Click here for an Interactive Summary of Findings table (opens in a new window).
GRADE Working Group grades of evidence
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect
Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect
Explanations:
a. Galan 2021 [14]; Gonzalez 2021 [15]; Lopez Medina 2021 [16]; Mohan 2021 [17]; Niaee et al 2021 [18]; Ravikirti 2021 [20]
b. Downgraded by one level for serious risk of bias. Due to Galan 2021 [14] and Niaee 2021 [18] having high risk of bias, and Gonzalez 2021 [15] and Ravikirti [20] having some concerns for risk of bias.
c. Downgraded by one level for serious inconsistency. There was substantial heterogeneity (I-squared=62%), and visually some trials have point effect estimates very far from others.
d. Downgraded by two levels for very serious imprecision, due to small absolute number of events, and CIs include important potential benefit and important potential harm.
e. Galan 2021 [14]; Mohan 2021 [17]; Pott-Junior 2021 [19]; Ravikirti 2021 [20].
f. Downgraded by one level for serious risk of bias, due to Pott-Junior 2021 [19] having high risk of bias, and Galan 2021 [14] and Ravikirti 2021 [20] having some concerns for risk of bias.
g. Downgraded by one level for serious inconsistency, due to substantial heterogeneity (I-squared=48%) and visually some trials having point effect estimates very far from each other.
h. Downgraded by two levels for very serious imprecision, due to small absolute number of events, and CIs including important potential benefit and important potential harm.
i. Ahmed 2020 [9]; Bukhari 2021 [11]; Chaccour 2021 [12]; Mohan 2021 [17]; Pott-Junior 2021 [19]; Ravikirti 2021 [20]
j. Downgraded by two levels for very serious risk of bias, due to Ahmed 2020 [9], Bukhari 2021 [11] and Pott-Junior 2021 [19] having high risk of bias for this outcome.
k. Downgraded by one level for serious inconsistency, due to substantial heterogeneity (I-squared=70%) and visually some trials having point effect estimates very far from those of other trials.
l. Downgraded by one level for serious imprecision, due to CIs overlapping no effect and inability to exclude important benefit.
m. Chaccour 2021 [12]; Chachar 2021 [13]; Lopez Medina 2021 [16]; Mohan 2021 [17]; Pott-Junior 2021 [19]
n. Downgraded by one level for serious risk of bias., due to Chaccour 2021 [12], Chachar 2021 [13] and Pott-Junior 2021 [19] having high risk of bias. Lopez-Medina 2021 [16] contributes 53% weight to the meta-analysis was at low risk of bias.
o. Downgraded by two levels for very serious imprecision, due to CIs overlapping including important potential benefit and important potential harm.
p. Gonzalez 2021 [15]; Lopez Medina 2021 [16]; Mohan 2021 [17].
q. Downgraded by one level for serious risk of bias, due to Gonzalez having high risk of bias.
r. Downgraded by two levels for very serious imprecision, due to CIs including important potential benefit and important potential harm.
Ivermectin has been shown to inhibit the replication of SARS CoV2 in vitro; it binds and destabilises the viral protein and prevents it from entering the nucleus [2]. However the drug dosages used in these laboratory studies far exceed those that have been used for other conditions [3]. Drug doses and levels required to achieve therapeutic effects in humans based on these studies may be safe, but this has not been studied in clinical trials [4]. An additional potential effect may be in modulating the immune system, though this is yet to be studied thoroughly in humans [5].
Although ivermectin is generally well tolerated, adverse effects like dizziness, tachycardia, postural hypotension, diarrhoea, arthralgia, and facial and peripheral oedema have been reported even with single doses as used in parasitic diseases [6]. Encephalopathy with permanent disability has been reported when ivermectin has been used in the treatment of onchocerciasis or other parasitic diseases [6]. It is predominantly metabolized in the liver (CYP3A4), which may lead to drug-drug interactions.
Due to lack of conclusive evidence from trials, World Health Organization recommends use of ivermectin only in clinical trials [7]. Use continues widely, including self-medication, especially in low- and middle-income countries due to easy availability and low cost of the drug [8].
