Click here for an Interactive Summary of Findings table (opens in a new window).
GRADE Working Group grades of evidence
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect
Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect
Explanations:
a. Gordon REMAP-CAP 2021 [3]; Hermine CORIMUNO-19 2020 [4]; Horby RECOVERY 2021 [5]; Rosas COVACTA 2021 [6]; Salama EMPACTA 2020 [7]; Salavarani 2020 [8]; Soin COVINTOC 2021 [9]; Stone 2020 [10]; Veiga TOCIBRAS 2021 [11]
b. Not downgraded for risk of bias: We feel the imbalance in the proportions on Remdesivir, used as a co-intervention, in the two groups is unlikely to affect the outcome.
c. Not downgraded for indirectness: The majority of participants had severe or critical illness in the REMAP-CAP and RECOVERY trials, which contributed the most data to this meta-analysis.
d. Downgraded by 1 level for serious imprecision, since the lower bound (2% reduction) of the 95% CI includes clinical benefit that may not be appreciable in clinical practice.
e. Gordon REMAP-CAP 2021 [3]; Hermine CORIMUNO-19 2020 [4]; Horby RECOVERY 2021 [5]; Rosas COVACTA 2021 [6]; Salama EMPACTA 2020 [7]; Salvarani 2020 [8]; Stone 2020 [10]; Veiga TOCIBRAS 2021 [11]
f. Downgraded by 1 level for serious risk of bias: some concerns due to deviation from intended interventions, outcome measurement and selection of reported results
g. Downgraded by 1 level for serious indirectness, since the outcome is a composite outcome of in-hospital survival, hospital discharge, 2-point ordinal scale improvement
h. Gordon REMAP-CAP 2021 [3]; Hermine CORIMUNO-19 2020 [4]; Horby RECOVERY 2021 [5]; Rosas COVACTA 2021 [6]; Salama EMPACTA 2020 [7]; Salvarani 2020 [8]; Stone 2020 [10]
i. Gordon REMAP-CAP 2021 [3]; Hermine CORIMUNO-19 2020 [4]; Horby RECOVERY 2021 [5]; Salama EMPACTA 2020 [7]; Stone 2020 [10]; Veiga TOCIBRAS 2021 [11]
j. Hermine CORIMUNO-19 2020 [4]; Rosas COVACTA 2021 [6]; Salama EMPACTA 2020 [7]; Salvarani 2020 [8]; Soin COVINTOC 2021 [9]; Stone 2020 [10]; Veiga TOCIBRAS 2021 [11]; Wang 2021 [12]
k. Downgraded by 1 level for serious risk of bias: some concerns regarding randomization, deviations from intended interventions and outcome measurement
l. Downgraded by 1 level for serious inconsistency: I²=64%
m. Downgraded by 1 level for serious imprecision due to a wide confidence interval consistent with the possibility for no effect and the possibility for harm
n. Gordon REMAP-CAP 2021 [3]; Hermine CORIMUNO-19 2020 [4]; Rosas COVACTA 2021 [6]; Salama EMPACTA 2020 [7]; Salvarani 2020 [8]; Soin COVINTOC 2021 [9]; Stone 2020 [10]; Veiga TOCIBRAS 2021 [11]; Wang 2020 [12]
o. Downgraded by 1 level for serious risk of bias: some concerns regarding randomization, deviations from intended interventions and outcome measurement
p. Downgraded by 1 level for serious indirectness: the absolute estimates of serious adverse events varied between trials, for e.g. 30% to 40% for the Rosas COVACTA 2021 trial [6] and about 3% for the Gordon REMAP-CAP 2021 trial [3]
† For Clinical Improvement, as the event rate is >20% [common event], OR may overestimate. Hence we calculated RR with 95% CI – 1.14 [1.08, 1.18].
The incidence of COVID-19 infection in India has reached unprecedented proportions leading to considerable mortality and morbidity among those infected. Health care systems are facing enormous pressures with regard to oxygen supplies, admission of patients into intensive care, and what are considered lifesaving drugs. Death due to COVID-19 is believed to be related to immune dysregulation, often leading to acute inflammation, a cytokine storm leading on to an acute lung injury, acute respiratory distress syndrome (ARDS), coagulopathy and multi-organ dysfunction.
Markedly elevated inflammatory markers (such as C-reactive protein, D-dimer and ferritin) and pro-inflammatory cytokines like IL-6 are associated with critical and fatal COVID-19 and it is believed that blocking this inflammatory pathway may prevent disease progression [1]. There is therefore, a huge demand for drugs that will improve outcomes even marginally.
A scoping search revealed many published systematic reviews investigating use of Tocilizumab in COVID-19. It was decided to select a high-quality systematic review to inform this evidence review. The criteria for selection of this systematic review was based on the following criteria:
- Included and only meta-analysed data from randomized controlled trials
- Had a recent latest search date (within the last three months)
- Included all available outcomes
- Matched the pre-defined PICO question closely
- Scored a high or moderate score on the AMSTAR 2 quality assessment tool
To identify all available systematic reviews pertaining to our PICO question, a systematic search of Pubmed, Cochrane and the Epistemonikos databases was conducted. The search strategy was designed and validated by the Group’s information specialist. The search results were screened independently by two reviewers against the pre-defined criteria set out above, using Rayyan. The reviews identified were prioritised based on quality according to the AMSTAR 2 score and the most recent search date.
A systematic search was also done to identify studies meeting the inclusion criteria for the review but published after its latest search. As this review will inform a recommendation for India, a separate search was conducted for studies of any design investigating use of Tocilizumab for COVID-19 in India.
