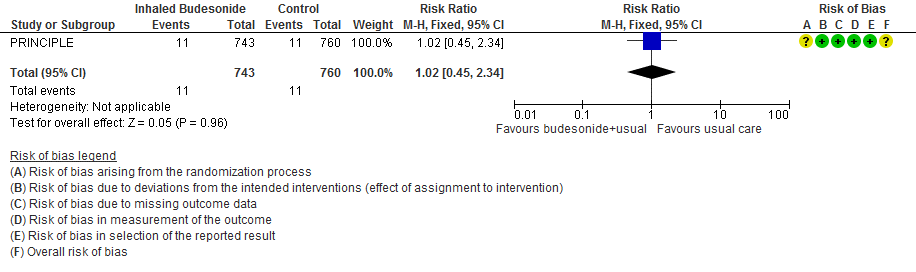
Summary of findings table 1: Inhaled budesonide started an average of 3 days after symptom onset compared to no budesonide for treatment of outpatients with non-severe COVID-19, with a mean age of 44 years
Explanations:
a. STOIC trial [7]
b. Downgraded by 1 level for serious risk of bias. The STOIC trial was assessed to be at high overall risk of bias (high risk of bias in measurement of outcome and selection of the reported result).
c. Downgraded by 1 level for serious imprecision. The lower boundary of the CI is 1.78, which may not be considered a clinically appreciable benefit.
d. Downgraded by 1 level for serious indirectness, as the study was conducted in one country and only recruited between surge situations. The pathway for referral for urgent care was very specific and might not be relevant to other countries
e. Downgraded by 1 level for serious imprecision. The optimal information size was not met for this outcome.
f. Downgraded by 1 level for serious imprecision, due to CI overlapping no effect and inability to exclude serious benefit or serious harm.
g. Downgraded by 2 levels for very serious imprecision, due to small absolute number of events, CI overlapping no effect and inability to exclude serious benefit or serious harm.
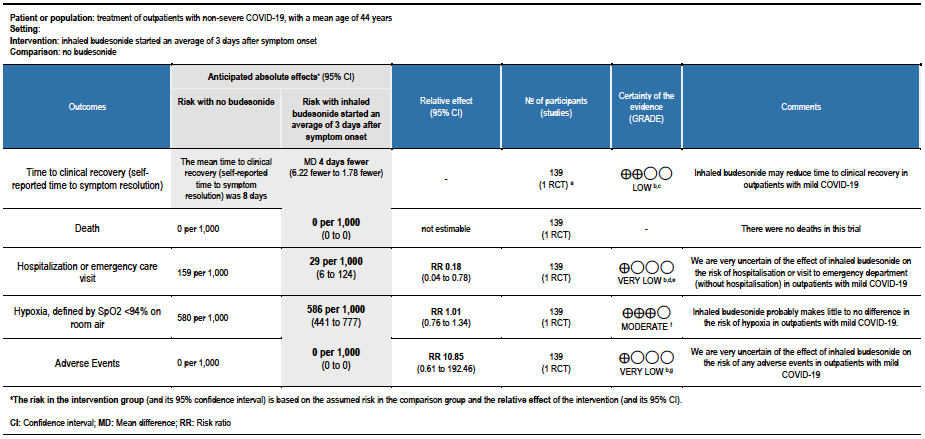
Summary of findings table 2: Inhaled budesonide started an average of 6 days after symptom onset compared to no budesonide for treatment of outpatients with non-severe COVID-19, with a mean age of 63 years
Explanations:
a. PRINCIPLE trial [8]
b. Downgraded by 1 level for serious risk of bias, due to early reporting of results for a time-to-event outcome without confirmation of meeting power calculation, and the effect of no placebo on an unblinded participant-reported outcome.
c. Downgraded due to reporting bias: the interim analysis was published using a subgroup of participants, and results were not published for all outcomes.
d. Downgraded by 1 level for serious imprecision, due to CI including no effect, and inability to exclude important benefit or serious harm with budesonide.
e. Downgraded by 1 level for serious imprecision, due to inability to exclude no benefit from budesonide.
f. Downgraded by 2 levels for very serious imprecision, due to few events, and CI including no effect, and inability to exclude important benefit and very serious harm with budesonide
Corticosteroids are potent anti-inflammatory agents [1], used widely in medicine. Inhaled corticosteroids have been the cornerstone of treatment in chronic respiratory diseases like asthma for decades [2], in whom they have been shown to reduce the number and severity of exacerbations [3].
