 clinicians have used and felt that there were too many side effects. At this point the group decided, they don’t know enough about the use of this drug in COVID-19. There is insufficient efficacy data and with safety concerns, it is something they would not recommend to stakeholders during prevailing uncertainties.
Feasibility
The feasibility of this intervention varies as this drug is available and can be administered. However in the absence of efficacy this drug should not be recommended for use and hence even if feasible should not be recommended at this time.
The group is of the opinion that this drug should not be used except in the context of randomized controlled trials. Although it is not considered a primary (first line) drug against SARS CoV-2 virus, Itolizumab was provided license for emergency use, during the COVID19 pandemic, in India. It was used as an alternative to Tocilizumab when there was a shortage of the latter in the market, although it should not have been considered as such.
However, the approval was granted for a restricted emergency use based on a relatively small phase II trial without conducting a conventional phase III trial when other efficacious immunomodulator options were not available and hence it is likely this would be revised in the near future. There is a strong recommendation against its use by the experts. In view of paucity of credible data, its use should be discouraged in covid 19 at this moment. However, there is a definite need for a large RCT to generate valid data before any attempt to change the current standard of care. At this moment, Itolizumab should not be used as an alternative to Tocilizumab or otherwise, based on its biological differences and paucity of valid data in its favor.
In the moderate to severe category the evidence about the effect of Itolizumab on reduction in progression of disease in requiring oxygen, NIV, mechanical ventilation or improvement in biomarkers was uncertain with low certainty of evidence. Hence, the committee recommends against use of Itolizumab in all subgroups of patients with COVID-19.
There is very limited data regarding the exact dose of Itolizumab for the treatment of COVID 19. The factors guiding further doses are also unclear as of now. The chosen dose was calculated based on experience in patients with chronic plaque psoriasis. The frequently observed side effects were infusion related reactions like chills, rigor, urticaria, wheezing, etc with some cases even reporting further oxygen decompensation in COVID 19 patients. Premedication with Hydrocortisone, and Pheniramine with slow rate of infusion could be tried to prevent infusion related reactions. Due to concern over frequent adverse events, the drug has been frequently discontinued and hence this precludes its use despite ready availability. The recommendation against the use of Itolizumab will be reviewed as and when new evidence emerges.
There is a severe paucity of evidence with regards to this drug. There is very limited evidence of efficacy, timing, cost effectiveness of Itolizumab in COVID-19 infection. Age, comorbidities, prior sensitization, immunocompromised status, dose and time of administration are other aspects that merit further study. We need more randomized controlled trials with larger numbers to evaluate the direct effects of Itolizumab and also as an alternative to Tocilizumab. We also need to follow up patients for delayed side effects. However, this also needs to be balanced against the knowledge that we do have suitable alternative options.
- Ministry of Health and Family Welfare. DCGI gives nod for restricted emergency use to Itolizumab for moderate to severe COVID-19 patients. Accessed on 15 November 2021 at URL https://pib.gov.in/PressReleasePage.aspx?PRID=1637926
- Kumar et al., A two-arm, randomized, controlled, multi-centric, open-label phase-2 study to evaluate the efficacy and safety of Itolizumab in moderate to severe ARDS patients due to COVID-19;
Covid Management Guidelines India Group – Anti-inflammatory Working Group – Itolizumab. Covid Guidelines India; Published online on 22nd November 2021; URL: https://indiacovidguidelines.org/itolizumab/ (accessed <date>)