This review aims to provide a summary of the available evidence from randomised clinical trials of ivermectin for treatment of acute COVID-19, for any dose or duration, so the Expert Working Group can provide a recommendation to guide clinicians and researchers regarding the appropriate use of this drug.
We used Cochrane rapid review methods. We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language published from March 2020 up to 15th April 2021. We also reviewed reference lists of systematic reviews and included studies. We contacted researchers to identify unpublished and ongoing studies.
We included randomized controlled trials (RCTs) testing ivermectin treatment of any dose or duration in people with acute COVID‐19, whether suspected or confirmed. Trials were included if the intervention arm did not combine ivermectin with another experimental drug, and if the comparator arm did not include ivermectin (this could involve use of placebo, standard care, or other potentially active drugs). We excluded trials that did not report any outcomes that could provide usable data for the review, those which were quasi-randomized and those lacking a comparator arms.
We planned to extract data for the following outcomes, pre-defined by the Expert Working Group:
- Critical (primary for this review):
- Mortality (all-cause) – at 28-30 days, or in-hospital
- Progression to:
- Oxygen therapy
- Ventilation: non-invasive or invasive
- Critical or Intensive care (any reason) - Duration of hospitalization
- Need for hospitalization (for out-patients)
- Adverse events: all and serious
- Important (secondary):
- Time to clinical improvement
- Time to negative PCR for SARS-CoV-2
- Negative PCR for SARS-CoV-2 by day 7 post-enrolment
- Complications of COVID-19:
- Thrombotic events
- Pulmonary function/fibrosis
- Long covid/post acute sequelae COVID
- Secondary infections
Two reviewers independently assessed eligibility of search results. One reviewer extracted data from each included study, and assessed risk of bias using the Cochrane Risk of bias (RoB) v2.0 tool. Data and RoB assessments were checked against a Cochrane systematic review team’s extractions and assessments (who used a consensus approach). In case of any discrepancies, the whole RoB assessment was scrutinised by the whole team for this review, to reach consensus.
We used RevMan 5.4 to perform meta‐analysis using a random‐effects model for outcomes where pooling of effect estimates was appropriate. We used risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). We planned to explore heterogeneity in the effect on mortality using subgroup analysis comparing between trials, based on COVID-19 illness severity of participants included and risk of bias. We used the I2 statistic to measure residual heterogeneity. We used GRADE methodology to assess the certainty in the evidence, and documented this in a ‘Summary of findings’ table using GradeProGDT.
We included 12 trials involving 1413 participants, all of whom were adults, and 718 of whom received ivermectin [9-20]. There were two trials each from Brazil, India and Pakistan; one trial reported from each of Bangladesh, Colombia, Iran, Mexico, Nigeria, and Spain. Eight were in hospitalized patients, one recruited outpatients only, one recruited both, and two did not report care setting. Disease severity, prevalence of comorbidities, and use of co‐interventions varied substantially between trials. The ‘Summary of characteristics of included trials’ table provides further details about the trials.
We found potential risks of bias across all domains; 10 of the 12 trials were at high risk of bias overall for at least one outcome. Risk of bias for each domain per trial is displayed alongside the Forest plots below. Studies excluded at full-text review are listed in the References section, with the reason provided in brackets [21-31].
The following comparisons were investigated in the trials. We compared outcomes for arms randomised to ivermectin vs. outcomes in arms with placebo, standard care, or agents considered inactive or ineffective against COVID-19. Where multiple arms contained ivermectin without another experimental agent, we combined results in those arms into a single ivermectin arm, but we did not double-count controls. Where another experimental agent undergoing investigation was combined with ivermectin, that trial arm was excluded from the analysis (e.g. doxycycline).
- Six trials [11-13;16;17;20] compared ivermectin vs. placebo (793 participants)
- Two [18;19] compared ivermectin vs. standard care (212 participants)
- One [9] compared ivermectin vs. placebo vs. a combination of ivermectin & doxycyline in three arms (72 participants; 24 participants in ivermectin & doxycycline arm excluded)
- One [15] compared ivermectin vs. placebo vs. hydroxychloroquine in three arms (106 participants)
- One [10] compared ivermectin vs. lopinavir/ritonavir (62 participants)
- One [14] compared ivermectin vs. chloroquine or hydroxychloroquine (168 participants)
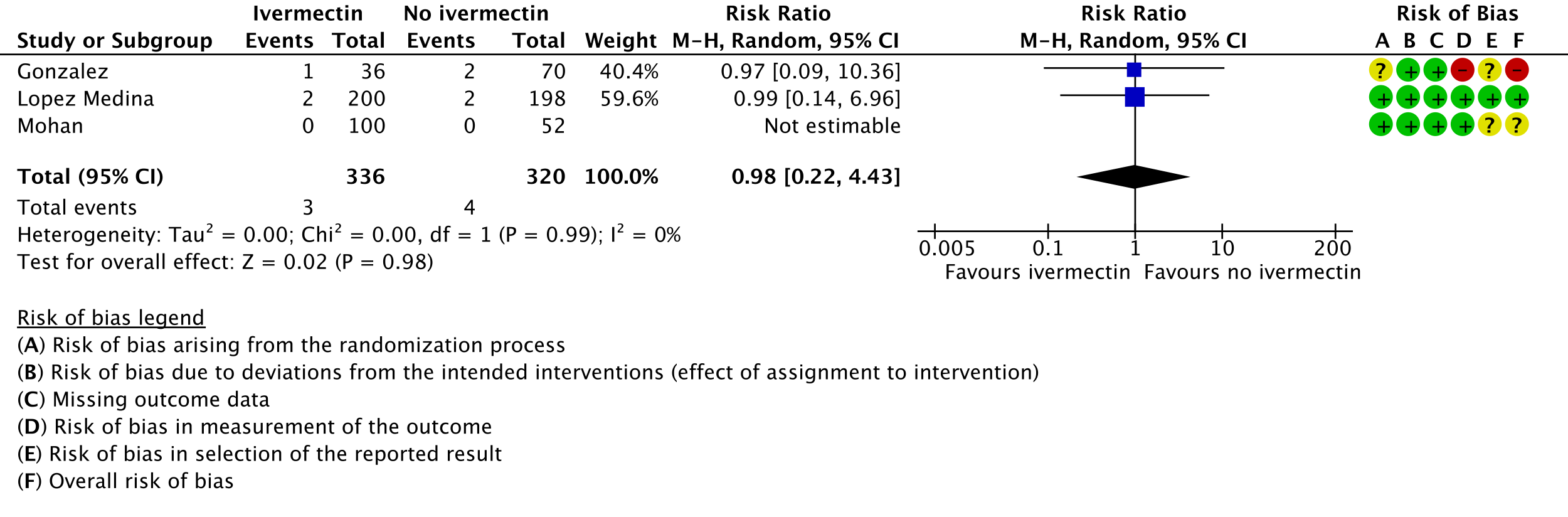
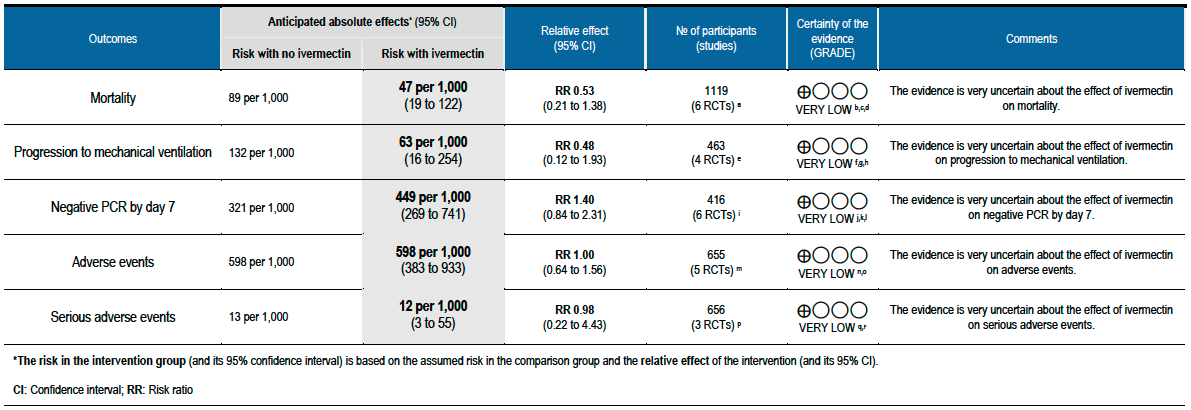
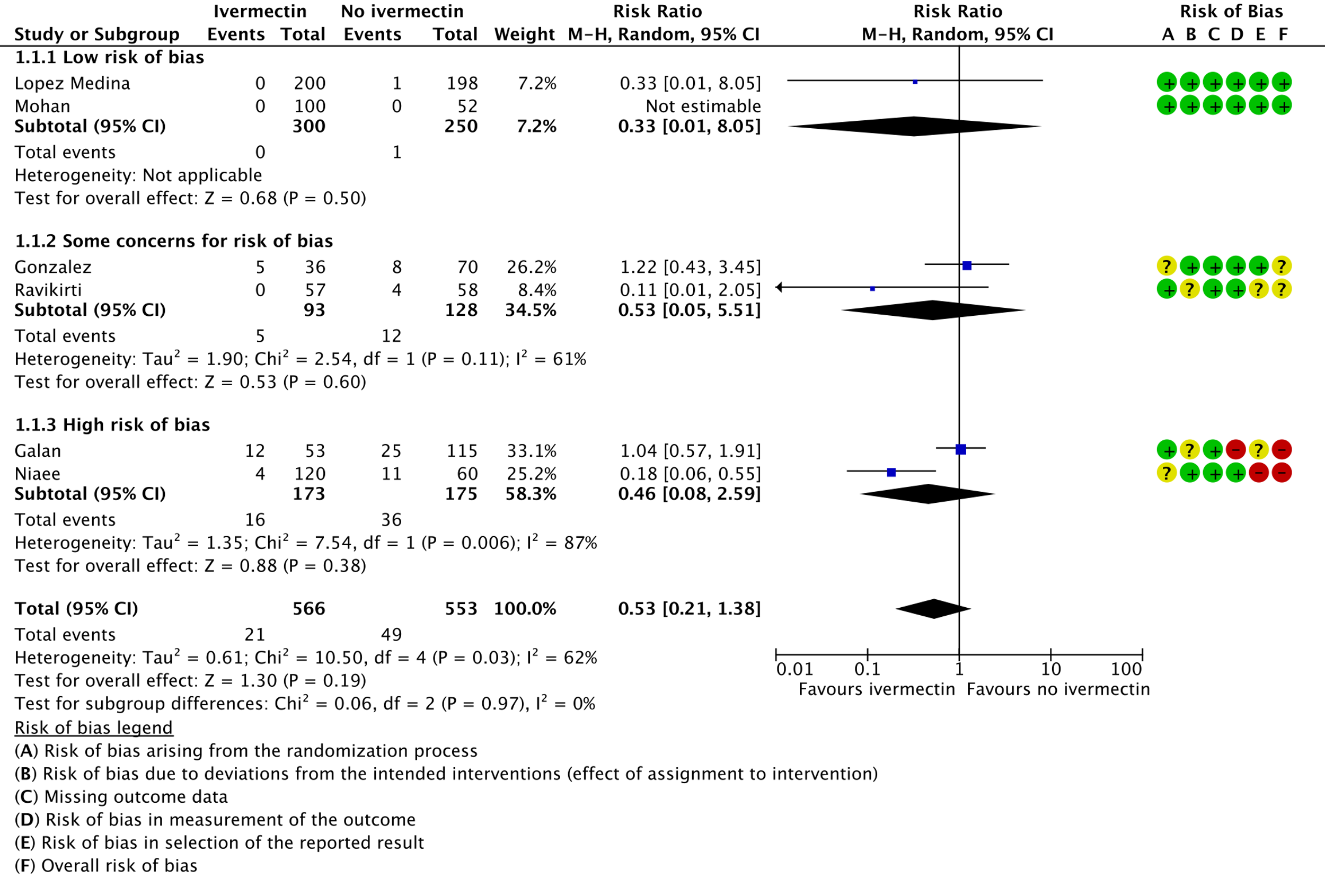
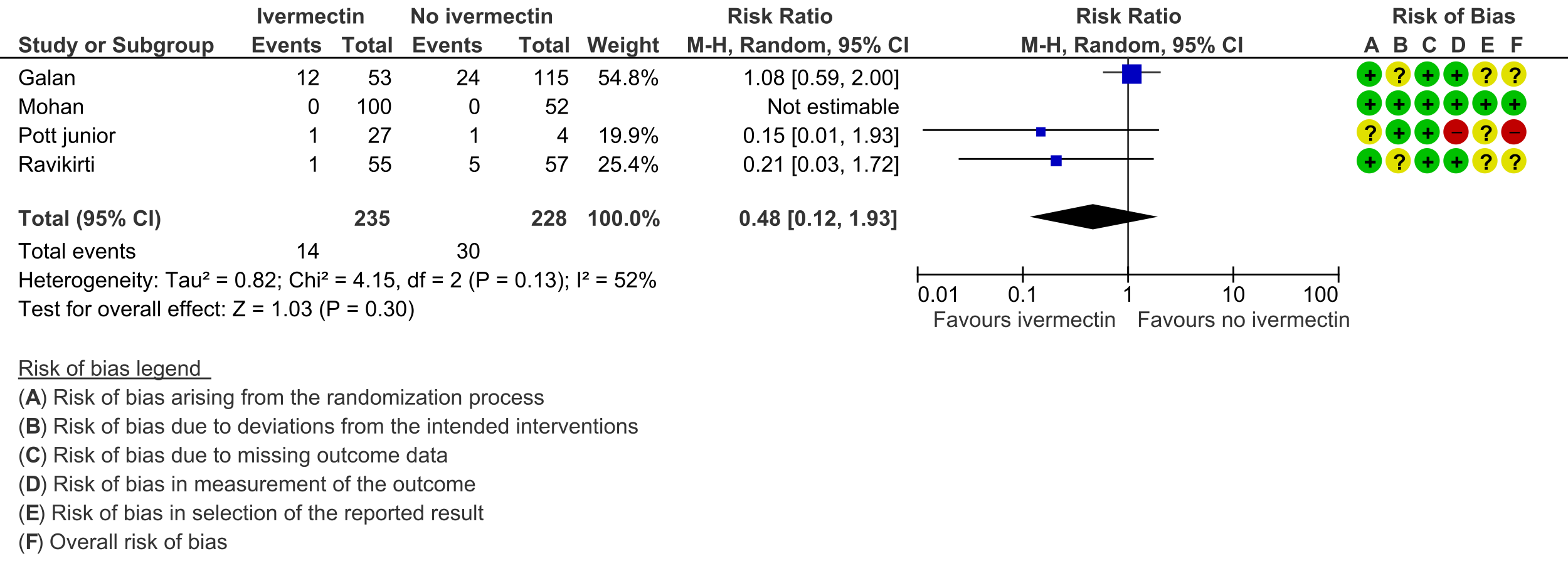
As presented in the ‘Summary of findings’ table, the evidence is very uncertain about the effect of ivermectin on mortality; progression to mechanical ventilation; negative PCR for SARS-CoV-2 by day 7; adverse events; and serious adverse events. For mortality, there were no significant differences observed when trials were stratified by COVID-19 illness severity or risk of bias.
One trial reported a higher risk of discontinuation of ivermectin vs. placebo due to an adverse event (RR 2.97; 95% CI 1.10 to 8.02; 1 trial [16]; 398 participants).