In studies in the present COVID-19 pandemic, in-vitro studies have shown that inhaled corticosteroids reduce the replication of SARS-CoV-2 in airway epithelial cells, in addition to the downregulation of expression of ACE2 and TMPRSS2 genes, which are critical for viral cell entry [4].
Systemic corticosteroids have been reported to reduce mortality and need for invasive ventilation in patients hospitalised with COVID-19 as demonstrated by the RECOVERY trial and subsequent trials [5].
It is likely that the beneficial effect of corticosteroids in severe viral respiratory infections is dependent on the selection of the right dose and duration. High doses may be more harmful than beneficial, especially in earlier disease [5], though this may be due to systemic effects, which might be avoided using local delivery to the respiratory epithelium, using the inhaled route.
Based on this, and interesting observations from observational studies of under-representation of patients with chronic respiratory diseases among patients with severe COVID-19 [6], multiple trials of inhaled corticosteroids in non-severe COVID-19 have been started. Two of these have reported, with resulting use of this intervention around the world [7;8].
This review aims to provide a summary of the available evidence from randomised controlled trials (RCTs) of inhaled corticosteroids for treatment of COVID-19, so the Expert Working Group can provide a recommendation to guide clinicians and researchers regarding appropriate use of this intervention.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Pubmed) and Epistemonikos for RCTs of any publication status and in any language. We performed all searches up to 12th May 2021. We contacted researchers to identify unpublished and ongoing studies.
We planned to extract data for the following pre-defined outcomes:
- Critical (primary for this review):
- Time to clinical recovery (including self-reported)
- Need for hospitalisation
- Need for oxygen support
- Important (secondary):
- Need for invasive or non-invasive ventilation
- All-cause mortality
- Adverse events:
- All
- Serious
- Oral candidiasis
- Secondary lung infection (clinician defined bacterial pneumonia)
Two reviewers independently assessed eligibility of search results using the online Rayyan tool. Data extraction was performed by one reviewer, and checked by another, using a piloted data extraction tool on Microsoft Word. Risk of bias (RoB) was assessed using the Cochrane RoB v2.0 tool, with review by a second reviewer and then discussion of each trial’s judgments with a third senior reviewer. We planned to use risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). We planned to meta-analyse if appropriate, i.e. if outcomes were measured and reported in a similar way for similar populations in each trial, using random-effects models with the assumption of substantial clinical heterogeneity (the appropriateness of which would be checked by the Methodology Committee), and the I2 test to calculate residual statistical heterogeneity, using Review Manager (RevMan) v5.4. We used GRADE methodology to make summary of findings tables on GradeProGDT.
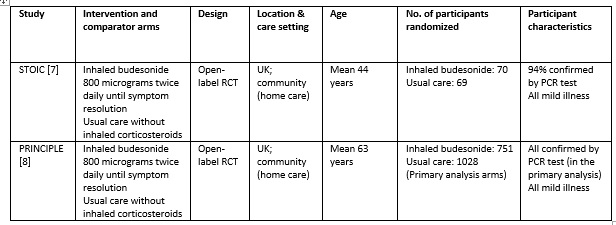
We found 1119 articles including 29 RCTs and 5 meta-analyses, of which only 2 RCTs matched the PICO question as pre-defined by the expert working group [7;8]. The trials included a total of 1799 symptomatic participants, all of whom were adult outpatients in the UK with illness satisfying the Guidelines Group’s criteria of mild COVID-19. However, as the trials did not report any outcomes in a way that allowed them to be pooled, meta-analysis was not performed. Additionally, as the trials recruited participants with substantially different characteristics, two separate summary of findings tables were made, one for each trial. Each trial and its results are described below; characteristics of the trials are summarised in the Summary of characteristics of included trials table.