Where possible, we used data extracted by authors of the selected review. Where outcomes data were not found in the review at all or in the format required, we re-extracted them from the primary trial reports directly, for each of the outcomes identified as critical or important by the Expert Working Group prior to commencing the review:
Critical (Primary) outcomes:
- All-cause mortality
- Clinical improvement at day 28
- Time to clinical recovery
- Progression mechanical ventilation/ECMO/death
- Any adverse events
- Serious adverse effects
Important (Secondary) outcomes
- Admission to critical care
- Time to ventilation
- Duration of ventilation
- Length of hospital stay
- Length of stay in critical care
- Organ support other than ventilation
- Change in CRP at 48 hours (as a percentage of the initial value)
We did not conduct a separate risk of bias assessment as it had already been performed in duplicate as part of the systematic review selected.
We performed our own meta-analysis using Review Manager (RevMan) 5.4 using a random‐effects model for each of the critical and important outcomes where pooling of effect estimates was appropriate. We used risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). We used the I2 statistic to measure residual heterogeneity. We used GRADE methodology to assess the certainty in the evidence, and documented this in a ‘Summary of findings’ table using GradeProGDT.
The review selected was the collaborative living network meta-analysis at covid-nma.org, which is published in the Cochrane Database of Systematic Reviews periodically as a review of IL-6 inhibitors for COVID-19, therefore including both tocilizumab and sarilumab [2].
Our updated search found 10 trials [3-12], that were already included in the COVID-NMA/Cochrane review, including an Indian study by Soin et al [12]. All participants were hospitalised adults, with an average age ranging from 54 to 65 years. There one trial reporting from each of Brazil, China, France, India, Italy, UK and USA; three were multi-country trials. Disease severity was mostly severe or critical (though some participants in the RECOVERY 2021 trial whom we labelled as at least moderate by the guidelines group’s criteria would probably be classified as severe [5]). The median or mean C-reactive protein was around or above 100mg/L in 8/10 trials, indicating significant systemic inflammation. Prevalence of comorbidities varied substantially between trials. Most trials used weight-based dosing of tocilizumab, up to a maximum of 800mg per dose; seven of the 10 trials offered treating clinicians the option of a second dose, though in trials that reported the numbers receiving this, a minority of participants received two doses. Most (7/10) trials were unblinded. Use of co‐interventions varied substantially between trials. The ‘Summary of characteristics of included trials’ table summarises key information about the trials; further detailed characteristics are available in the Cochrane review [2].
Most trials were judged to be at low risk of bias or had some concerns about a few domains; none were at high risk of bias overall for the outcomes considered. Risk of bias for each domain per trial is displayed alongside the Forest plots below.
The effect of Tocilizumab was evaluated for critical outcomes as follows.
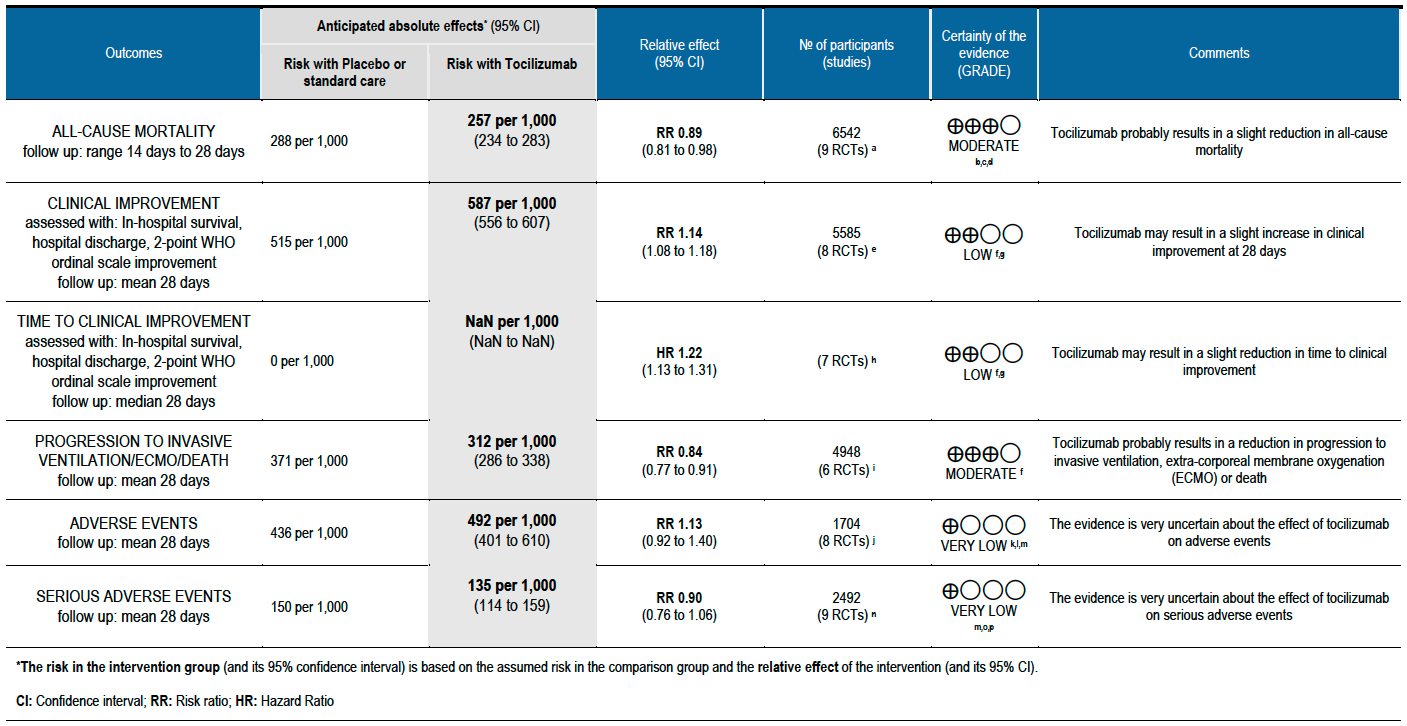
All-cause mortality (follow up till 14-28 days): Moderate certainty evidence from 9 RCTs [3-11] that included 6542 people with severe to critical categories of COVID-19 disease indicates that tocilizumab added to corticosteroids reduced all-cause mortality on average by 11% (95% CI 19% to 2%) compared to usual care and steroids. Based on the data, we anticipate that for 1000 people with severe and critical COVID disease treated with placebo or standard of care (that includes steroids) 288 deaths due to any cause are likely. We anticipate that there would probably be 32 fewer (from 6 fewer to 55 fewer) deaths per 1000 people if tocilizumab is added to steroids and standard care in people with severe to critical COVID disease.
Clinical improvement (at day 28; defined as in-hospital survival, hospital discharge or 2-point improvement on the WHO ordinal scale): Low certainty evidence from 8 RCTs [3-8;10;11] with 5585 severe and critically ill adult in-patients with COVID-19 suggests that tocilizumab added to steroids may increase the odds of clinical improvement compared to placebo or usual care with steroids (odds ratio [OR] 1.33; 95% CI 1.19 to 1.48). For every 1000 patients treated with placebo or usual care and steroids, 515 are likely to clinically impove. Adding tocilizumab may result in 72 more (from 41 more to 93 more) per 1000 people showing clinical improvement. As this event was common (>20%), in which case OR may overestimate an effect size, we calculated RR, which was 1.14 (95% CI 1.08 to 1.18).
Time to clinical improvement (follow up median 28 days (defined as in hospital survival, hospital discharge or 2-point improvement on the WHO ordinal scale): Low certainty evidence from 7 RCTs [3-8;10] suggests that the time to clinical improvement was reduced by around one day when tocilizumab was added to steroids (medians ranged from 6 to 20 days) compared to standard care and steroids (5 to >28 days) [hazard ratio (HR) 1.22; 95% CI 1.13 to 1.31].
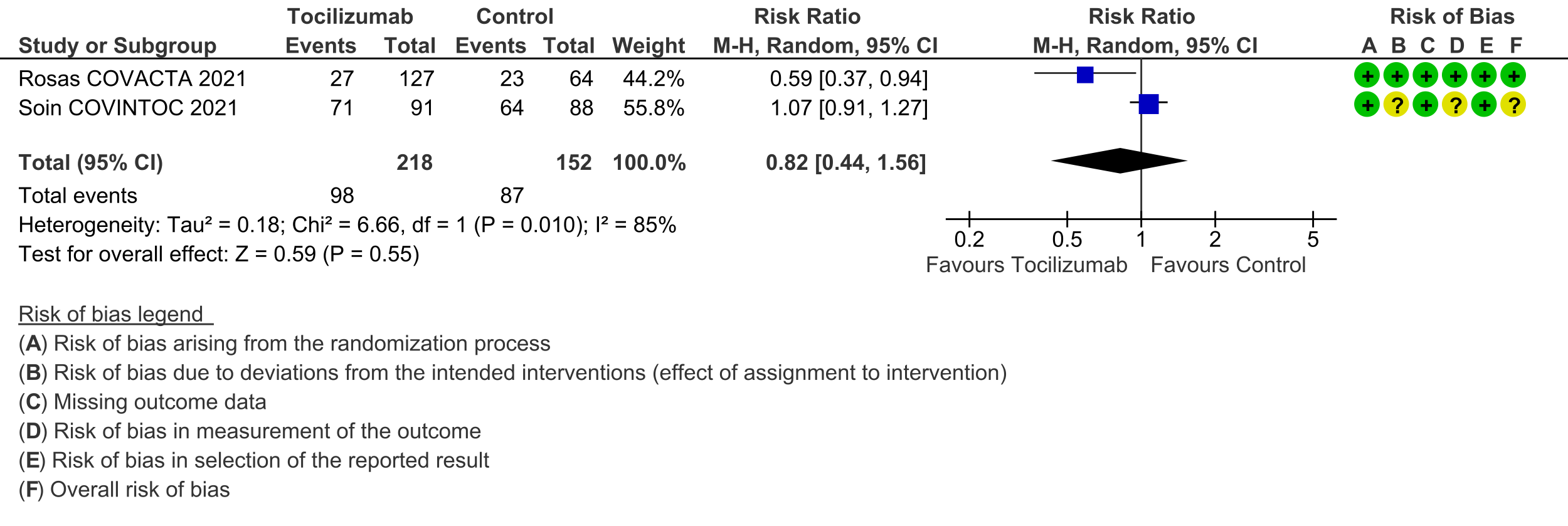
Progression to invasive ventilation or extra-corporeal membrane oxygenation (ECMO) or death (these were reported as a composite outcome in one large trial [3]): Moderate certainty evidence from 6 RCTs [3-5;7;10;11] indicates that from among 4948 severe or critically ill patients with COVID-19, the proportions progressing to invasive ventilation, extra-corporeal membrane oxygenation (ECMO) or death (due to not being mechanically ventilated) was reduced on average by 16% (95% CI 23% to 9%) when tocilizumab was added to steroids and usual care compared to only usual care and steroids. This suggests that for every 1000 patients who are severe or critically ill with COVID-19 and treated with standard care and steroids but not given tocilizumab, 371 will probably progress to this outcome compared to 59 fewer (from 85 fewer to 33 fewer) per 1000 patients also given tocilizumab.
Adverse events (follow up till day 28): Two trials (REMAP-CAP [3] and RECOVERY [5]) did not report adverse events. Very low certainty evidence from 8 RCTs [4; 6-12] that included 1704 severely or critically ill patients suggested that adverse events may be little or no different when tocilizumab was added, or not added, to steroids and usual care (RR 1.13; 95% CI 0.92 to 1.40).
Serious adverse events (follow up till day 28): These were reported by 9 RCTs [3;4;6-12]. There was again very low certainty in the evidence, though the effect estimate from meta-analysis found no significant difference between the tocilizumab and control groups: RR 0.9 (95% CI 0.76 to 1.06.
Secondary outcomes: Length of hospital stay favoured the tocilizumab group with a HR of 1.28 (4 trials [3;6;7;10]; 95% CI 1.13 to 1.44). Admission to critical care was not different between the two groups (OR 0.8; 2 trials [6;9]; 95% CI 0.50 to 1.28). Ventilator-free days and length of stay in critical care were variably expressed as median, rate ratio or adjusted odds ratio and hence could not be pooled. However, the median difference of ventilator-free days varied from 0 to 5.5 days shorter in the tocilizumab groups in 4 trials [3;6;9;11], and length of critical care stay in 3 trials ranged from a median difference of 2 to 5.5 days shorter in the tocilizumab groups [3;6;9]. Secondary infections in participants in the tocilizumab groups ranged from 1.7% to 20.6%; in the control groups this was 5.7% to 24.5%; none of the trials reported a higher incidence of secondary infections in the tocilizumab group [4;6-11].
Subgroup analyses: While formal subgroup analyses could not be performed, we attempted to inform the optimal use of tocilizumab using the following approaches:
(a) We found that the median CRP across the studies varied, ranging widely from 6-160mg/L. The median CRP was >100 mg/L for the two largest trials: it was 150mg/L in REMAP-CAP [3] and 143mg/L in RECOVERY [5]. The other inflammatory marker evaluated was Ferritin in 2 trials [8;11], which was not reported to demonstrate any meaningful correlation.
(b) REMAP-CAP was the only study to document time to administration of tocilizumab after admission to a critical care unit, with a median of 13.6 hours [3]. Time of administration since symptom onset was also noted in 5 trials and varied from a median of 8 to 11 days [3;4;6;8;10].
(c) To determine efficacy with steroids vs. no steroids: this outcome was reported in subgroup analyses performed by 2 trials [4;7]. Both favoured administration of Tocilizumab with steroids though these trials had fewer numbers with a low event rate. In addition, >80% of participants in the 2 largest trials had Tocilizumab added on to their steroids [3;5].
(d) Data were not available for other subgroup analyses for time since onset of respiratory support (though see point (a) above), lung involvement on CT scan, single vs. two doses, and other markers like procalcitonin and pentraxin.
| Study | Intervention & comparator arms | Design | Location | Age, average in years | No. of participants randomized | Participant characteristics |
|---|---|---|---|---|---|---|
| Gordon REMAP‐CAP 2021 [3] | Tocilizumab (8 mg/kg, maximum 800 mg; (Note that the sarilumab | Unblinded RCT | Australia, Ireland, Netherlands, | Tocilizumab: 61.5 Standard care: 61.1 | Tocilizumab: 353 Standard care: 402 | COVID-19 severity: 69% severe; 28% Median CRP: 130-150 mg/L |
| Hermine CORIMUNO‐19 2020 [4] | Tocilizumab (8 mg/kg; optional 2nd | Unblinded RCT | France | 64.8 | Tocilizumab: 64 Standard care: 67 | COVID-19 severity: 42% moderate; 58% Median CRP: 120-127 mg/L |
| Horby RECOVERY 2021 [5] | Tocilizumab (400-800 mg depending on weight; optional 2nd dose after 12-24 hours - number | Unblinded RCT | UK | 63.6 | Tocilizumab: 2022 Standard care: 2094 | COVID-19 severity: < 45% moderate; at Median CRP: 143-144 mg/L (> 75 was an |
| Rosas COVACTA 2021 [6] | Tocilizumab (8 mg/kg, maximum 800 mg; | Double-blinded RCT | Canada, Denmark, France, Germany, Italy, | 60.8 | Tocilizumab: 301 Standard care: 151 | COVID-19 severity: < 30% moderate; at Median CRP: 150-157 mg/L |
| Salama EMPACTA 2020 [7] | Tocilizumab (1 dose of 8 mg/kg, maximum | Double-blinded RCT | Brazil, Kenya, Mexico, Peru, South Africa, | 55.9 | Tocilizumab: 259 Standard care: 129 | COVID-19 severity: 62% moderate; 26% Median CRP: 125-143 mg/L |
| Salvarani 2020 [8] | Tocilizumab (8 mg/kg, maximum 800 mg; optional | Unblinded RCT | Italy | 60 | Tocilizumab: 60 Standard care: 66 | COVID-19 severity: all severe Median CRP: 6.5-10.5 |
| Soin COVINTOC 2021 [9] | Tocilizumab (6 mg/kg, maximum 480 mg; | Unblinded RCT | India | Tocilizumab: 56 Standard care: 54 | Tocilizumab: 91 Standard care: 88 | COVID-19 severity: 40% moderate; 50% Mean CRP: 88-110 mg/L |
