Explanations:
a. Downgraded by one level serious bias; high ROB
b. Downgraded by one level for serious inconsistency: as I square is 59%
c. Downgraded by one level serious imprecision; wide CI and clinically non significant
d. Downgraded by one level for serious RoB in two studies
e. Downgraded by one level serious inconsistency; I square is 82%
f. Downgraded by one level for serious imprecision; Lower limit of CI overlaps the line of no difference
g. Downgraded by two levels very serious imprecision; Three studies reporting median and IQR with favoring intervention group, Single study reporting Mean and SD favoring control group.
h. Downgraded by one level serious imprecision; wide CI and OIS criteria was not met
i. Downgraded by one level serious inconsistency; I square is 69%
j. Downgraded by one level serious imprecision; Lower limit of CI overlaps the line of no difference
k. Downgraded by one level serious imprecision; wide CI
Interferons are a biological molecule secreted by immune and infected epithelial cells in response to cell injury, viral infections and tumors. It plays a key role as a part of the innate immune response.2-4 They have immunomodulatory and antiviral effects and have been used for the treatment of Multiple sclerosis and Hepatitis C infection.5 Interferons are classified on the basis of receptors as Class I, II and III. With the start of COVID-19 pandemic, multiple repurposed drugs have been tested for its efficacy and Interferons were one of the potential agents in this category.6 In the pre COVID -19 pandemic era, studies have shown the antiviral effects of Interferons on Rhino virus, Respiratory syncytial virus and Influenza virus infections.7 This review aims to provide a summary of the available evidence from randomized clinical trials of Interferons for treatment of COVID-19, which could guide clinicians and researchers regarding the appropriate use of this drug in COVID-19 in the future.
We searched PubMed, Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language. We also reviewed reference lists of systematic reviews and included studies. We performed all searches up to 10th December 2021.
We searched the above databases and found 43 records.
After removing duplicates and excluding that which did not match our PICO question, 12 RCTs were screened and 10 studies assessed for eligibility. One meta-analysis was reviewed; however it included RCTs which were already selected by our search. A total of 9 RCTs were included for meta-analysis.
We extracted data for the following outcomes, as pre-defined by the Expert Working Group:
Primary outcome:
a) All-cause mortality
b) Time to clinical improvement
Secondary outcomes
a) Duration of hospital stay
b) Progression to ICU
c) Progression to O2 therapy/Ventilator
d) Adverse events
Two reviewers independently assessed eligibility of search results using the online Rayyan tool. Data extraction was performed by one reviewer, and checked by another, using a piloted data extraction tool on Microsoft Word. Risk of bias (RoB) was assessed using the Cochrane RoB v2.0 tool, with review by a second reviewer and compared with covidnma database . If there was a difference in more than one domain it was assessed by a third independent author. We planned to use risk ratios (RR) fordichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). We planned to meta-analyze if appropriate, i.e., if outcomes were measured and reported in a similar way for similar populations in each trial, using random-effects models with the assumption of substantial clinical heterogeneity (the appropriateness of which would be checked by the Methodology Committee), and the I2 statistic to calculate residual statistical heterogeneity, using Review Manager (RevMan) v5.4. We used GRADE methodology to make summary of findings tables on GRADEPro GDT.
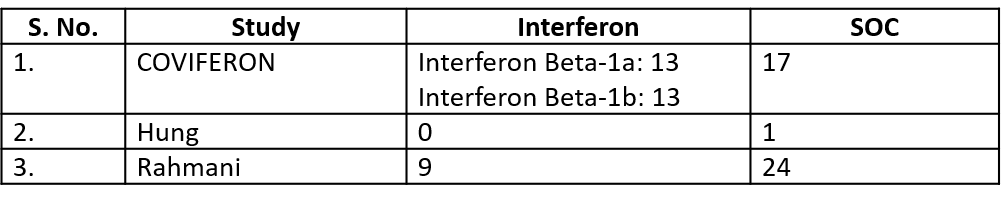
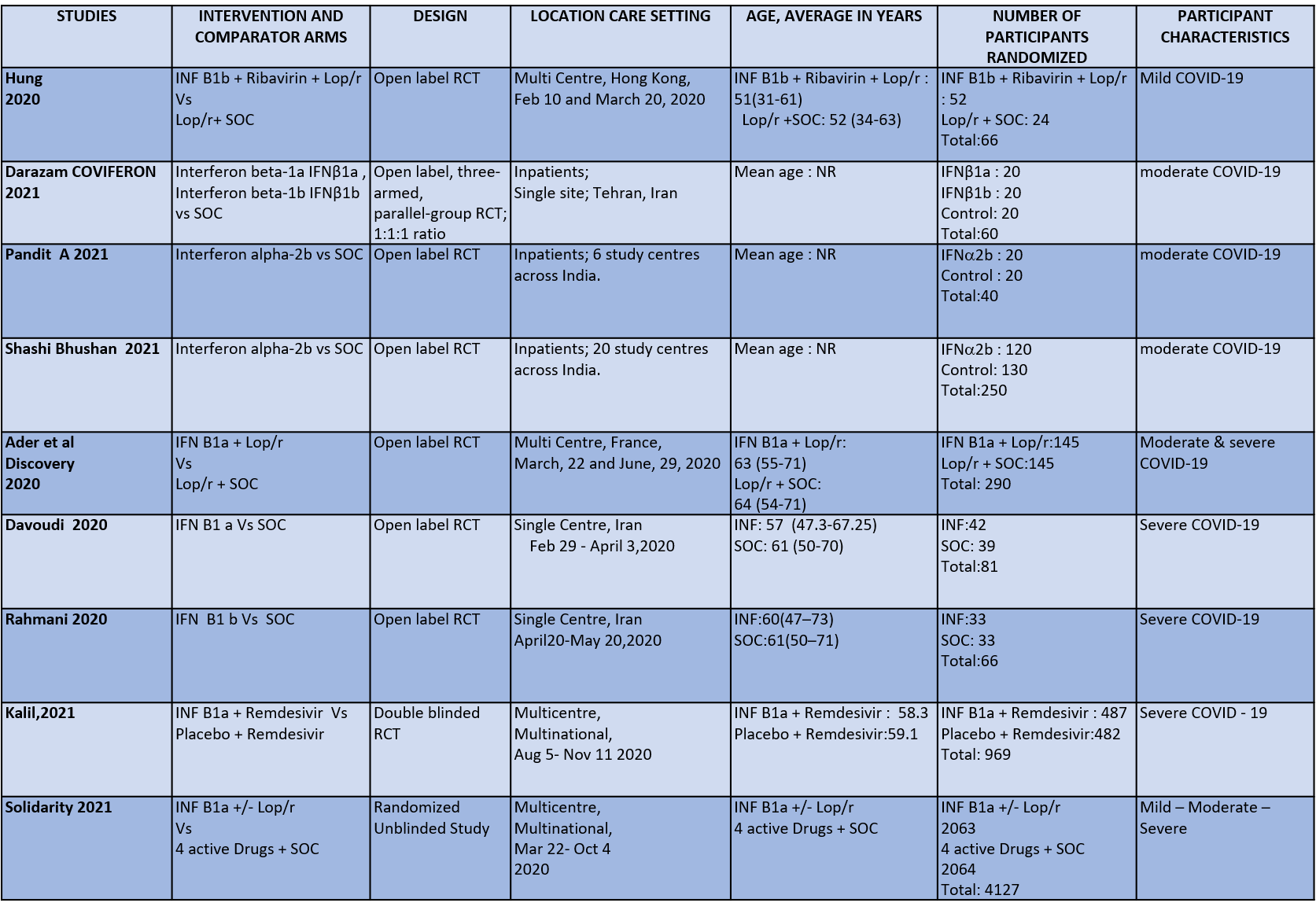
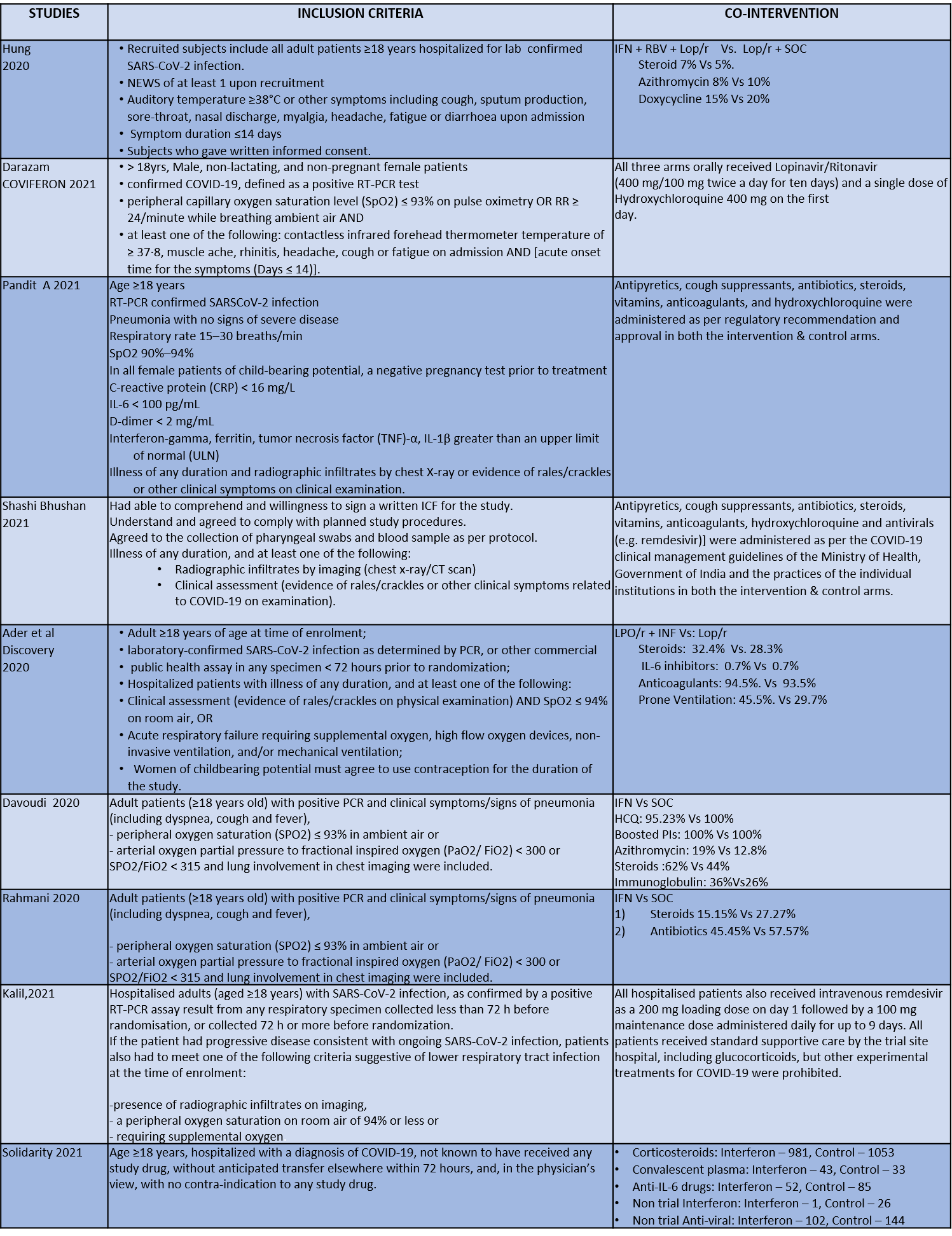
We found 9 RCTs that matched the PICO question as pre-defined by the expert working group. The trials included a total of 5949 participants, all of whom were adults. Two trials were reported from India8-9, three from Iran9-12, two from Russia15,16, and one from France13, all of which were of varying disease severity ranging from moderate to severe. Also, the trials had participants who were enrolled to varying interventions i.e. some studies compared interferon with standard of care or placebo whereas others with active comparators. All trials were done on hospitalized patients with confirmed COVID-19 infection.
Each trial and its results are described below; characteristics of the trials are summarized in the “Summary of characteristics of included trials” table. All the trials had varying risk of bias across all domains. Risk of bias for each domain per trial is displayed alongside the forest plots below.
The following comparisons were investigated in the trials (we compared outcomes for arms randomized to Interferon vs. standard of care or active comparators).
● Five trials8-12 compared Interferon with standard of care(440 participants)
● Four trials13-16 compared Interferon with an active comparator (e.g.: Remdesivir, Ribavirin, Lopinavir/Ritonavir, Tocilizumab)
Our expert working group classified mortality, time to clinical improvement as primary outcomes and duration of hospitalization, progression to oxygen therapy/ventilation or progression to ICU and adverse events as secondary outcomes.
Primary Outcomes
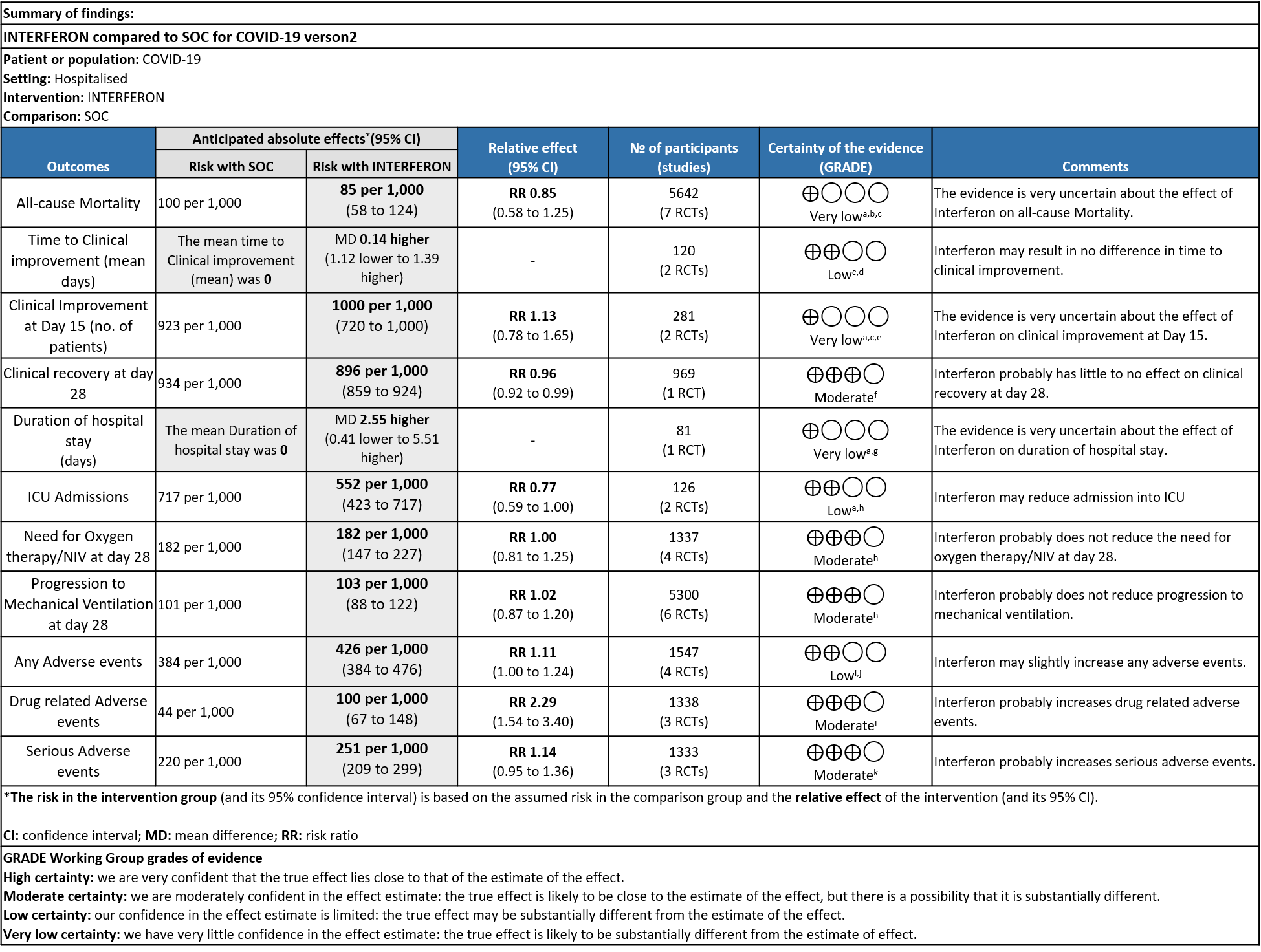
As presented in the ‘Summary of findings’ table, there is low or very low certainty evidence regarding the effect of Interferon on mortality, time to clinical improvement (not requiring oxygen or hospitalised care), duration of hospital stay, progression to ICU care and any adverse events. The evidence is of moderate quality for outcomes of progression to oxygen or ventilation (non-invasive or invasive), all adverse events, drug-related adverse events and serious adverse events.
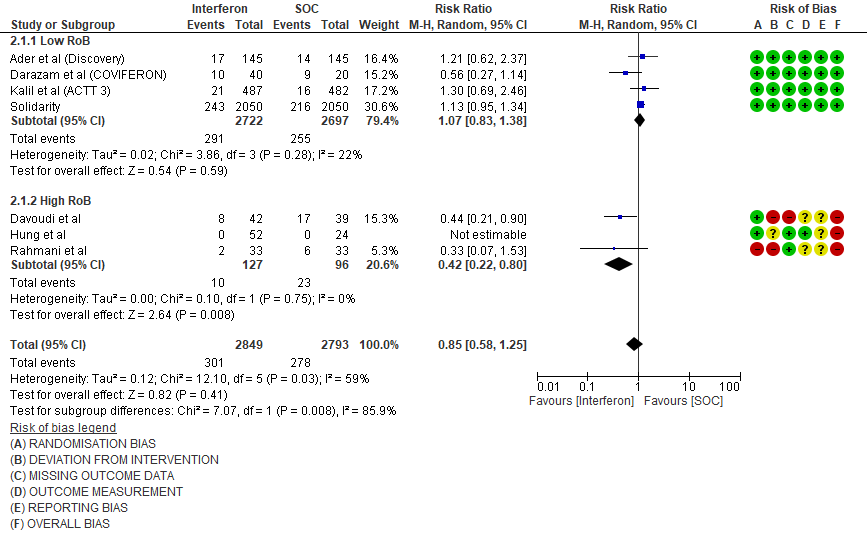
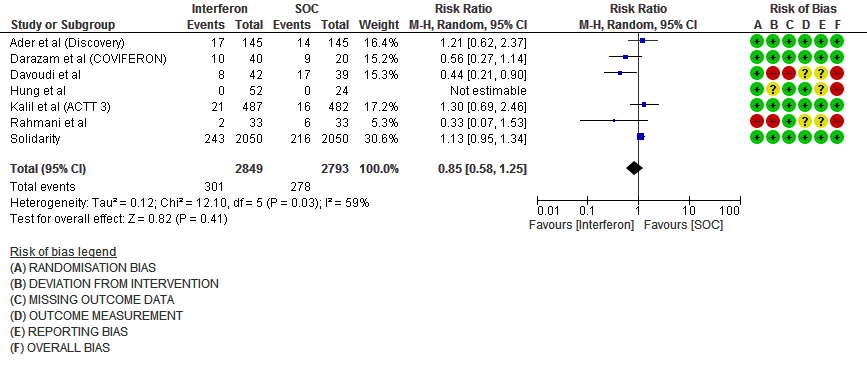
a) All-cause mortality: Very low certainty of evidence in 5642 patients in seven RCTs10-16 found a very uncertain effect of Interferon on all-cause mortality (RR 0.85; 95% CI 0.58 to 1.25). There were no significant differences observed even when trials were stratified by severity, risk of bias or comparators.
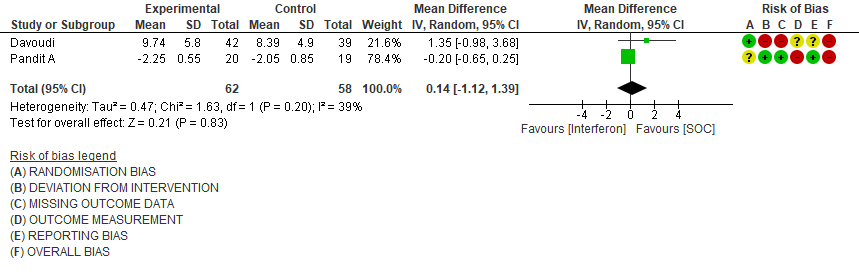
b) Time to Clinical improvement [mean days](time to achieve >2 point reduction in the WHO ordinal score):Low certainty of evidence in 121 patients intwo RCTs8,11suggests that Interferon may result in no difference in mean time to clinical improvement(MD 14 days higher; 1.12 lower to 1.39 higher days).
Secondary Outcomes
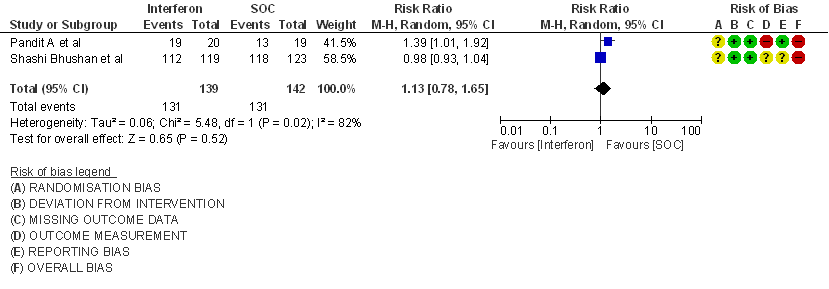
a) Clinical Improvement at Day 15: Very Low certainty evidence in 281 patients from two RCT8,9, found that the evidence is very uncertain about the effect of interferon on clinical improvement at day 15 (RR 1.13; 95% CI 0.78 to 1.65).
b) Clinical recovery at Day 28 (time to achieve WHO score 1,2,3 or not requiring hospitalization for oxygen or medical care): Moderate certainty evidence in 969 patients from one RCT15, found that Interferon made little to no difference in clinical recovery at day 28 (RR 0.96; 95% CI 0.92 to 0.99).
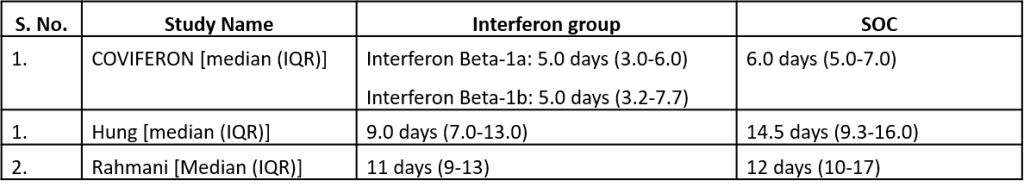
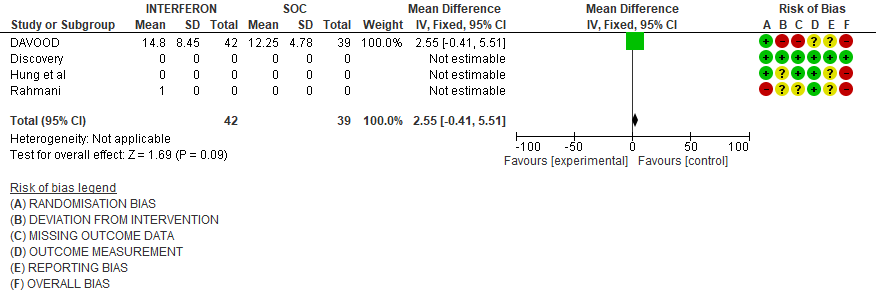
c). Duration of hospital stay(days): Very low certainty of evidence in 81 patients from one RCT11suggested that the effect of interferon on the mean duration of hospitalization was
higher in patients receiving Interferon MD 2.55 days higher (0.41 days lower to 5.51 higher).
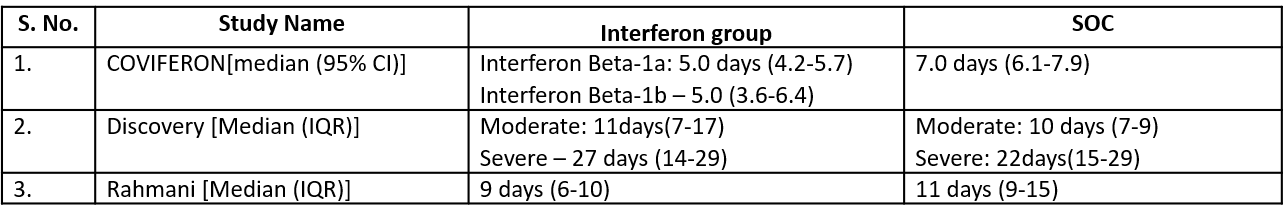
Three studies10,12,14 reported duration of hospital stay in median (IQR).
Three studies10,12,14 reported duration of hospital stay in median (IQR)
d) Progression to oxygen therapy/NIV at Day 28: Moderate certainty evidence in 1337 patients from 4 RCTs10,11,13,15, found that Interferon probably does not reduce the need for oxygen therapy/NIV at day 28(RR 1.00; 95% CI 0.81 to 1.25).
e. Progression to Invasive mechanical ventilation at Day 28: Moderate certainty evidence in 5300 patients from six RCTs10-13,15-16, found that Interferon probably does not reduce progression to mechanical ventilation at day 28 (RR 1.02; 95% CI 0.87 to 1.20).
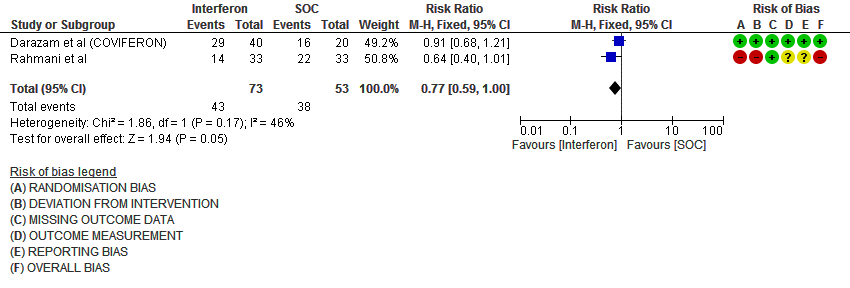
f. Progression to intensive care: Low certainty evidence in 126 patients from two RCTs10,12, found that Interferon may reduce admission into ICU (RR 0.77; 95% CI 0.59 to 1.00).
g. Any Adverse events: Low certainty evidence in 1547 patients from four RCTs8-9,13,15, found that Interferon may slightly increase adverse events(RR 1.11; 95% CI 1.00 to 1.24).
h. Drug-related Adverse events: Moderate certainty evidence in 1338 patients from three RCTs11,13,15, found that Interferon probably increases drug-related adverse events when compared to standard of care or active comparator(RR 2.29; 95% CI 1.54 to 3.40).
i. Serious Adverse events: Moderate certainty evidence in 1333 patients from three RCTs13-15, found that Interferon probably increases serious adverse events when compared to standard of care or active comparator (RR 1.14; 95% CI 0.95 to 1.36).
Disaggregated data could not be obtained for co-morbidities, inflammatory markers or different age groups. Subgroups of pregnancy, lactation, renal failure, liver failure were excluded from most trials. No data were available separately for immunocompromised individuals in any of the trials.
Forest plots:
Each outcome has been pooled and evaluated separately as a sub group with studies which had a low risk of bias and high risk of bias.
1.Mortality
2. Admission into intensive care (ICU)
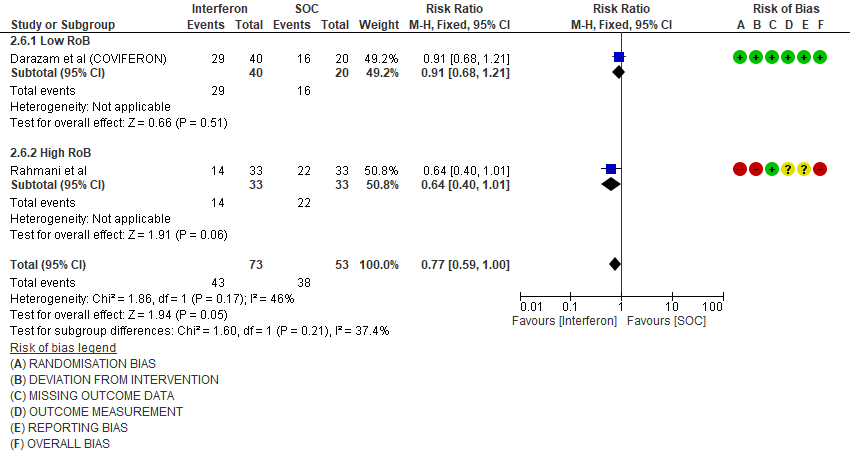
3. Progression to Oxygen Therapy
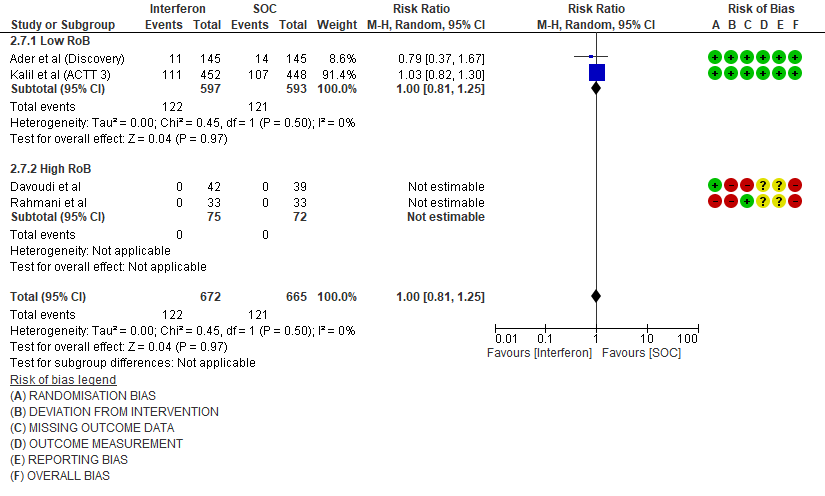
4. Progression to NIV/IMV
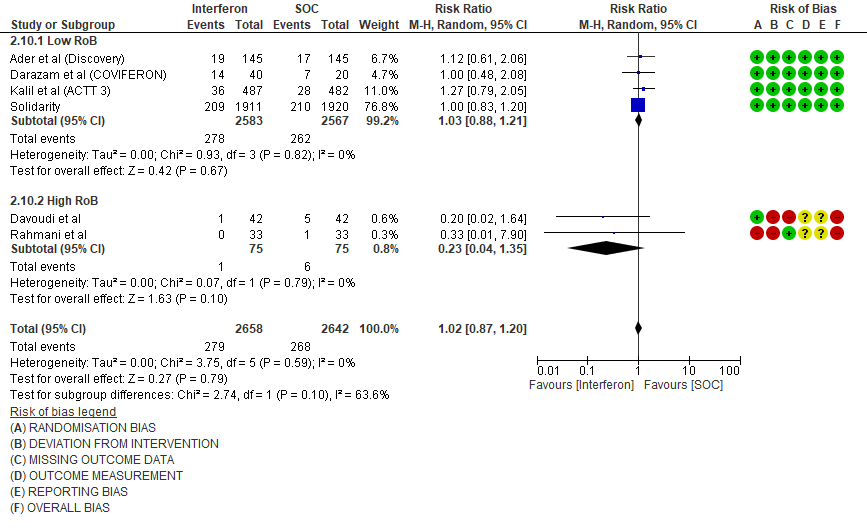
5. Adverse events
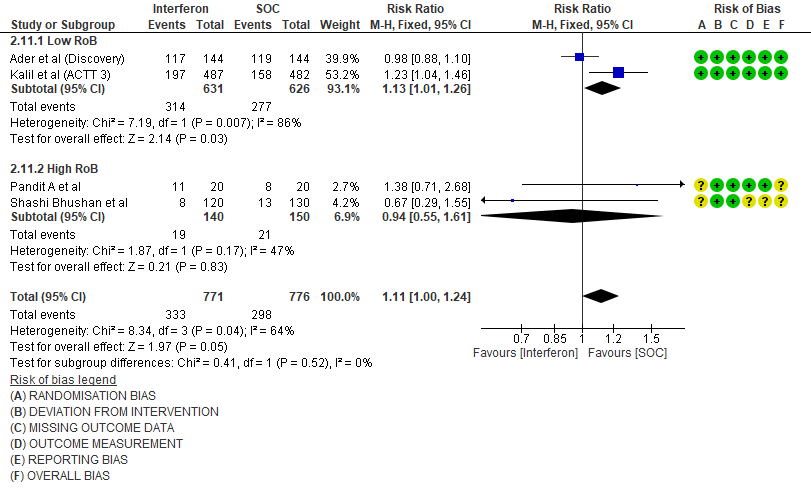
6. Drug related adverse events
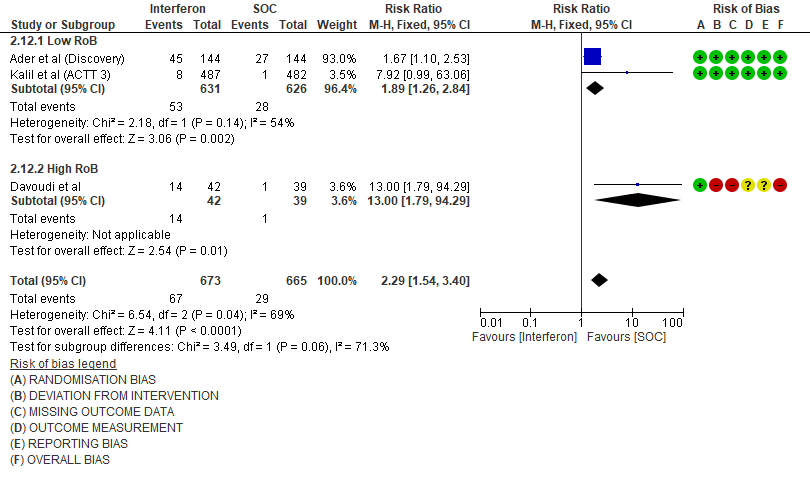
7. Serious Adverse events
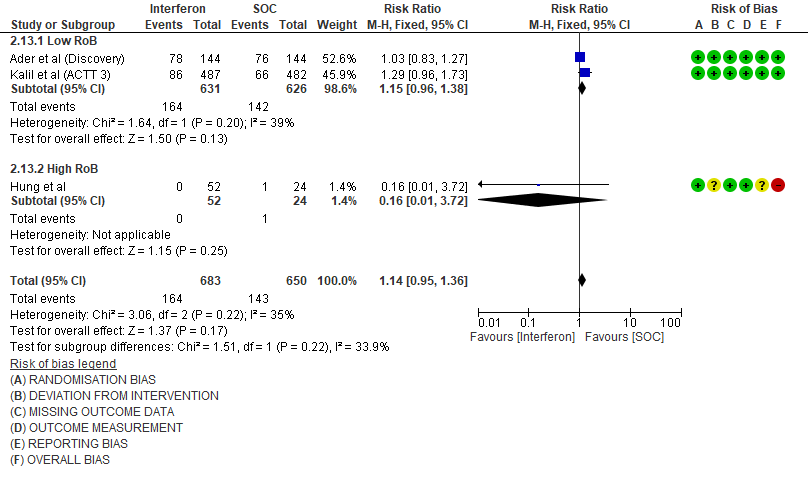
1.Mortality
2. Time to Clinical improvement [mean days](time to achieve >2 point reduction in the WHO ordinal score)
3. Clinical Improvement at Day 15
4. Clinical recovery at Day 28 (time to achieve WHO score 1,2,3 or not requiring hospitalization for oxygen or medical care)
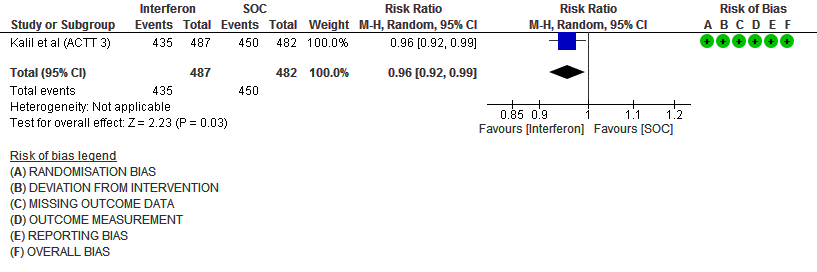
5. Duration of Hospital stay (days)
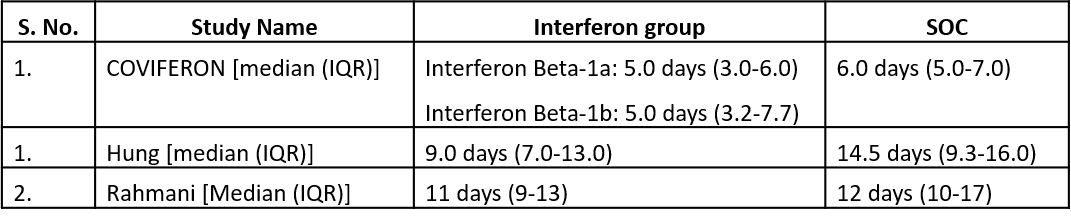
6. Progression to intensive care (ICU)
7. Progression to oxygen therapy/NIV at Day 28
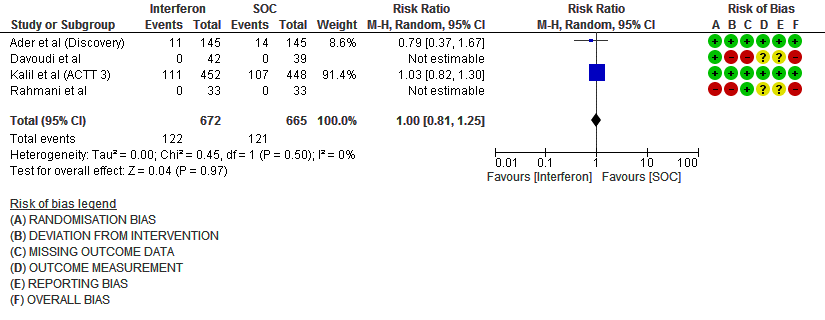
8. Progression to Invasive mechanical ventilation at Day 28
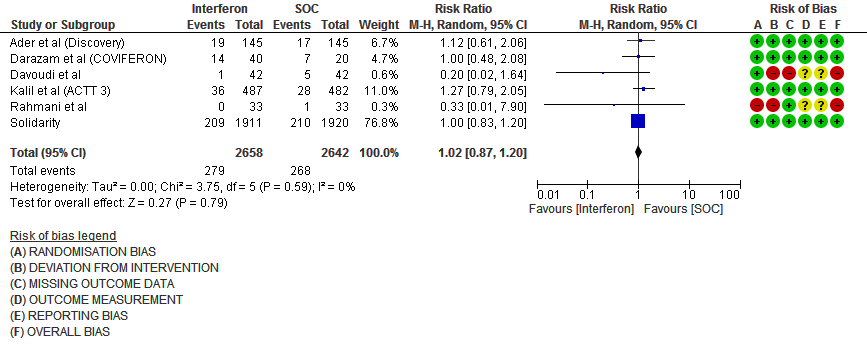
9. Adverse events (no. of patients)
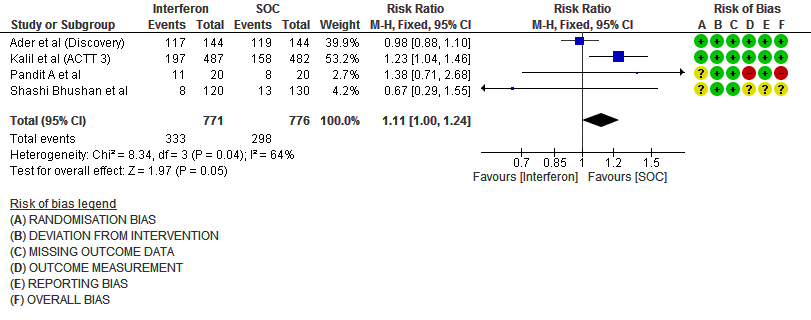
Number of events
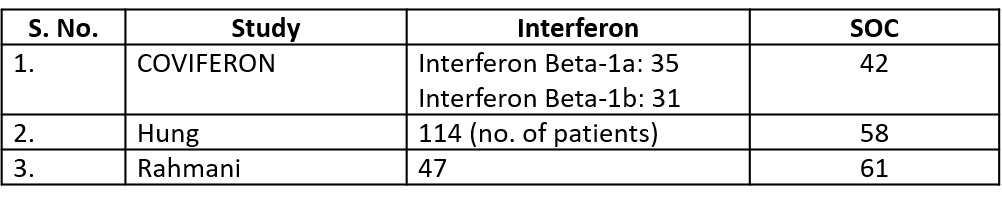
10. Drug related adverse events (no. of patients)
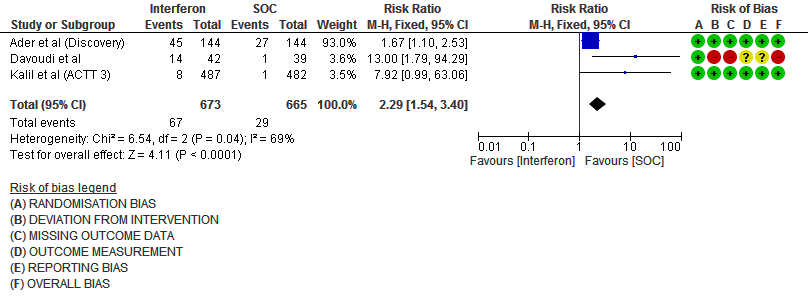
11. Serious Adverse events (no. of events)
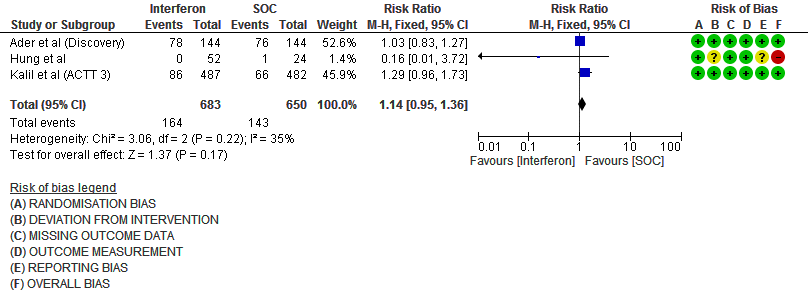
Events