STOIC trial
The STOIC trial [7] was a community-based, open-label randomized trial with two arms: one with inhaled budesonide 800 micrograms twice daily using a dry-powder Turbohaler inhaler device (until resolution of symptoms) and the other received usual care without inhaled corticosteroids. Initially, 73 participants were randomized to each arm; 70 and 69 completed the trial and were included in the primary analysis, respectively. All patients were aged 18 years or above, and had mild COVID-19 within 7 days of symptom onset. All the baseline characteristics were similar, including symptoms, age, comorbidities and positivity for PCR for SARS-CoV-2 (94% overall). The primary outcome of the study was COVID-19 related urgent care visit, which included emergency department visit and hospitalisation. This was a composite outcome and a breakdown was not available.
The secondary outcomes were clinical recovery (self-reported symptom resolution), intensity of viral symptoms (questionnaire based), oxygen saturations measured using a pulse oximeter and temperature (both self-monitored) and SARS-CoV-2 viral load.
The mean age of participants was 44 years, and they were enrolled at a median of 3 days since symptom onset. They had a median of 1 pre-existing comorbidity.
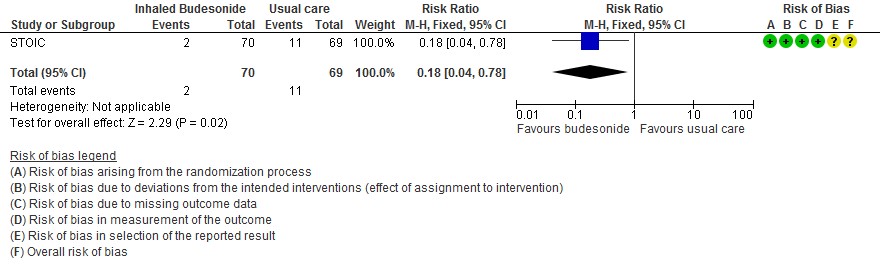
The intention to treat (ITT) analysis showed that urgent care visits occurred in 2 participants (3%) in the inhaled budesonide group versus 11 (15%) in the usual care group.
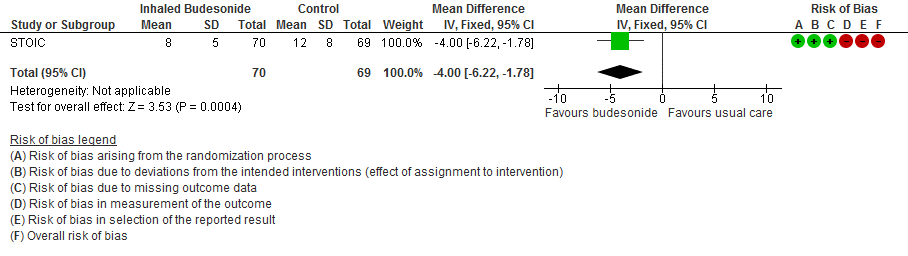
The self-reported time to clinical recovery was an average of 1 day shorter in the inhaled budesonide group: median 7 days (95% CI 6 to 9) versus 8 days (95% CI 7 to 11).
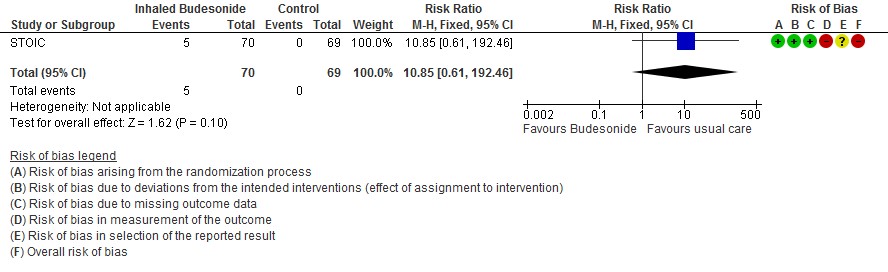
The other outcomes were similar in both the groups; there were 5 adverse events reported in the inhaled budesonide group which were mild (4 sore throat, 1 dizziness), thought the dizziness prompted discontinuation of the inhaled budesonide.
We found potential risks of bias across all domains except domain 2, which investigates deviations from the intended intervention. Risk of bias for each domain per trial and outcome is displayed alongside the Forest plots below.
STOIC (mean age 44 years, median 3 days from symptom onset, mild COVID-19):
- Inhaled budesonide may reduce time to clinical recovery in outpatients with mild COVID-19, with a mean difference of 4 days fewer in the inhaled budesonide vs. the usual care group (95% CI 6.22 fewer to 1.78 fewer; low certainty in the evidence).
- There were no deaths in this trial.
- The effect of inhaled budesonide on the risk of hospitalisation or visit to emergency department (without hospitalisation) is very uncertain in outpatients with mild COVID-19 (RR 0.18; 95% CI 0.04 to 0.78; very low certainty in the evidence).
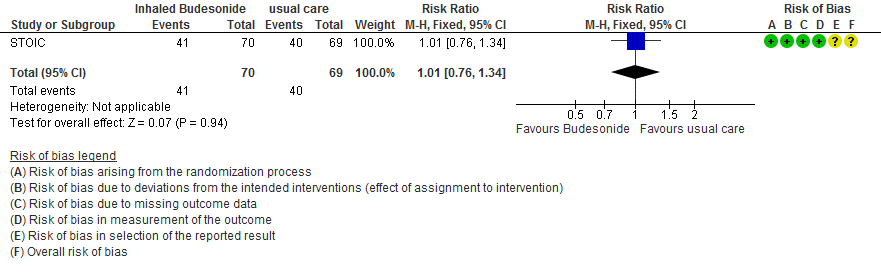
- Inhaled budesonide probably has little to no effect on risk of progression to hypoxia (<94% oxygen saturation breathing room air) in outpatients with mild COVID-19 (RR 1.01; 95% CI 0.76 to 1.34; moderate certainty in the evidence).
- We are very uncertain of the effect of inhaled budesonide on the risk of adverse events in outpatients with mild COVID-19 (RR 10.85; 95% CI 0.61 to 192.46; very low certainty in the evidence).
PRINCIPLE Trial
The PRINCIPLE trial [8] was available in a pre-print, non-peer reviewed document reporting results from an interim analysis. While also an open-label randomised trial comparing inhaled budesonide with usual care in people with mild COVID-19, in comparison to the STOIC trial, this trial enrolled older people (>65 years age, or >50 years with comorbidities), who could have had symptom onset up to 14 days before enrolment. The dose and administration method was the same as the STOIC trial (800 micrograms twice daily via a dry-powder Turbohaler), but 14 days of use was planned.
The trial recruited 751 participants in the inhaled budesonide arm versus 1028 in the usual care arm, who had a positive RT-PCR test for SARS-CoV-2. The inhaled budesonide arm included 65.6% participants with an age above 65 years, compared to 58.5% in the usual care arm. Overall average age was 63 years. Of the usual care arm, 15% had chronic respiratory diseases, compared to 8.4% in the inhaled budesonide arm. All the other baseline characteristics were similar. The median time from symptom onset to enrolment was 6 days. Around 80% of participants had comorbidity.
The co-primary outcomes were time to self-reported recovery and a composite outcome of hospitalisation or death due to COVID-19.
The secondary outcomes were how well the participants felt (self-reported ratings on a 10-point scale), incidence of contact with health services, oxygen administration, intensive care unit admission and mechanical ventilation.