| Stone 2020 [10] | Tocilizumab (1 dose of 8 mg/kg, maximum | Double-blinded RCT | USA | 60 | Tocilizumab: 161 Standard care: 82 | COVID-19 severity: 80% moderate; 4% Median CRP: 94-116 mg/L (CRP > 50 mg/L |
| Veiga TOCIBRAS 2021 [11] | Tocilizumab (1 dose of 8 mg/kg, maximum | Unblinded RCT | Brazil | 57.4 | Tocilizumab: 65 Standard care: 64 | COVID-19 severity: 52% moderate; 32% Mean CRP: 160-193 mg/L |
| Wang 2020 [12] | Tocilizumab (400 mg; optional 2nd dose after | Unblinded RCT | China | 63 | Tocilizumab: 33 Standard care: 32 | COVID-19 severity: 57% moderate; 43% Median CRP: 6-10 mg/L |
1. Mortality, all-cause: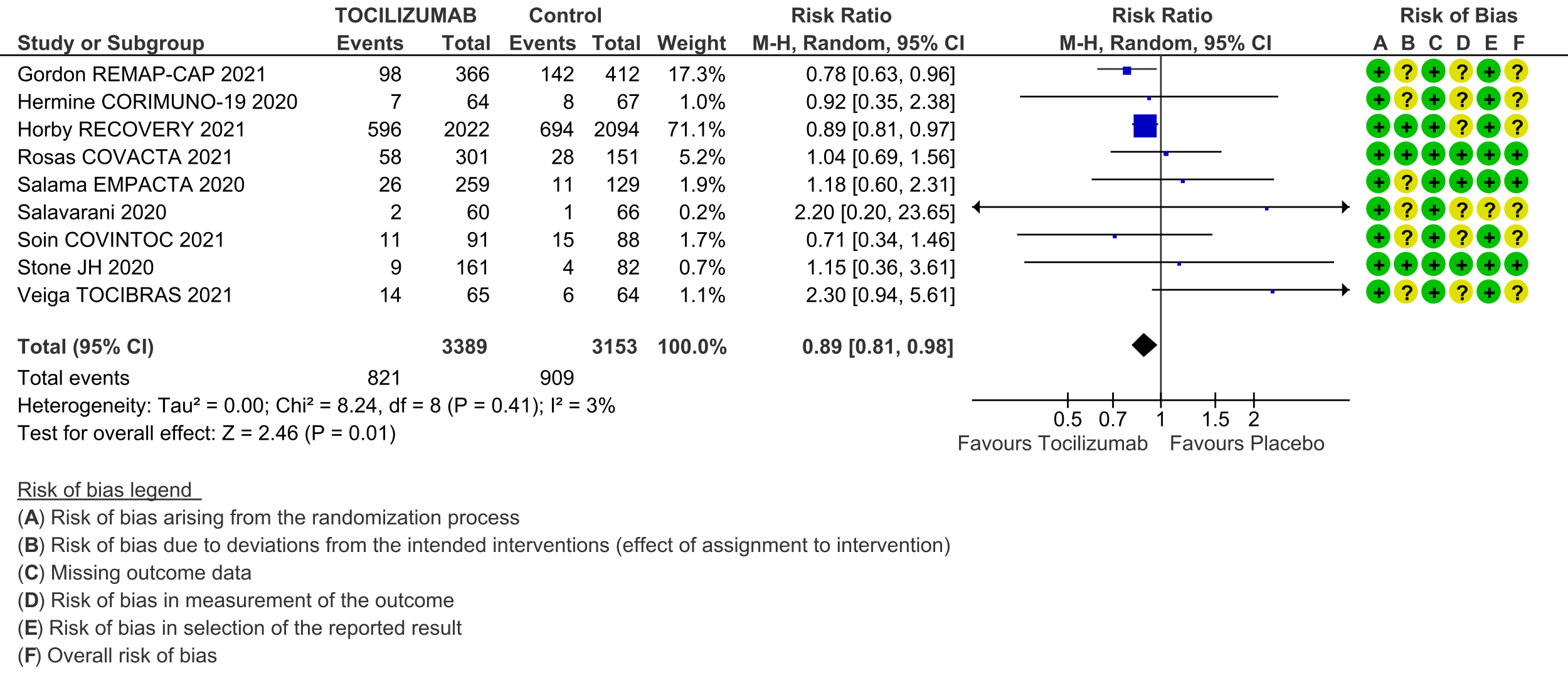
2. Clinical improvement:
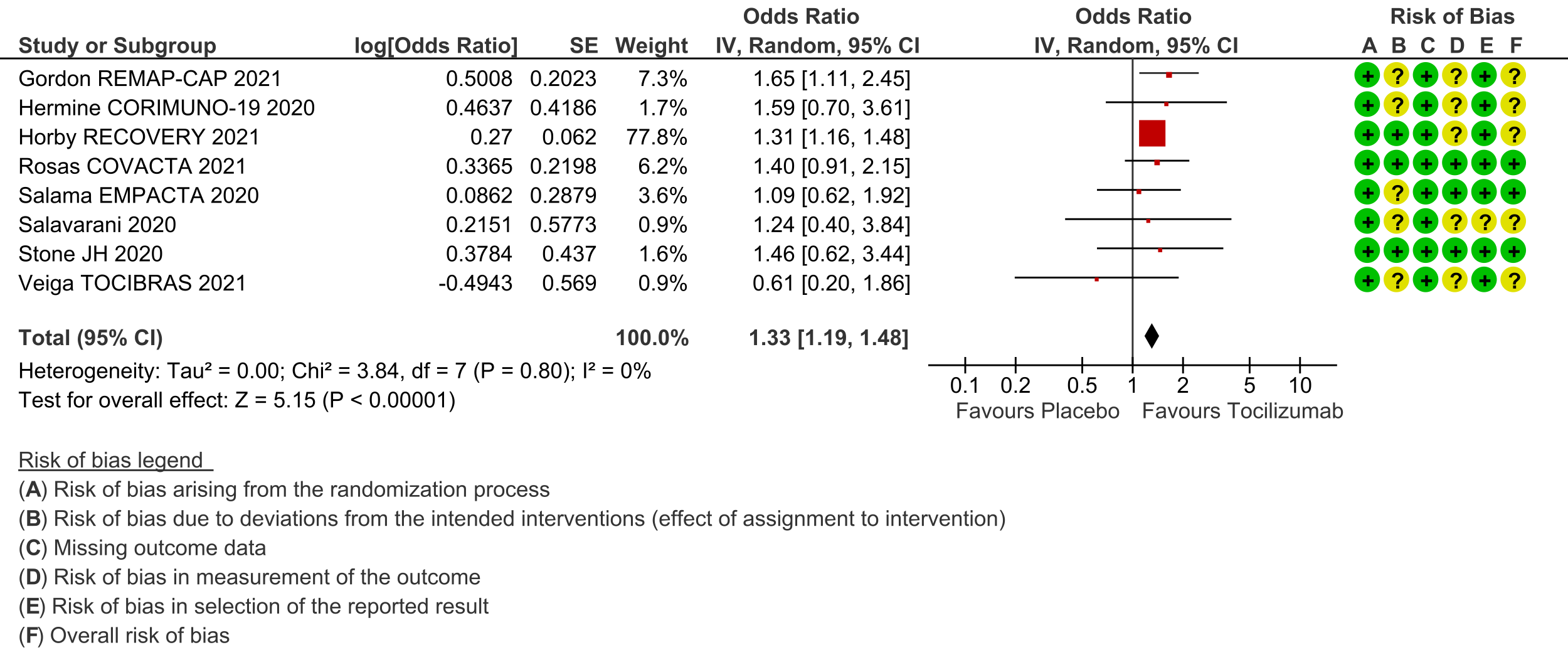
3. Time to clinical improvement:
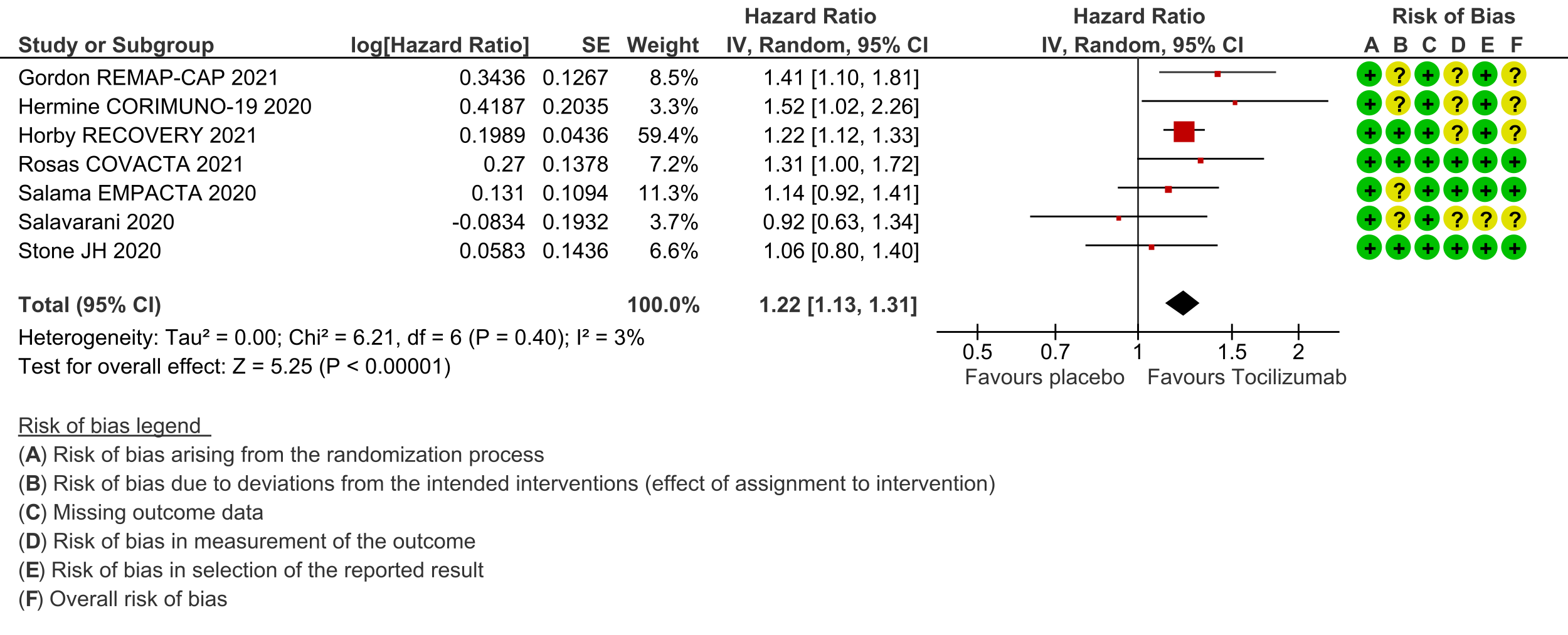
4. Progression to invasive ventilation, ECMO or death:
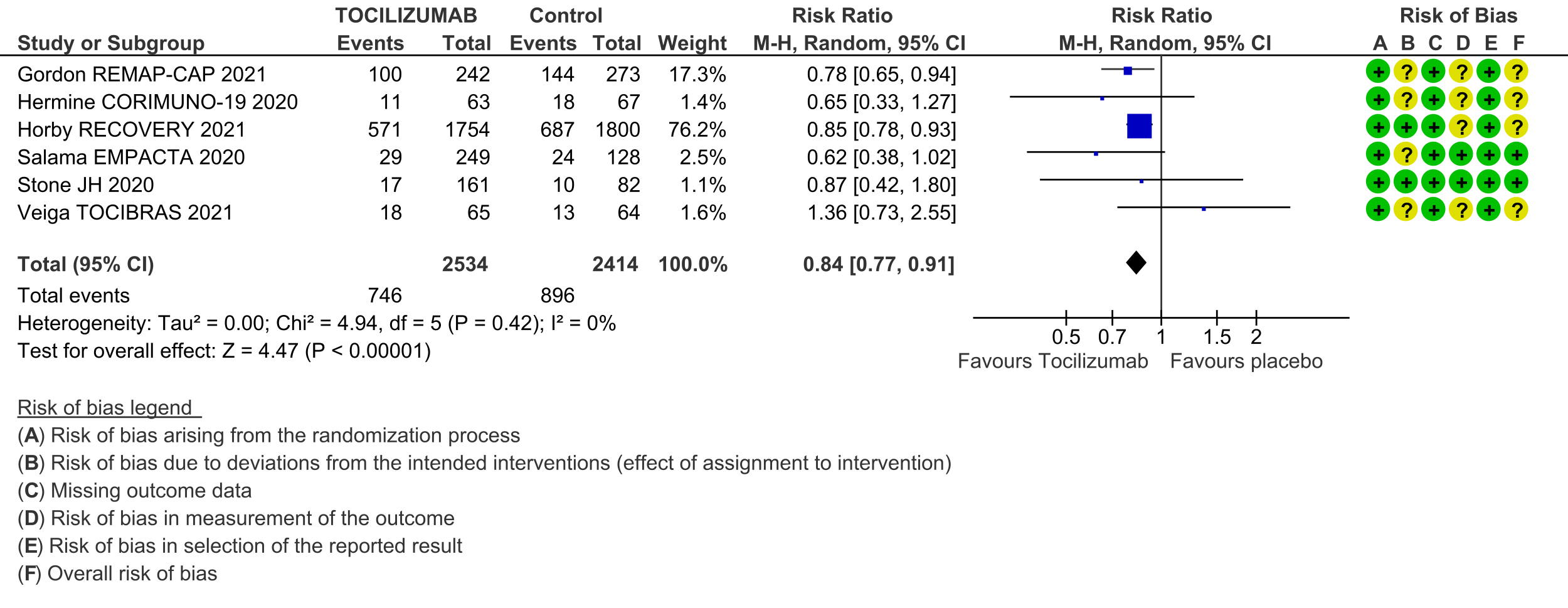
5. Adverse events:
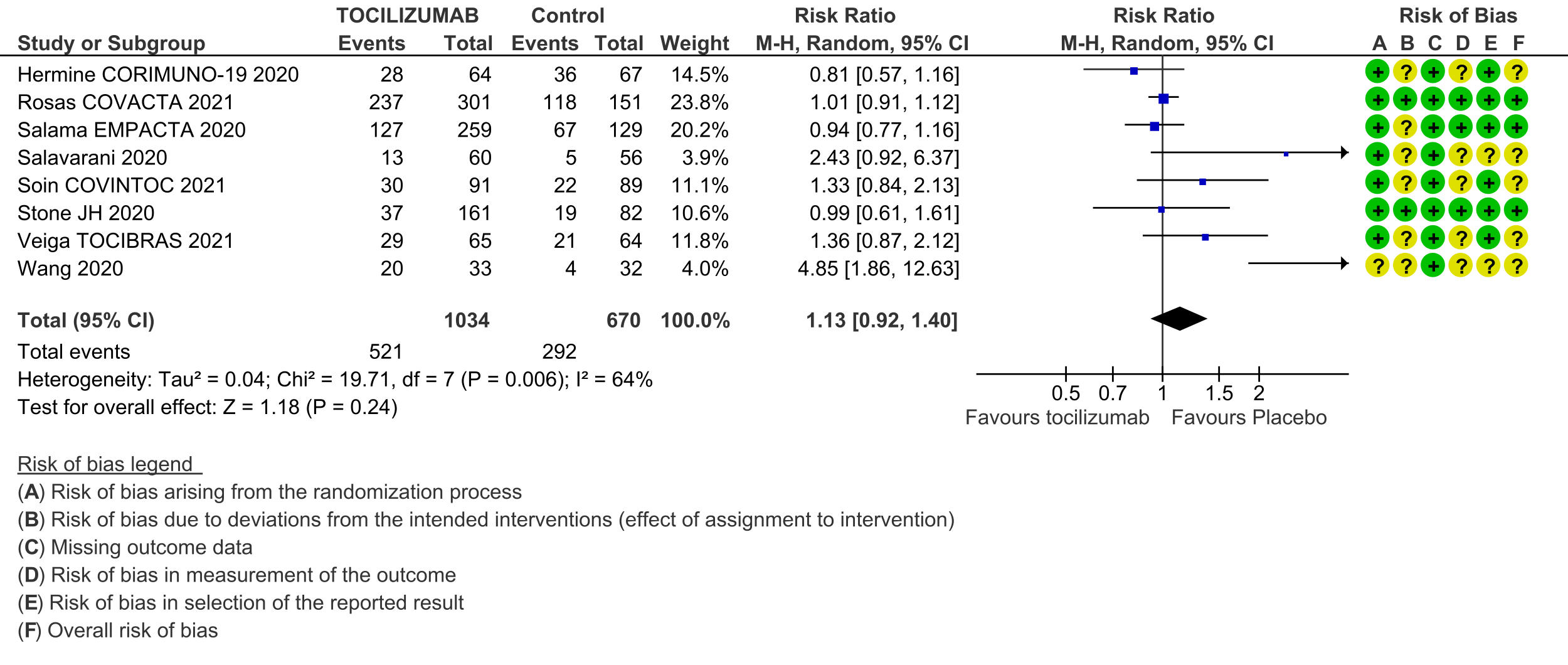
6. Serious adverse events:
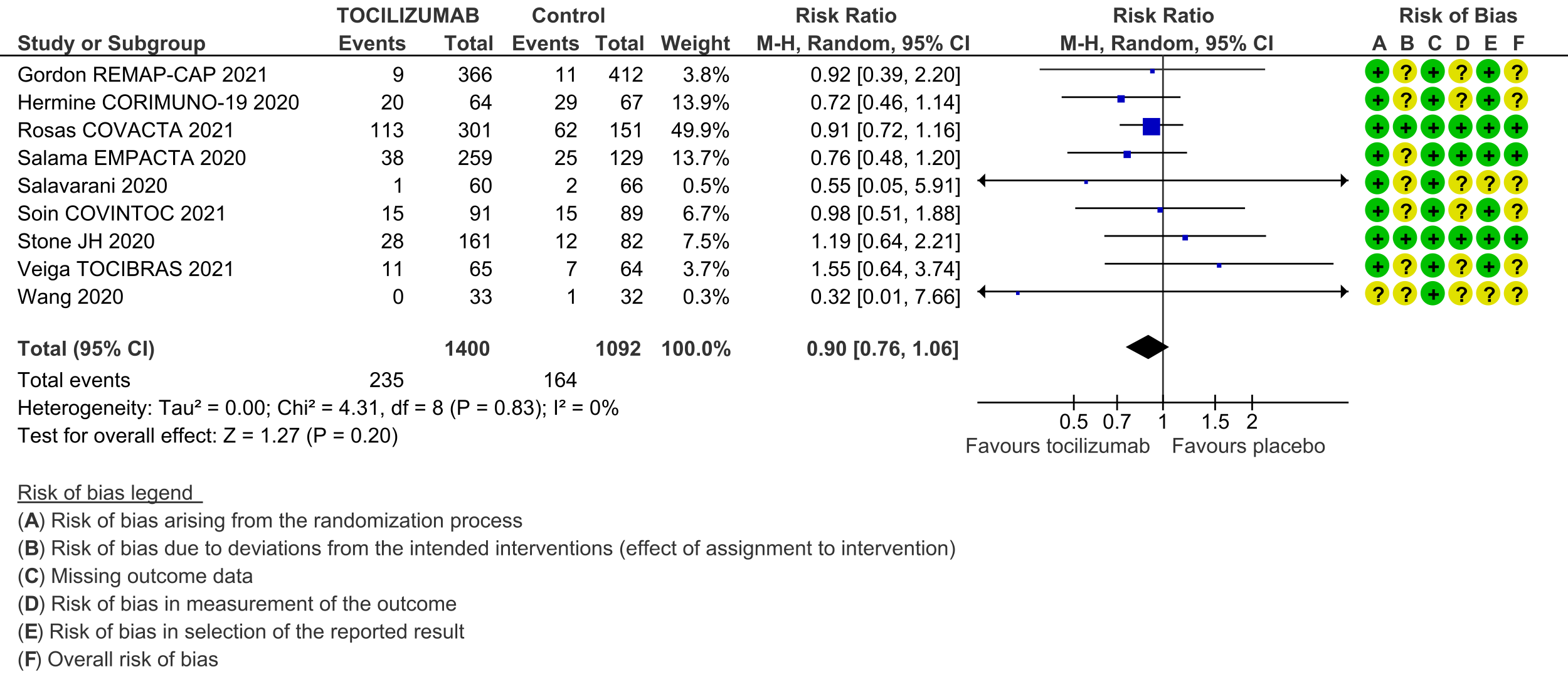
7. Length of stay in hospital:
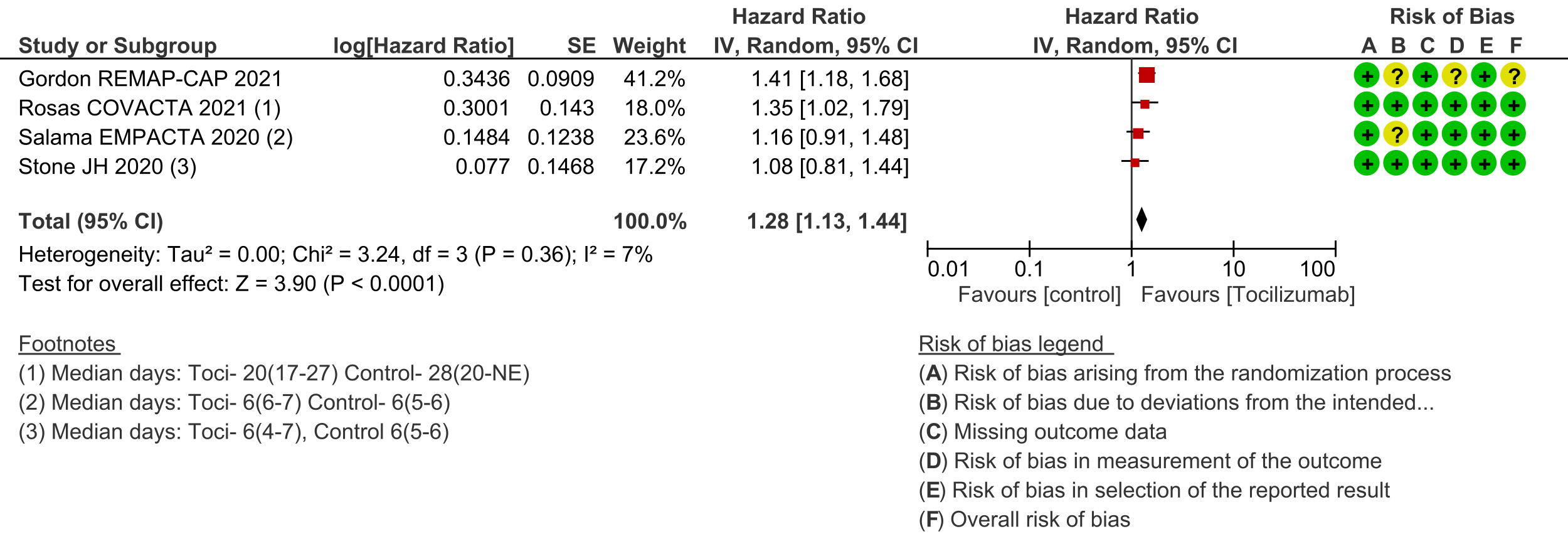
8. Admission to critical care: