The evidence from randomized controlled trials is very uncertain about the effect of Ivermectin on mortality, progression to mechanical ventilation, time to achieving negative PCR for SARS-CoV-2 and adverse events. Further evidence is needed to ensure its efficacy and safety, from well-conducted large trials.
Currently, its use may distract from use of other therapies for which there is better evidence. Indiscriminate use might also reduce its availability for other conditions where its benefit is established, such as parasitic infections.
Taking this into account, we recommend against use of ivermectin outside of a randomized controlled trial.
Date of latest search: 15th April 2021.
Date of completion & presentation to Expert Working Group: 29th April 2021.
Date of planned review: 15th November 2021.
Evidence synthesis team: Harshdeep Acharya, Audrin Lenin, Richard Kirubakaran, Priscilla Rupali and Bhagteshwar Singh.
Valuable assistance with performing the evidence synthesis was provided by the author team of an ongoing Cochrane systematic review: Maria Popp, Miriam Stegemann, Maria-Inti Metzendorf, Peter Kranke, Patrick Meybohm, Nicole Skoetz and Stephanie Weibel [1].
Click here for an Interactive Summary of Findings table (opens in a new window).
GRADE Working Group grades of evidence
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect
Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect
Explanations:
a. Galan 2021 [14]; Gonzalez 2021 [15]; Lopez Medina 2021 [16]; Mohan 2021 [17]; Niaee et al 2021 [18]; Ravikirti 2021 [20]
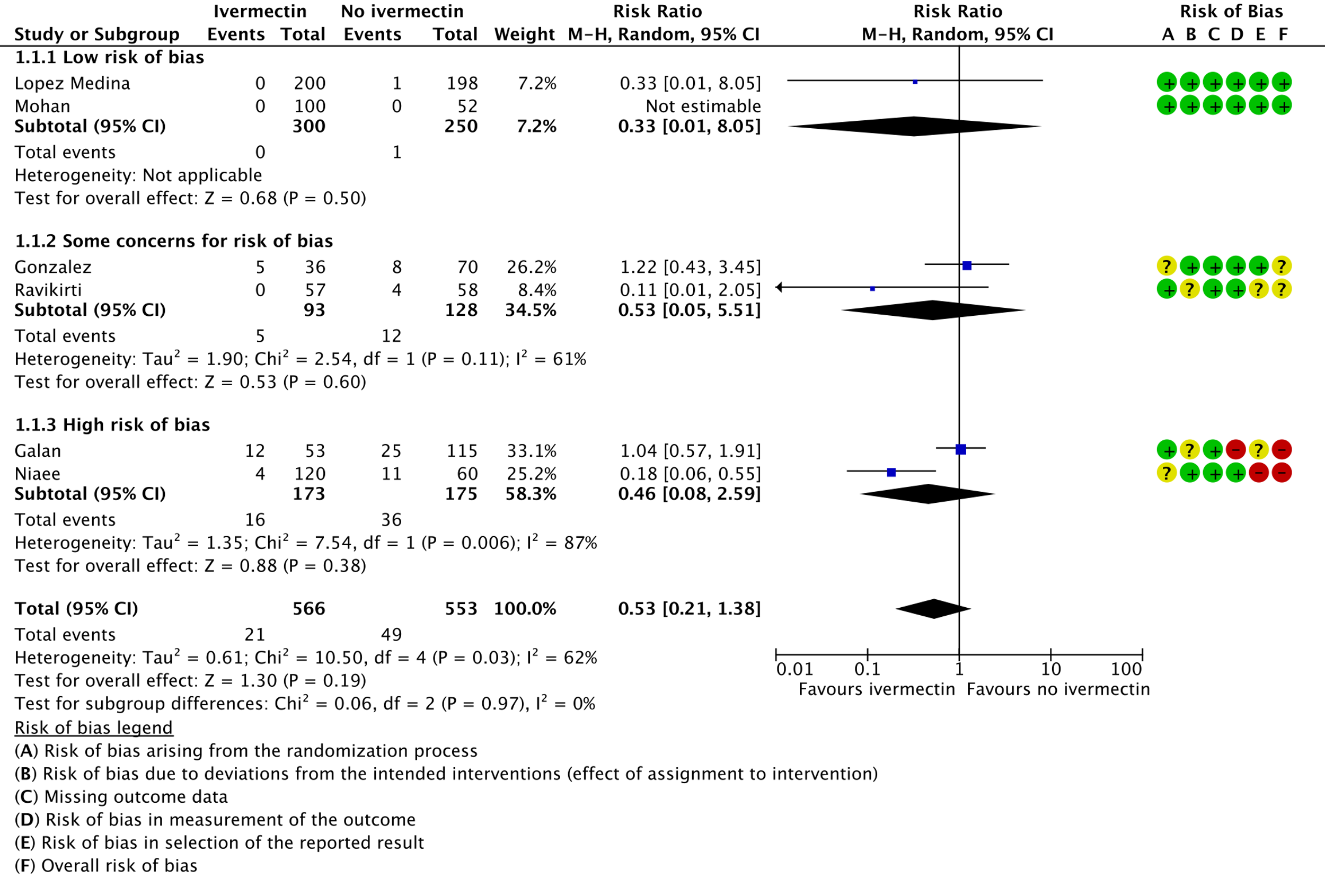
b. Downgraded by one level for serious risk of bias. Due to Galan 2021 [14] and Niaee 2021 [18] having high risk of bias, and Gonzalez 2021 [15] and Ravikirti [20] having some concerns for risk of bias.
c. Downgraded by one level for serious inconsistency. There was substantial heterogeneity (I-squared=62%), and visually some trials have point effect estimates very far from others.
d. Downgraded by two levels for very serious imprecision, due to small absolute number of events, and CIs include important potential benefit and important potential harm.
e. Galan 2021 [14]; Mohan 2021 [17]; Pott-Junior 2021 [19]; Ravikirti 2021 [20].
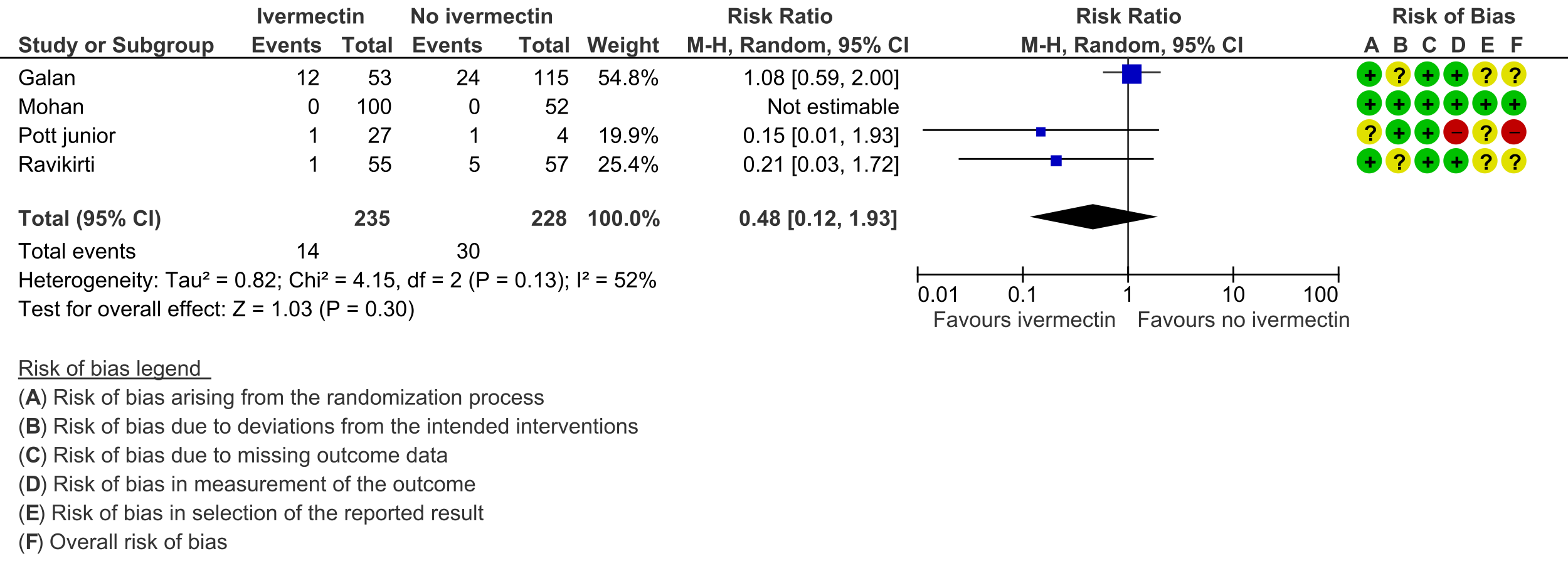
f. Downgraded by one level for serious risk of bias, due to Pott-Junior 2021 [19] having high risk of bias, and Galan 2021 [14] and Ravikirti 2021 [20] having some concerns for risk of bias.
g. Downgraded by one level for serious inconsistency, due to substantial heterogeneity (I-squared=48%) and visually some trials having point effect estimates very far from each other.
h. Downgraded by two levels for very serious imprecision, due to small absolute number of events, and CIs including important potential benefit and important potential harm.
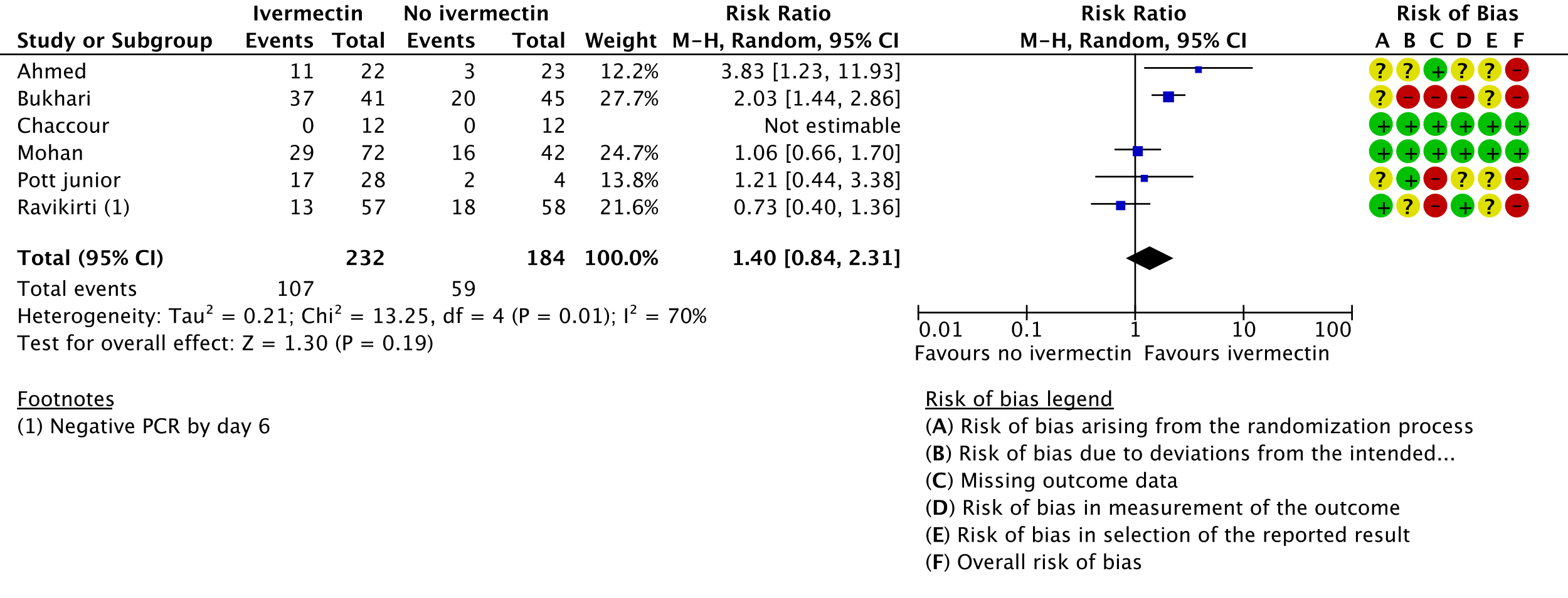
i. Ahmed 2020 [9]; Bukhari 2021 [11]; Chaccour 2021 [12]; Mohan 2021 [17]; Pott-Junior 2021 [19]; Ravikirti 2021 [20]
j. Downgraded by two levels for very serious risk of bias, due to Ahmed 2020 [9], Bukhari 2021 [11] and Pott-Junior 2021 [19] having high risk of bias for this outcome.
k. Downgraded by one level for serious inconsistency, due to substantial heterogeneity (I-squared=70%) and visually some trials having point effect estimates very far from those of other trials.
l. Downgraded by one level for serious imprecision, due to CIs overlapping no effect and inability to exclude important benefit.
m. Chaccour 2021 [12]; Chachar 2021 [13]; Lopez Medina 2021 [16]; Mohan 2021 [17]; Pott-Junior 2021 [19]
n. Downgraded by one level for serious risk of bias., due to Chaccour 2021 [12], Chachar 2021 [13] and Pott-Junior 2021 [19] having high risk of bias. Lopez-Medina 2021 [16] contributes 53% weight to the meta-analysis was at low risk of bias.
o. Downgraded by two levels for very serious imprecision, due to CIs overlapping including important potential benefit and important potential harm.
p. Gonzalez 2021 [15]; Lopez Medina 2021 [16]; Mohan 2021 [17].
q. Downgraded by one level for serious risk of bias, due to Gonzalez having high risk of bias.
r. Downgraded by two levels for very serious imprecision, due to CIs including important potential benefit and important potential harm.
Ivermectin has been shown to inhibit the replication of SARS CoV2 in vitro; it binds and destabilises the viral protein and prevents it from entering the nucleus [2]. However the drug dosages used in these laboratory studies far exceed those that have been used for other conditions [3]. Drug doses and levels required to achieve therapeutic effects in humans based on these studies may be safe, but this has not been studied in clinical trials [4]. An additional potential effect may be in modulating the immune system, though this is yet to be studied thoroughly in humans [5].
Although ivermectin is generally well tolerated, adverse effects like dizziness, tachycardia, postural hypotension, diarrhoea, arthralgia, and facial and peripheral oedema have been reported even with single doses as used in parasitic diseases [6]. Encephalopathy with permanent disability has been reported when ivermectin has been used in the treatment of onchocerciasis or other parasitic diseases [6]. It is predominantly metabolized in the liver (CYP3A4), which may lead to drug-drug interactions.
Due to lack of conclusive evidence from trials, World Health Organization recommends use of ivermectin only in clinical trials [7]. Use continues widely, including self-medication, especially in low- and middle-income countries due to easy availability and low cost of the drug [8].
This review aims to provide a summary of the available evidence from randomised clinical trials of ivermectin for treatment of acute COVID-19, for any dose or duration, so the Expert Working Group can provide a recommendation to guide clinicians and researchers regarding the appropriate use of this drug.
We used Cochrane rapid review methods. We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language published from March 2020 up to 15th April 2021. We also reviewed reference lists of systematic reviews and included studies. We contacted researchers to identify unpublished and ongoing studies.
We included randomized controlled trials (RCTs) testing ivermectin treatment of any dose or duration in people with acute COVID‐19, whether suspected or confirmed. Trials were included if the intervention arm did not combine ivermectin with another experimental drug, and if the comparator arm did not include ivermectin (this could involve use of placebo, standard care, or other potentially active drugs). We excluded trials that did not report any outcomes that could provide usable data for the review, those which were quasi-randomized and those lacking a comparator arms.
We planned to extract data for the following outcomes, pre-defined by the Expert Working Group:
- Critical (primary for this review):
- Mortality (all-cause) – at 28-30 days, or in-hospital
- Progression to:
- Oxygen therapy
- Ventilation: non-invasive or invasive
- Critical or Intensive care (any reason) - Duration of hospitalization
- Need for hospitalization (for out-patients)
- Adverse events: all and serious
- Important (secondary):
- Time to clinical improvement
- Time to negative PCR for SARS-CoV-2
- Negative PCR for SARS-CoV-2 by day 7 post-enrolment
- Complications of COVID-19:
- Thrombotic events
- Pulmonary function/fibrosis
- Long covid/post acute sequelae COVID
- Secondary infections
Two reviewers independently assessed eligibility of search results. One reviewer extracted data from each included study, and assessed risk of bias using the Cochrane Risk of bias (RoB) v2.0 tool. Data and RoB assessments were checked against a Cochrane systematic review team’s extractions and assessments (who used a consensus approach). In case of any discrepancies, the whole RoB assessment was scrutinised by the whole team for this review, to reach consensus.
We used RevMan 5.4 to perform meta‐analysis using a random‐effects model for outcomes where pooling of effect estimates was appropriate. We used risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). We planned to explore heterogeneity in the effect on mortality using subgroup analysis comparing between trials, based on COVID-19 illness severity of participants included and risk of bias. We used the I2 statistic to measure residual heterogeneity. We used GRADE methodology to assess the certainty in the evidence, and documented this in a ‘Summary of findings’ table using GradeProGDT.
We included 12 trials involving 1413 participants, all of whom were adults, and 718 of whom received ivermectin [9-20]. There were two trials each from Brazil, India and Pakistan; one trial reported from each of Bangladesh, Colombia, Iran, Mexico, Nigeria, and Spain. Eight were in hospitalized patients, one recruited outpatients only, one recruited both, and two did not report care setting. Disease severity, prevalence of comorbidities, and use of co‐interventions varied substantially between trials. The ‘Summary of characteristics of included trials’ table provides further details about the trials.
We found potential risks of bias across all domains; 10 of the 12 trials were at high risk of bias overall for at least one outcome. Risk of bias for each domain per trial is displayed alongside the Forest plots below. Studies excluded at full-text review are listed in the References section, with the reason provided in brackets [21-31].
The following comparisons were investigated in the trials. We compared outcomes for arms randomised to ivermectin vs. outcomes in arms with placebo, standard care, or agents considered inactive or ineffective against COVID-19. Where multiple arms contained ivermectin without another experimental agent, we combined results in those arms into a single ivermectin arm, but we did not double-count controls. Where another experimental agent undergoing investigation was combined with ivermectin, that trial arm was excluded from the analysis (e.g. doxycycline).
- Six trials [11-13;16;17;20] compared ivermectin vs. placebo (793 participants)
- Two [18;19] compared ivermectin vs. standard care (212 participants)
- One [9] compared ivermectin vs. placebo vs. a combination of ivermectin & doxycyline in three arms (72 participants; 24 participants in ivermectin & doxycycline arm excluded)
- One [15] compared ivermectin vs. placebo vs. hydroxychloroquine in three arms (106 participants)
- One [10] compared ivermectin vs. lopinavir/ritonavir (62 participants)
- One [14] compared ivermectin vs. chloroquine or hydroxychloroquine (168 participants)
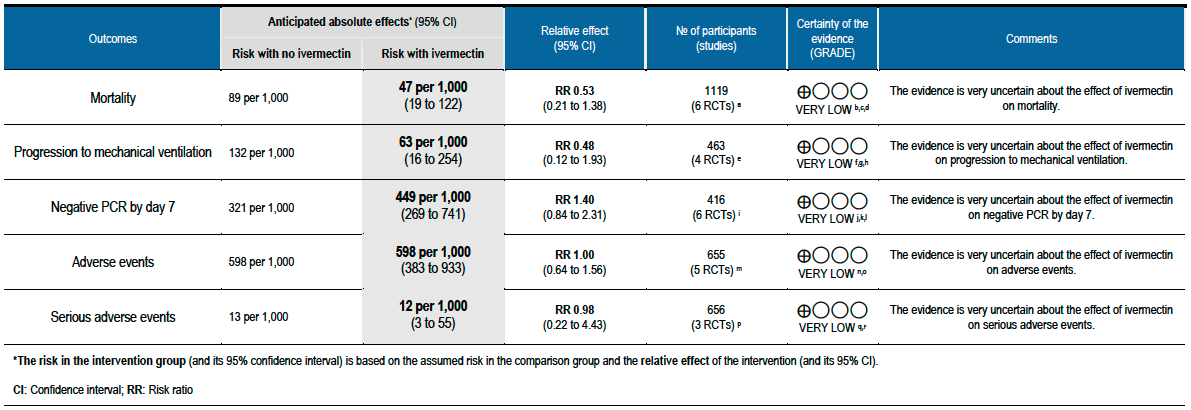
As presented in the ‘Summary of findings’ table, the evidence is very uncertain about the effect of ivermectin on mortality; progression to mechanical ventilation; negative PCR for SARS-CoV-2 by day 7; adverse events; and serious adverse events. For mortality, there were no significant differences observed when trials were stratified by COVID-19 illness severity or risk of bias.
One trial reported a higher risk of discontinuation of ivermectin vs. placebo due to an adverse event (RR 2.97; 95% CI 1.10 to 8.02; 1 trial [16]; 398 participants).
95% confidence intervals for pooled effect estimates for all of the following outcomes not included in the summary of findings table included potential benefit and potential harm from ivermectin: need for critical or intensive care (2 trials [14;20]; 283 participants); discharge from hospital by day 10 post-enrolment (1 trial [20]; 115 participants); deterioration by 2 points on 8-point clinical ordinal scale (1 trial [16]; 398 participants); lack of fever on day 7 (1 trial [9]; 36 participants); lack of symptoms on day 7 (1 trial [13]; 50 participants); and thrombotic events (1 trial [15]; 106 participants). We were unable to pool data for time to clinical improvement as they were not reported in a way that was amenable to meta-analysis (3 trials [12;16;17]; 149 participants).
No comparative data could be extracted for risk of progression to oxygen therapy; need for hospitalisation in outpatients; or post-acute COVID-19 pulmonary function/fibrosis or other sequelae; or secondary infections.
Lack of uniform criteria for COVID-19 severity, substantial overlap and lack of clear reporting of severity in the included trials prevented a meaningful subgroup analysis by severity.
Furthermore, a lack of within trial comparison prevented subgroup analysis by age, duration of symptoms or dose of ivermectin. In addition, data of safety and efficacy in specific subgroups such as pregnancy, children, liver and kidney disease were not available in the trials included in the rapid review.
| Study | Intervention and comparator arms | Design | Location Care setting | Age, average in years | No. of participants randomized | Participant characteristics |
|---|---|---|---|---|---|---|
| Ahmed 2020 [9] | Ivermectin alone (12 mg once daily for5 days) vs. Ivermectin and doxycycline (12 mg ivermectin single dose and 200 mg doxycycline on day 1, followed by 100 mg every 12 h for the next 4 days) vs. placebo | Double-blinded RCT | Dhaka, Bangladesh Inpatients | Not reported | Ivermectin: 24 Ivermectin+Doxycyline: 24 (not included in analysis). Placebo: 24 | RT-PCR positive admitted patients with symptoms for < 7 days |
| Babalola 2021 [10] | Ivermectin 6mg twice a week vs. Ivermectin 12mg twice a week vs.? Lopinavir/Ritonavir daily | Open label RCT | Lagos, Nigeria Care setting not reported | Ivermectin: 44 Lpv/Rtn: 44.8 | Ivermectin: 42 Lpv/Rtn: 20 | RT-PCR positive COVID-19 patients who were asymptomatic or had mild/moderate symptoms |
| Bukhari 2021 [11] | Ivermectin 12mg single dose vs. standard of care | Open label RCT | Lahore, Pakistan Inpatients | Ivermectin: 42.24 SoC: 38.98 | Ivermectin: 41 Placebo: 45 | RT-PCR positive COVID-19 with mild to moderate disease |
| Chaccour 2021 [12] | Ivermectin 400mcg/kg single dose vs. placebo | Double-blinded RCT | Barcelona, Spain Care setting not reported | Ivermectin: 37 Placebo: 37 | Ivermectin: 12 Placebo: 12 | RT-PCR positive COVID-19 with mild disease and duration of symptoms less than 5 days |
| Chachar 2021 [13] | Ivermectin 12mg three doses vs. standard of care | Open label RCT | Lahore, Pakistan Outpatients | Ivermectin: 40.6 SoC: 43.08 | Ivermectin: 25 Placebo: 25 | RT-PCR positive COVID-19 with mild disease |
| Galan 2021 [14] | Ivermectin 14mg 2 doses vs. Chloroquine vs. Hydroxychloroquine | Double blinded RCT | Boa Vista, Brazil Inpatients | Ivermectin: 53.2 CQ: 54.8 HCQ: 51.9 | Ivermectin: 53 CQ: 54 HCQ: 61 | RT-PCR or antigen positive COVID-19 with severe disease |
| Gonzalez 2021 [15] | Ivermectin 12/18mg vs. HCQ 400mg vs. Placebo | Double blinded RCT | Mexico Inpatients | Ivermectin: 56 HCQ: 48.9 Placebo: 53.8 | Ivermectin: 36 HCQ: 33 Placebo: 37 | RT-PCR positive COVID-19 with mild to severe illness |
| Lopez Medina 2021 [16] | Ivermectin, 300 mcg/kg per day for 5 days vs. placebo | Double blinded RCT | Colombia Inpatients & outpatients | Ivermectin: 37 Placebo: 37 | Ivermectin: 200 Placebo: 198 | RT-PCR positive COVID-19 with mild disease |
| Mohan 2021 [17] | Ivermectin 12mg in 20ml 40% ethanol vs. Ivermectin 24mg in 20ml 40% ethanol vs. placebo | Double-blinded RCT | New Delhi, India Inpatients | Ivermectin: 35.3 Placebo: 35.3 | Ivermectin: 80 Placebo: 40 | RT-PCR positive, non-severe COVID-19 with oxygen saturation more than 90% |
| Niaee et al 2021 [18] | 6 arms: Single doseivermectin (200mcg/Kg) vs. three low interval doses of ivermectin (200, 200, 200 mcg/Kg on day 1,3 and 5) vs. single dose ivermectin (400mcg/Kg), vs. three high interval doses of ivermectin (400, 200, 200 mcg/Kg on day 1,3 and 5) vs. placebo vs. standard of care | Double-blinded RCT | Qazvin, Iran Inpatients | Ivermectin: 55.5 Control: 56.5 | Ivermectin: 120 Control: 60 | RT-PCR or CT thorax positive COVID-19 with mild to severe disease |
| Pott-Junior 2021 [19] | Ivermectin 100 mcg/kg vs. Ivermectin 200 mcg/kg vs. Ivermectin 400 mcg/kg vs. standard of care | Open label RCT | Sao Carlos, Brazil Inpatients | Ivermectin: 48.75 SoC: 54.2 | Ivermectin: 28 SoC: 4 | RT-PCR positive COVID-19 with ECOG score of 0?1 and NEWS of 0?4 |
| Ravikirti 2021 [20] | Ivermectin 12mg for 2 days vs. placebo | Double-blinded RCT | Patna, India Inpatients | Ivermectin: 50.7 Placebo: 54.2 | Ivermectin: 57 Placebo: 58 | RT-PCR positive COVID-19 with mild to moderate disease |
1. Mortality, stratified by risk of bias:
2. Progression to mechanical ventilation:
3. Negative PCR by day 7:
4. Adverse events:
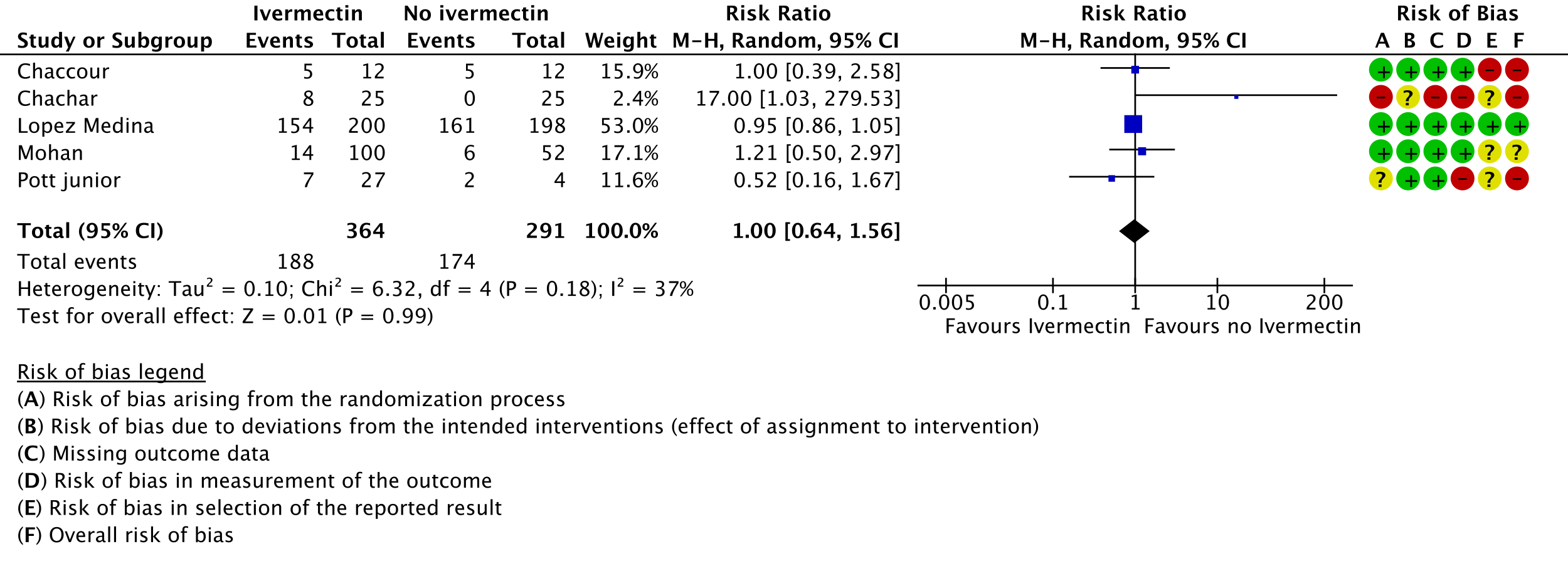
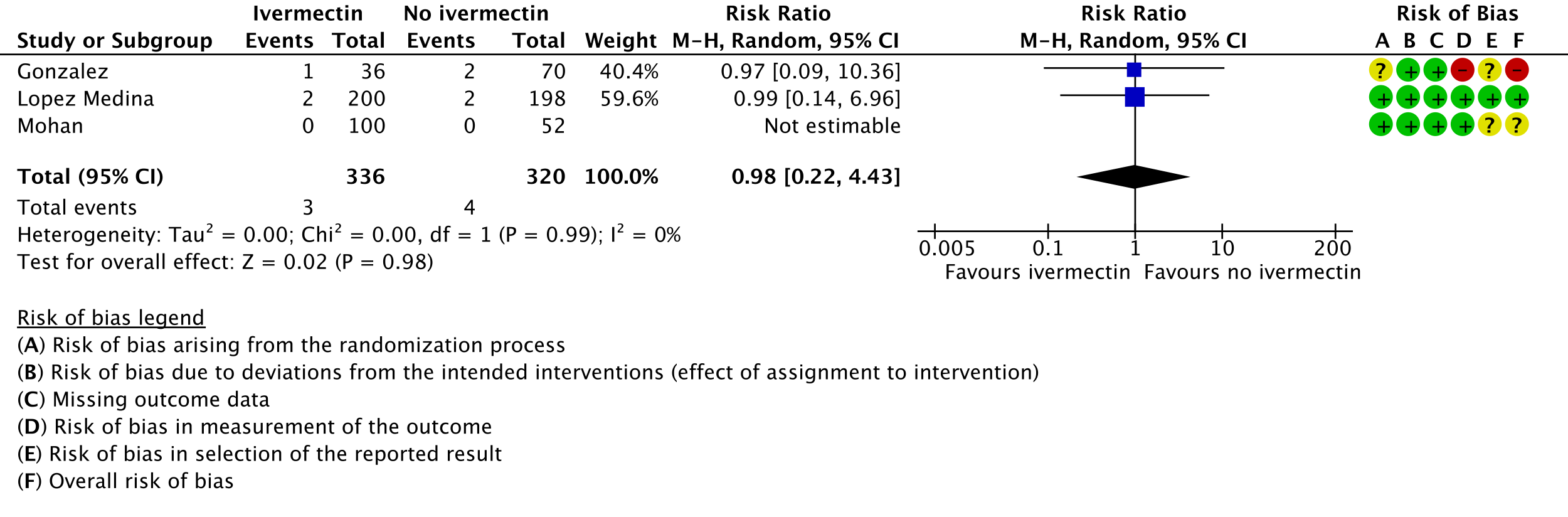
5. Serious adverse events:
The Antiviral Expert Working Group met on 29th April 2021 to consider ivermectin as a treatment for COVID-19. Conflict of interest declarations were reviewed by the Steering Committee; none were found to be relevant to ivermectin.
A summary and then more detailed explanations of the Expert Working Group's judgements follow.
Summary of judgements
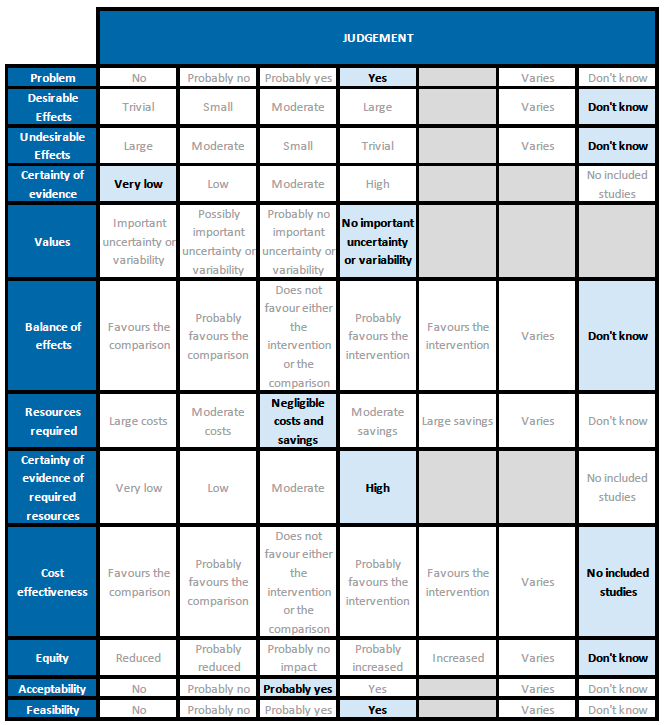
Problem
There is some evidence in the literature regarding use of ivermectin for treatment of COVID-19, but reports of trials and reviews are conflicting. Given current demand for effective treatments, examination of the evidence and a recommendation based on this is a priority. The PICO question now applies for all patients with COVID-19 (initial PICO was for hospitalised patients).
Desirable effects
The 95% CI for the pooled effect estimates for mortality, progression to mechanical ventilation and negative PCR at day 7 included 1 (no difference); and the certainty of the evidence was very low. This means that we do not know whether the desirable effects with ivermectin are any different from placebo or the other interventions compared.
Undesirable effects
All 95% CI for the pooled effect estimates for adverse events and serious adverse events include 1 (no difference), indicating that we do not know if adverse events are any different with ivermectin compared to placebo or the interventions compared in the trials. This drug has been widely used and adverse effects at the dose used have not been reported commonly.
The panel felt that drug-drug interactions are possible. For example, ivermectin can increase the concentration of warfarin, and one panel member reported a case where a patient with COVID disease who was on warfarin and given ivermectin developed severe bleeding. The panel also cautioned that use with dexamethasone can reduce ivermectin concentrations.
There is also the indirect risk of harm from relying on this treatment and not using treatments with better evidence, such as good supportive care.
Certainty of evidence
Using GRADE methods, the team rated the certainty in the evidence as very low for all outcomes in the Summary of findings table. The panel agreed.
Values
The review included critical and important outcomes. Results were not reported for all, but results were pooled and GRADE applied for four critical outcomes and one important outcome including death and adverse events.
Balance of effects
Since the certainty of evidence was very low for desirable and undesirable effects of ivermectin, this means we do not know if ivermectin has an overall desirable effect compared to an undesirable effect when given to people with COVID disease.
Ivermectin is part of the current protocol recommended by the Ministry of Health & Family Welfare for mild COVID-19 disease. Some reviews of the evidence have suggested a large beneficial effect [32;33], but the current review does not reflect this. Trials at higher risk of bias were the ones that reported a benefit. They also include three trials that were excluded in this as they were not randomized trials [21;24;29]. The panel discussed whether an intervention should be given if there is no observed benefit, as there is a risk to giving any intervention (see the section on Undesirable effects above).
Resources required
Cost of a dose, or a course as used in trials (up to 7 days of usual daily dose of up to 12mg) is low.
Certainty of evidence of required resources
Ivermectin is a low-cost treatment widely used in low- and middle-income settings for other conditions. We did not use published evidence on costs for this judgement, as ivermectin has been used extensively by the members of the expert working group for other conditions.
Cost effectiveness
None of the included trials assessed cost-effectiveness.
Equity
There is not enough information to make this judgement.
Acceptability
The panel felt that many well-informed clinicians and patients may not accept an intervention for which the evidence for beneficial and adverse events were uncertain. However, in the context of a surge with few evidence-based therapeutic options, other clinicians and patients would feel a relatively safe, cheap oral drug might be acceptable in the absence of evidence for benefit. The panel finally judged that ivermectin would probably be accepted, under such circumstances.
Feasibility
Evidence about feasibility was not specifically examined for COVID-19, but the panel is aware of reports of mass-drug administration of ivermectin for other indications.
The panel noted that it would be feasible to administer ivermectin in India for COVID-19, if it was found effective. However, we do not have data right now to suggest this. The panel felt a recommendation for use would only detract from other interventions which have reported an unequivocal benefit.
Ivermectin is widely available, relatively inexpensive and has low risk of adverse drug reactions. It may be used citing that lack of evidence of benefit is not evidence of lack of benefit. However, there are likely indirect harms. Its use may lead to a false sense of security. This could hamper close monitoring, and cause delay in the use of other interventions for which better evidence of benefit exists.
This recommendation is based on the evidence to date, and may be updated as new evidence emerges. If ivermectin is subsequently assessed to be effective and safe in the treatment of COVID-19, it should be relatively feasible to implement in management protocols.
Our conditional recommendation against use of ivermectin applies to all subgroups of patients with COVID-19.
This conditional recommendation against use of ivermectin may be revisited as evidence emerges. In addition to evidence of benefit, with its widespread use in India, there may be additional real-world reports regarding undesirable effects, which the group will monitor.
Further evidence for the use of Ivermectin in the treatment of COVID-19 needs to be generated from well-conducted randomized double-blind placebo-controlled trials which are adequately powered and have a low risk of bias. Utility in pre-specified patient sub-groups like mild, moderate, or severe infection, special populations (immunosuppressed, pregnant, children, comorbid conditions), and various phases of the illness will also need to be addressed. Safety of the dose required to achieve an antiviral/immunomodulatory effect also needs to be evaluated.
- Popp M, Stegemann M, Metzendorf M-I, Kranke P, Meybohm P, Skoetz N, Weibel S. Ivermectin for preventing and treating COVID‐19. Cochrane Database of Systematic Reviews 2021, Issue 4. Art. No.: CD015017. DOI: https://doi.org/10.1002/14651858.CD015017.
- Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020 Jun;178:104787.
- Virginia D Schmith, Jie Jessie Zhou, Lauren R L Lohmer et al. The Approved Dose of Ivermectin Alone is not the Ideal Dose for the Treatment of COVID-19. Clin Pharmacol Ther. 2020 Oct;108(4):762-765. doi: 10.1002/cpt.1889.
- Guzzo CA, et al. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J Clin Pharmacol. 2002;42(10):1122–33. https://doi.org/10.1177/009127002401382731.
- Zhang X, Song Y, Ci X, et al. Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice. Inflamm Res 2008; 57(11): 524-9. http://dx.doi.org/10.1007/s00011-008-8007-8
- Chandler RE. Serious Neurological Adverse Events after Ivermectin-Do They Occur beyond the Indication of Onchocerciasis?. Am J Trop Med Hyg. 2018;98(2):382-388. https://doi.org/10.4269/ajtmh.17-0042
- Therapeutics and COVID-19: living guideline [Internet]. [cited 2021 Apr 26]. Available from: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-therapeutics-2021.1
- Molento MB. COVID-19 and the rush for self-medication and self-dosing with ivermectin: A word of caution. One Health. 2020 Dec;10:100148.
- Ahmed S, Karim MM, Ross AG, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2021 Feb;103:214-216. https://doi.org/10.1016/j.ijid.2020.11.191.
- Babalola OE, Bode O, Ajayi AA, et al. Ivermectin shows clinical benefits in mild to moderate COVID19: A randomised controlled double-blind, dose-response study in Lagos. QJM: An International Journal of Medicine, 2021;hcab035. https://doi.org/10.1093/qjmed/hcab035.
- Bukhari KHS, Asghar A, Perveen N, et al. Efficacy of ivermectin in COVID-19 patients with mild to moderate disease. medRxiv [preprint]. 2021.02.02.21250840; https://doi.org/10.1101/2021.02.02.21250840.
- Chaccour C, Casellas A, Blanco-Di Matteo A, et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalMedicine. 2021 Feb;32:100720. https://doi.org/10.1016/j.eclinm.2020.100720.
- Chachar AZK, Khan KA, Asif M, et al. Effectiveness of ivermectin in SARS-CoV-2/COVID-19 patients. International Journal of Sciences. 09(2020):31-35. https://doi.org/10.18483/ijSci.2378.
- Galan LEB, Santos NMD, Asato MS, et al. Phase 2 randomized study on chloroquine, hydroxychloroquine or ivermectin in hospitalized patients with severe manifestations of SARS-CoV-2 infection. Pathog Glob Health. 2021 Mar 8:1-8. https://doi.org/10.1080/20477724.2021.1890887.
- Gonzalez JLB, Gámez MG, Enciso EAM, et al. Efficacy and safety of Ivermectin and Hydroxychloroquine in patients with severe COVID-19. A randomized controlled trial. medRxiv [preprint]. 2021.02.18.21252037. https://doi.org/10.1101/2021.02.18.21252037.
- López-Medina E, López P, Hurtado IC, et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: A randomized clinical trial. JAMA. 2021 Apr 13;325(14):1426-1435. https://doi.org/10.1001/jama.2021.3071.
- Mohan A, Tiwari P, Suri T, et al. Ivermectin in mild and moderate COVID-19 (RIVET-COV): a randomized, placebo-controlled trial. Research Square [preprint]. https://doi.org/10.21203/rs.3.rs-191648/v1.
- Niaee MS, Gheibi N, Namdar P, et al. Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: A randomized multi-center clinical trial. Research Square [preprint]. https://doi.org/10.21203/rs.3.rs-109670/v1.
- Pott-Junior H, Bastos Paoliello MM, Miguel AQC, et al. Use of ivermectin in the treatment of Covid-19: A pilot trial. Toxicol Rep. 2021;8:505-510. https://doi.org/10.1016/j.toxrep.2021.03.003.
- Ravikirti, Roy R, Pattadar C, et al. Ivermectin as a potential treatment for mild to moderate COVID-19 – A double blind randomized placebo-controlled trial. medRxiv [preprint]. 2021.01.05.21249310. https://doi.org/10.1101/2021.01.05.21249310.
- Podder CS, Chowdhury N, Sina MI, et al. Outcome of ivermectin treated mild to moderate COVID-19 cases: a single-centre, open-label, randomised controlled study. IMC Journal of Medical Science Issue: Vol.14 No.2 - July 2020. (Quasi-RCT)
- Chahla RE, Ruiz LM, Ortega ES, et al. A Randomized trial – intensive treatment based in Ivermectin and Iota-Carrageenan as pre-exposure prophylaxis for covid- 19 in healthcare agents. https://doi.org/10.1101/2021.03.26.21254398. (Prevention trial)
- Chowdhury ATMM, Shahbaz M, Karim MR, et al. A Randomized Trial of Ivermectin-Doxycycline and Hydroxychloroquine-Azithromycin therapy on COVID19 patients. https://doi.org/10.21203/rs.3.rs-38896/v1 (Ivermectin-Doxycycline combination as intervention)
- Elgazzar A, Eltaweel A, Youssef SA, et al. Efficacy and Safety of Ivermectin for Treatment and prophylaxis of COVID-19 Pandemic. https://doi.org/10.21203/rs.3.rs-100956/v3 (Not a RCT)
- Hashim HA, Maulood MF, Rasheed AM, et al. Controlled randomized clinical trial on using Ivermectin with Doxycycline for treating COVID-19 patients in Baghdad, Iraq. https://doi.org/10.1101/2020.10.26.20219345 (Ivermectin-Doxycycline combination as intervention)
- Houssam RaaD et al. In vivo use of ivermectin (IVR) for treatment for corona virus infected patients: a randomized controlled trial. (Not yet recruiting)
- Krolewiecki, Alejandro and Lifschitz, et al. Antiviral Effect of High-Dose Ivermectin in Adults with COVID-19: A Pilot Randomised, Controlled, Open Label, Multicentre Trial. http://dx.doi.org/10.2139/ssrn.3714649 (Outcomes not reported in a usable way)
- Reaz Mahmud et al. Clinical Trial of Ivermectin Plus Doxycycline for the Treatment of Confirmed Covid-19 Infection. (Ivermectin-Doxycycline combination as intervention)
- Okumuş N, Demirtürk N, ÇETİNKAYA RA, et al. Evaluation of the Effectiveness and Safety of Adding Ivermectin to Treatment in Severe COVID-19 Patients. https://doi.org/10.21203/rs.3.rs-224203/v1 (Quasi-RCT)
- Mohammadsadegh Rezai et al. Effectiveness of Ivermectin in the Treatment of Coronavirus Infection in Patients admitted to Educational Hospitals of Mazandaran in 2020. (Results not yet published)
- Shoumann WM, Hegazy AA, Nafae RM, et al. Use of Ivermectin as a Potential Chemoprophylaxis for COVID-19 in Egypt: A Randomised Clinical Trial. Journal of Clinical and Diagnostic Research. 2021 Feb, Vol-15(2): OC27-OC32. https://doi.org/10.7860/JCDR/2021/46795.14529 (Prevention trial)
- Bryant A, Lawrie TA, Dowswell T, et al. Ivermectin for prevention and treatment of COVID-19 infection: a systematic review and meta-analysis. Research Square [preprint]. https://doi.org/10.21203/rs.3.rs-317485/v1.
- Kory P, Meduri GU, Varon J, Iglesias J, Marik PE. Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19. Am J Ther. 2021;28(3):e299-e318. https://doi.org/10.1097/MJT.0000000000001377.
Covid Management Guidelines India Group - Anti-viral Working Group. Ivermectin. Covid Guidelines India; Published online on May 15, 2021; URL: https://indiacovidguidelines.org/ivermectin/ (accessed ).