The median time to self-reported recovery was 11 days in the inhaled budesonide arm versus 14 days in the usual care arm. The incidence of hospitalisation or death was 59/692 (8.5%) in the budesonide arm versus 100/968 (10.3%) in the usual care arm.
PRINCIPLE (mean age 63 years, median 6 days from symptom onset, mild COVID-19):
- Inhaled budesonide may reduce time to self-reported clinical recovery in outpatients with mild COVID-19 (hazard ratio (HR) 1.21; 95% CI 1.08 to 1.36; low certainty in the evidence).
- Inhaled budesonide may result in little to no difference in the risk of hospitalisation or death in outpatients with mild COVID-19 (RR 0.83; 95% CI 0.61 to 1.12; low certainty in the evidence).
- Inhaled budesonide probably reduces risk of need for oxygen support in outpatients with mild COVID-19 (RR 0.69; 95% CI 0.48 to 1.00; moderate certainty in the evidence).
- Inhaled budesonide may have little or no effect on need for invasive or non-invasive ventilation in outpatients with mild COVID-19 (RR 1.02; 95% CI 45 to 2.34; low certainty in the evidence).
- RR of intensive care unit admission was 0.54 (95% CI 0.24 to 1.21).
Forest plots
STOIC trial
Time to clinical recovery
Hospitalization and ED visits
Progression to hypoxia (SpO2 ≤94% in 14 days)
Adverse events
PRINCIPLE
Time to clinical recovery
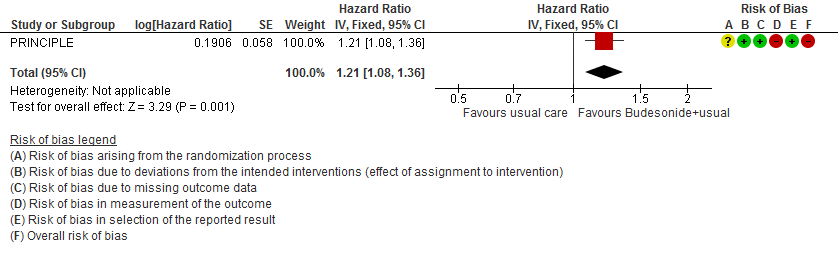
Hospitalization or death
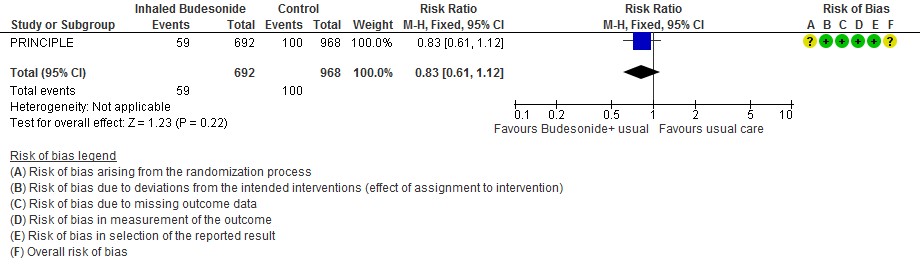
Need for oxygen support
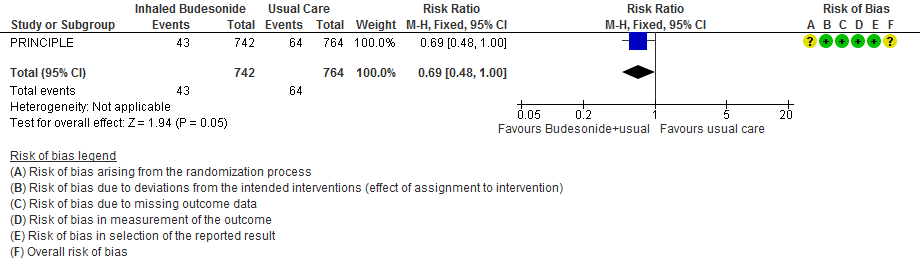
Need for invasive mechanical ventilation