95% confidence intervals for pooled effect estimates for all of the following outcomes not included in the summary of findings table included potential benefit and potential harm from ivermectin: need for critical or intensive care (2 trials [14;20]; 283 participants); discharge from hospital by day 10 post-enrolment (1 trial [20]; 115 participants); deterioration by 2 points on 8-point clinical ordinal scale (1 trial [16]; 398 participants); lack of fever on day 7 (1 trial [9]; 36 participants); lack of symptoms on day 7 (1 trial [13]; 50 participants); and thrombotic events (1 trial [15]; 106 participants). We were unable to pool data for time to clinical improvement as they were not reported in a way that was amenable to meta-analysis (3 trials [12;16;17]; 149 participants).
No comparative data could be extracted for risk of progression to oxygen therapy; need for hospitalisation in outpatients; or post-acute COVID-19 pulmonary function/fibrosis or other sequelae; or secondary infections.
Lack of uniform criteria for COVID-19 severity, substantial overlap and lack of clear reporting of severity in the included trials prevented a meaningful subgroup analysis by severity.
Furthermore, a lack of within trial comparison prevented subgroup analysis by age, duration of symptoms or dose of ivermectin. In addition, data of safety and efficacy in specific subgroups such as pregnancy, children, liver and kidney disease were not available in the trials included in the rapid review.
| Study | Intervention and comparator arms | Design | Location Care setting | Age, average in years | No. of participants randomized | Participant characteristics |
|---|---|---|---|---|---|---|
| Ahmed 2020 [9] | Ivermectin alone (12 mg once daily for5 days) vs. Ivermectin and doxycycline (12 mg ivermectin single dose and 200 mg doxycycline on day 1, followed by 100 mg every 12 h for the next 4 days) vs. placebo | Double-blinded RCT | Dhaka, Bangladesh Inpatients | Not reported | Ivermectin: 24 Ivermectin+Doxycyline: 24 (not included in analysis). Placebo: 24 | RT-PCR positive admitted patients with symptoms for < 7 days |
| Babalola 2021 [10] | Ivermectin 6mg twice a week vs. Ivermectin 12mg twice a week vs.? Lopinavir/Ritonavir daily | Open label RCT | Lagos, Nigeria Care setting not reported | Ivermectin: 44 Lpv/Rtn: 44.8 | Ivermectin: 42 Lpv/Rtn: 20 | RT-PCR positive COVID-19 patients who were asymptomatic or had mild/moderate symptoms |
| Bukhari 2021 [11] | Ivermectin 12mg single dose vs. standard of care | Open label RCT | Lahore, Pakistan Inpatients | Ivermectin: 42.24 SoC: 38.98 | Ivermectin: 41 Placebo: 45 | RT-PCR positive COVID-19 with mild to moderate disease |
| Chaccour 2021 [12] | Ivermectin 400mcg/kg single dose vs. placebo | Double-blinded RCT | Barcelona, Spain Care setting not reported | Ivermectin: 37 Placebo: 37 | Ivermectin: 12 Placebo: 12 | RT-PCR positive COVID-19 with mild disease and duration of symptoms less than 5 days |
| Chachar 2021 [13] | Ivermectin 12mg three doses vs. standard of care | Open label RCT | Lahore, Pakistan Outpatients | Ivermectin: 40.6 SoC: 43.08 | Ivermectin: 25 Placebo: 25 | RT-PCR positive COVID-19 with mild disease |
| Galan 2021 [14] | Ivermectin 14mg 2 doses vs. Chloroquine vs. Hydroxychloroquine | Double blinded RCT | Boa Vista, Brazil Inpatients | Ivermectin: 53.2 CQ: 54.8 HCQ: 51.9 | Ivermectin: 53 CQ: 54 HCQ: 61 | RT-PCR or antigen positive COVID-19 with severe disease |
| Gonzalez 2021 [15] | Ivermectin 12/18mg vs. HCQ 400mg vs. Placebo | Double blinded RCT | Mexico Inpatients | Ivermectin: 56 HCQ: 48.9 Placebo: 53.8 | Ivermectin: 36 HCQ: 33 Placebo: 37 | RT-PCR positive COVID-19 with mild to severe illness |
| Lopez Medina 2021 [16] | Ivermectin, 300 mcg/kg per day for 5 days vs. placebo | Double blinded RCT | Colombia Inpatients & outpatients | Ivermectin: 37 Placebo: 37 | Ivermectin: 200 Placebo: 198 | RT-PCR positive COVID-19 with mild disease |
| Mohan 2021 [17] | Ivermectin 12mg in 20ml 40% ethanol vs. Ivermectin 24mg in 20ml 40% ethanol vs. placebo | Double-blinded RCT | New Delhi, India Inpatients | Ivermectin: 35.3 Placebo: 35.3 | Ivermectin: 80 Placebo: 40 | RT-PCR positive, non-severe COVID-19 with oxygen saturation more than 90% |
| Niaee et al 2021 [18] | 6 arms: Single doseivermectin (200mcg/Kg) vs. three low interval doses of ivermectin (200, 200, 200 mcg/Kg on day 1,3 and 5) vs. single dose ivermectin (400mcg/Kg), vs. three high interval doses of ivermectin (400, 200, 200 mcg/Kg on day 1,3 and 5) vs. placebo vs. standard of care | Double-blinded RCT | Qazvin, Iran Inpatients | Ivermectin: 55.5 Control: 56.5 | Ivermectin: 120 Control: 60 | RT-PCR or CT thorax positive COVID-19 with mild to severe disease |
| Pott-Junior 2021 [19] | Ivermectin 100 mcg/kg vs. Ivermectin 200 mcg/kg vs. Ivermectin 400 mcg/kg vs. standard of care | Open label RCT | Sao Carlos, Brazil Inpatients | Ivermectin: 48.75 SoC: 54.2 | Ivermectin: 28 SoC: 4 | RT-PCR positive COVID-19 with ECOG score of 0?1 and NEWS of 0?4 |
| Ravikirti 2021 [20] | Ivermectin 12mg for 2 days vs. placebo | Double-blinded RCT | Patna, India Inpatients | Ivermectin: 50.7 Placebo: 54.2 | Ivermectin: 57 Placebo: 58 | RT-PCR positive COVID-19 with mild to moderate disease |
1. Mortality, stratified by risk of bias:
2. Progression to mechanical ventilation:
3. Negative PCR by day 7:
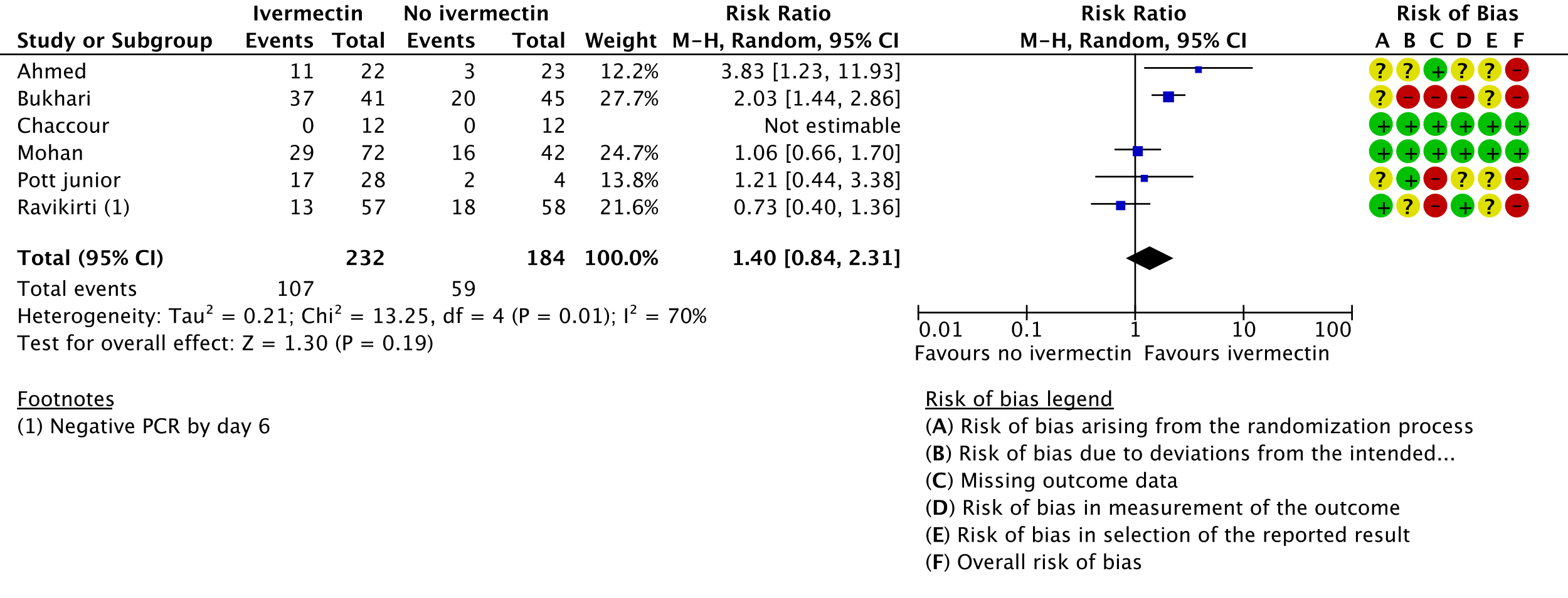
4. Adverse events:
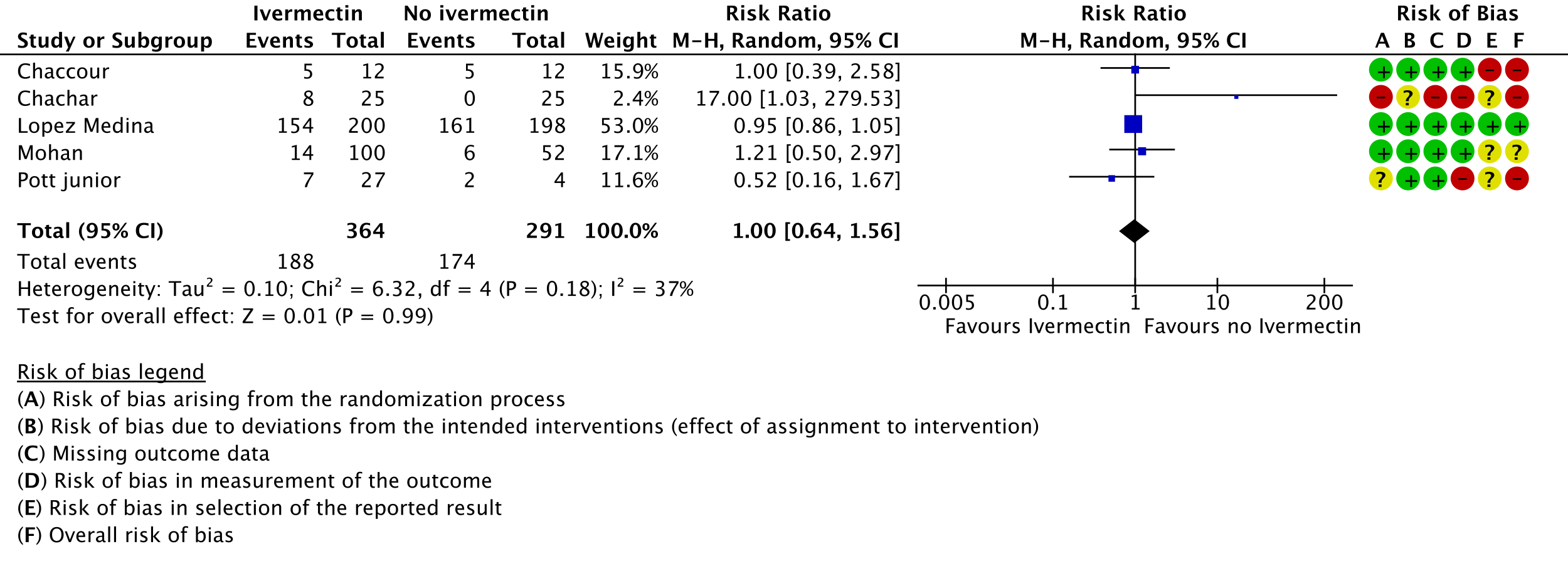
5. Serious adverse events: