Explanations
a. Downgraded by one level (indirectness). Arabi et al contributes to 33% of the weight of outcome (only severe patients included).
b. Downgraded by one level (serious imprecision) [11% benefit and 53% harm increases uncertainty]
c. Downgraded by one level: Risk of Bias (serious)
d. Downgraded by one level due to: Wide confidence interval, only a single study used to assess this outcome
e. Downgraded by one level due to serious risk of attrition and reporting bias.
f. Downgraded by one level due to high heterogeneity: I2= 81%
g. Downgraded by one level: for serious imprecision for the given outcome (wide CI)
h. Downgraded by one level: Risk of bias unclear to high in most of the studies
i. Downgraded by one level: Wide confidence interval and optimal information size (OIS) criteria not met
j. Downgraded by one level: One study showing higher incidence in the standard of carearm has high risk of attrition and reporting bias
k. Downgraded by one level due to high heterogeneity:I2 = 59%
l. Downgraded by 2 levels: CI very wide
SARS‐CoV‐2 is a novel coronavirus that has caused a pandemic since December 2019. Over 350 million people have been diagnosed with COVID‐19, and as of 25th January 2022, over 5.6 million people have died.4 Severe disease is characterized by hypoxia and progressive acute respiratory distress syndrome appears to be the main driver for mortality, although patients can experience a syndrome with clinical and laboratory features of severe systemic inflammation, termed a “cytokine storm”. At the other end of the spectrum, asymptomatic infection is not uncommon; national Italian data described this in approximately 10% of all people with a confirmed COVID‐19 diagnosis, though more recent estimates have been higher5. More recently, wide‐ranging longer‐term morbidity has been described in the absence of a severe initial illness.6
This review aims to provide a summary of the available evidence from randomized controlled trials (RCT) of Hydroxychloroquine/Chloroquine for treatment of COVID-19, for any duration, which could guide clinicians and researchers regarding the appropriate use of this drug in the future.
We searched PubMed and Epistemonikos, for studies of any publication status and in any language. We also reviewed reference lists of systematic reviews and included studies. We performed all searches up to 31st July 2021.
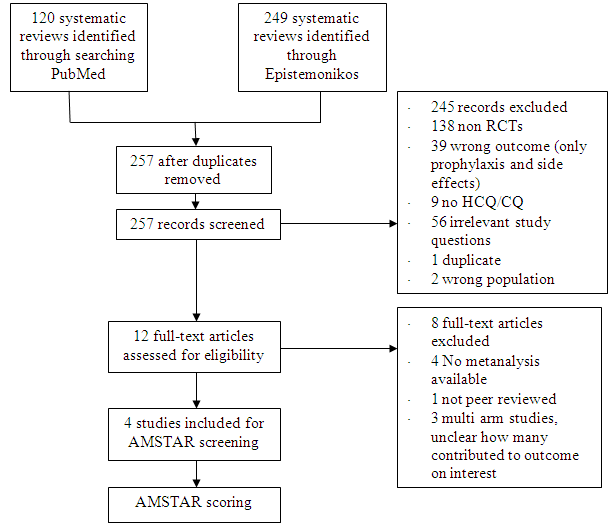
After searching the above databases and removing duplicates, we found 257 records for systematic reviews. After excluding non-RCTs, non-peer reviewed studies and systematic reviews which did not match our PICO question, four systematic reviews were selected. 3 of these were excluded since the AMSTAR scoring for these was found to be of critically low quality. One Cochrane review7 was included for the analysis. The AMSTAR score for the systematic review was found to be of moderate quality. The systematic review selected had 14 RCTs. 4 of these were excluded as they did not match the PICO question. We also went ahead and searched new RCTs to update the Cochrane review. 16 new RCTs were found which matched our PICO. A total of 26 RCTs were included for meta-analysis.
Data extraction was performed in context with the following PICO:
Population:
Patients who are symptomatic with confirmed or suspected COVID-19:
-
-
- Inpatients: Critical, Severe, Non-severe
- Outpatients
-
Intervention: HCQ or Chloroquine any dose, duration
Control: Standard of care/placebo
Outcomes:
Critical
- All- Cause mortality
- Progression to Mechanical ventilation
- Progression to non-invasive ventilation
- Participants with serious adverse events.
Important:
- Negative Respiratory PCR at D14 from randomization
- Time to clinical improvement
- Participants with any adverse events
- Participants with prolonged QTc on ECG
Two reviewers independently assessed eligibility of search results using the online Rayyan tool. Data extraction was performed by one reviewer, and checked by another, using a piloted data extraction tool on Microsoft Word. Risk of Bias (RoB) assessment for the 10 studies in the Cochrane review were included directly from the Cochrane Review7 . RoB for the 16 new RCTs was assessed using the Cochrane RoB v1.0 tool. For all outcomes, one review author performed the assessment, and cross-checked it against the assessment by another review author (who used consensus approach). The whole RoB assessment was scrutinised by the whole author team for this review. We planned to use risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). We planned to meta-analyze if appropriate, i.e., if outcomes were measured and reported in a similar way for similar populations in each trial, using random-effects models with the assumption of substantial clinical heterogeneity (the appropriateness of which would be checked by the Methodology Committee), and the I2 test to calculate residual statistical heterogeneity, using Review Manager (RevMan)v5.4. We used GRADE methodology to make summary of findings tables on GRADEPro GDT.
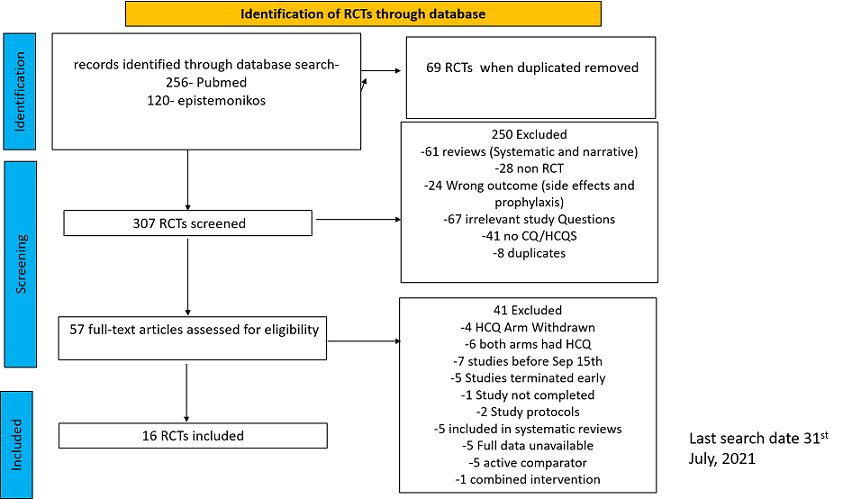
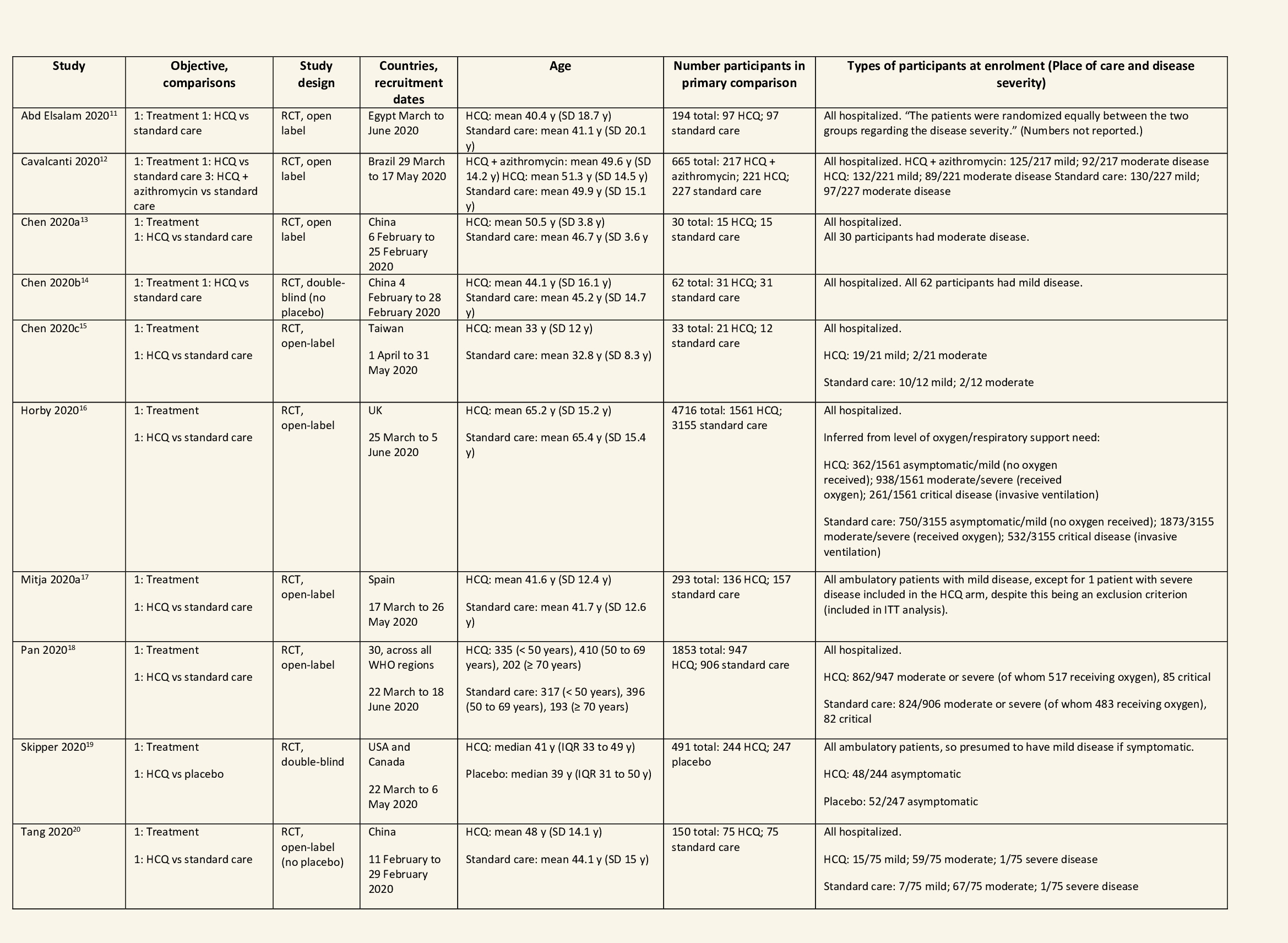
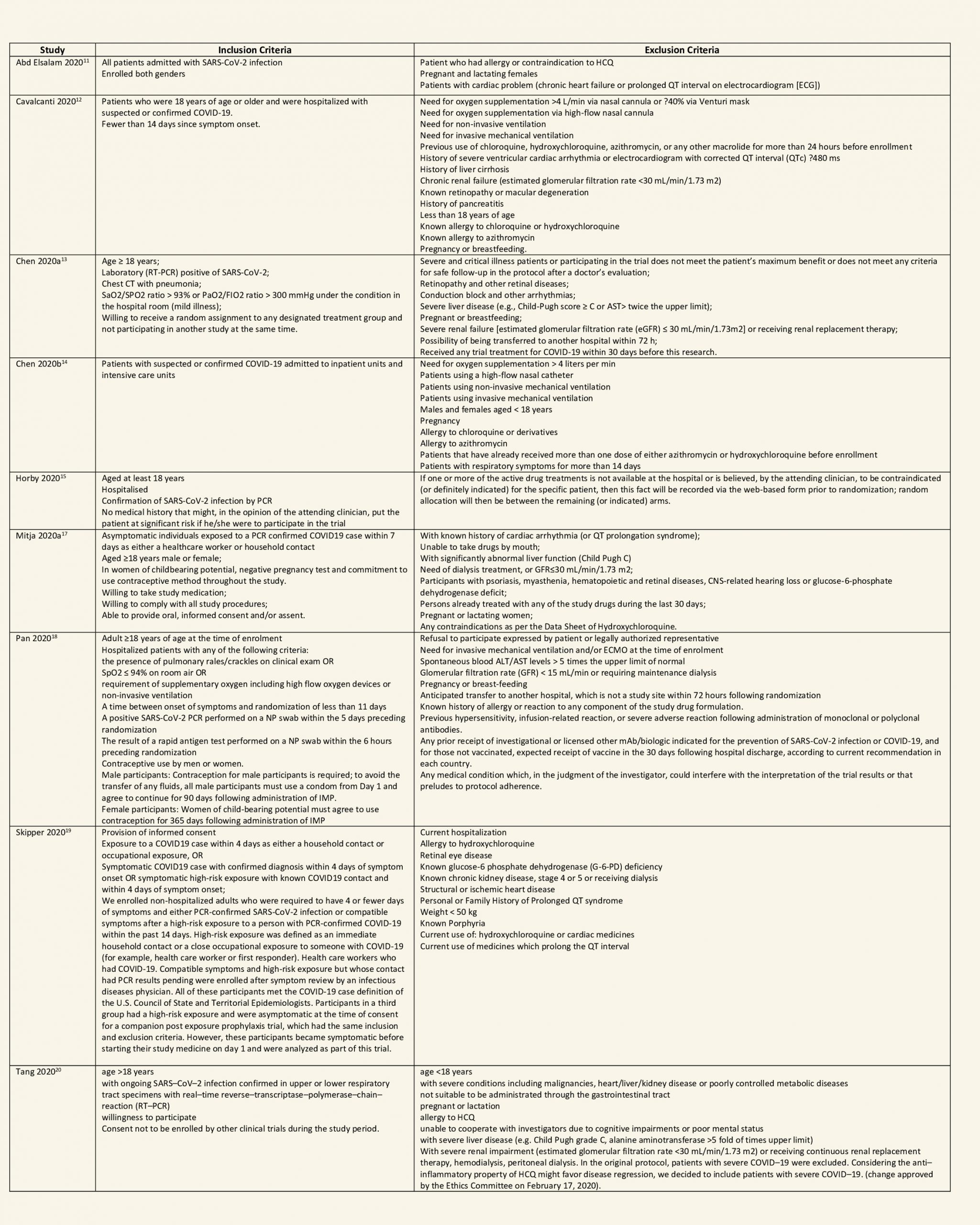
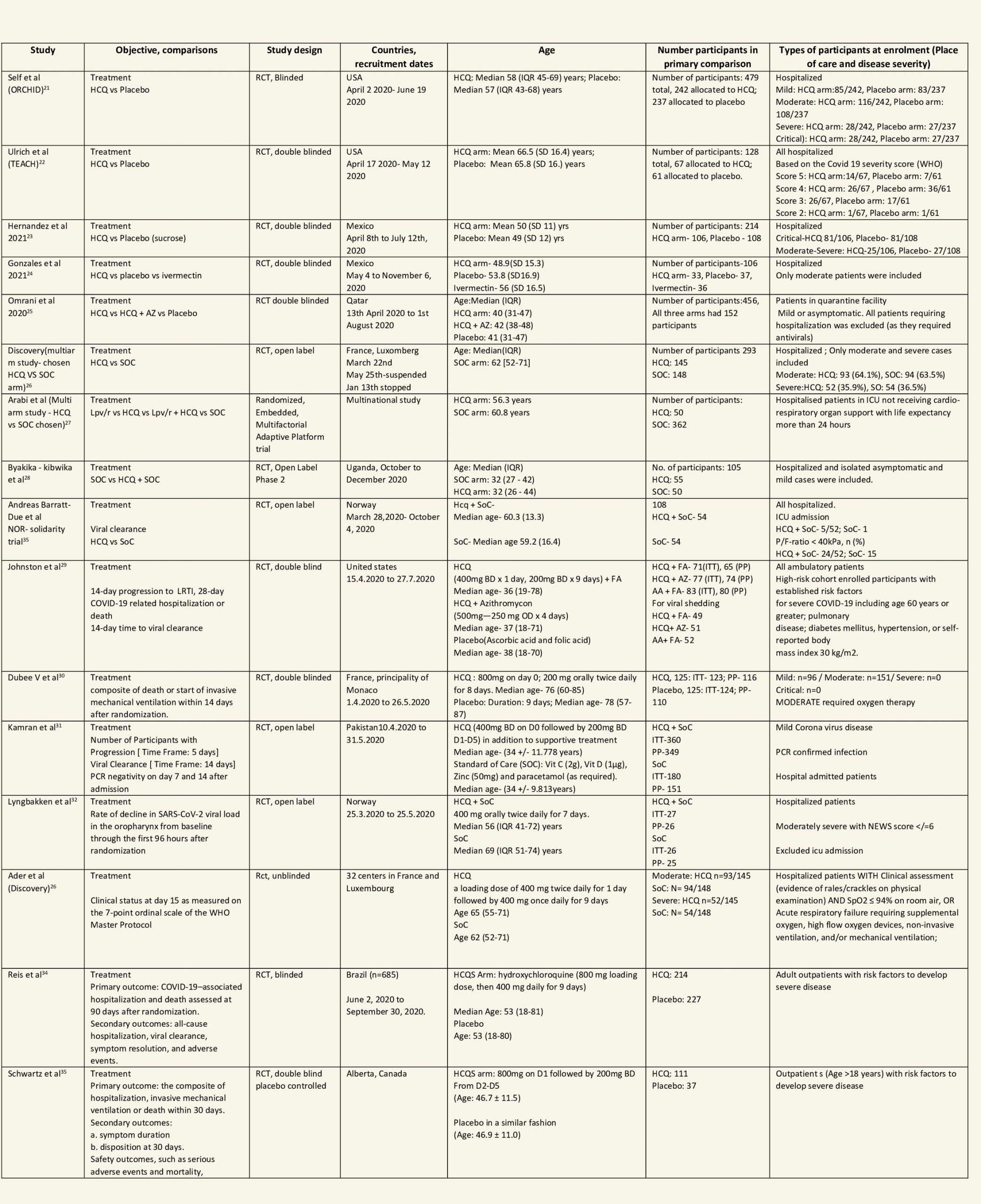
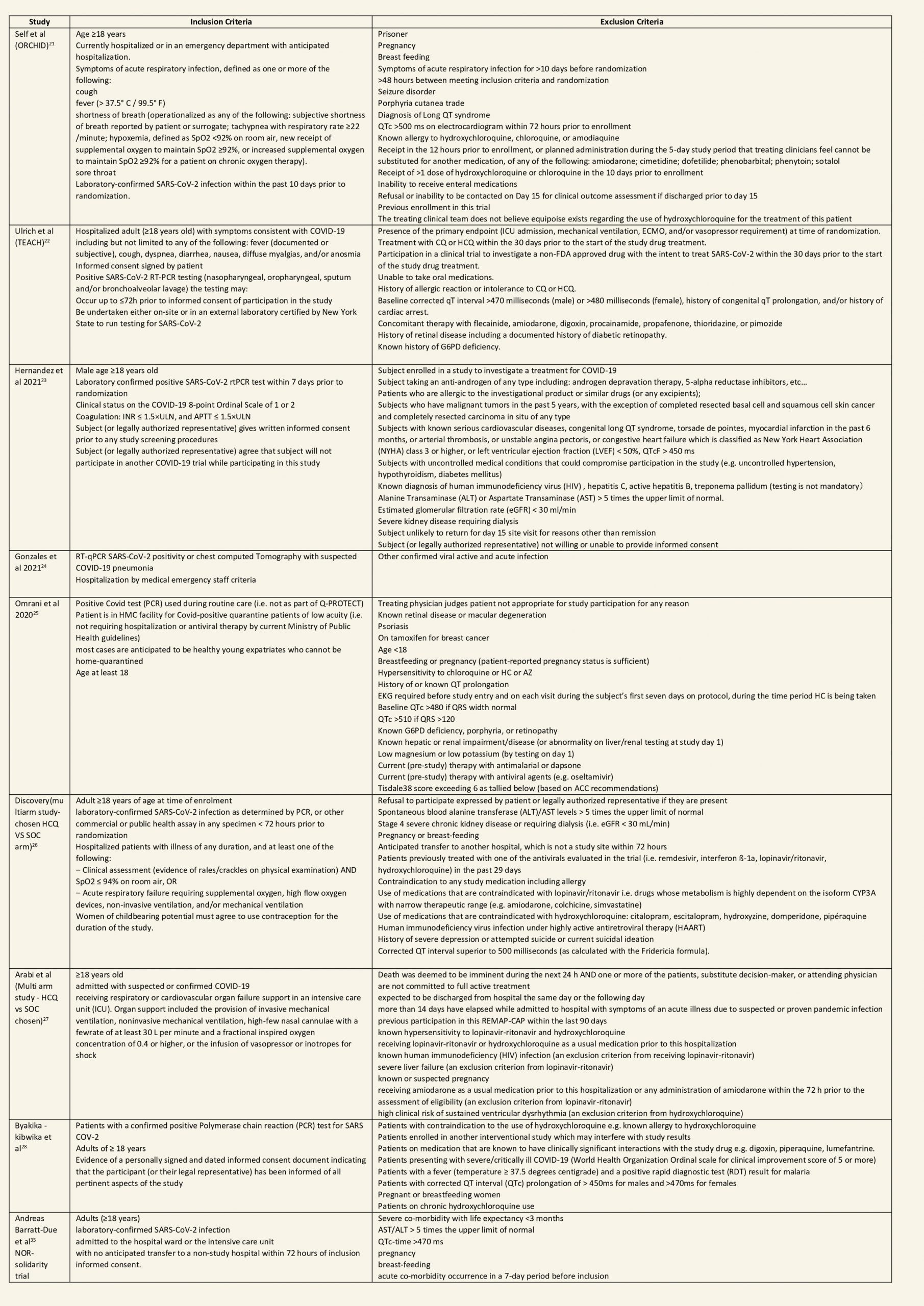
We found 26 RCTs (10 from the Cochrane review and 16 new RCTs) that matched the PICO question. The severity of disease varied from mild to critical. A few important details of these trials are provided below:
- Trials using placebo: 9/26
- Trials recruiting hospitalized participants: 20/26
- Severity of included participants:
-
- Mild disease only: 7/26
- Moderate disease only: 3/26
- Only severe disease: 1/26
- The remaining trials included participants with illness of mixed severity
-
Each trial and its results are described below; characteristics of the trials are summarized in the Summary of characteristics of included trials table. All the trials have varying risk of bias across all domains. Risk of bias for each domain per trial is displayed alongside the forest plots below.
Hydroxychloroquine/Chloroquine compared to Standard of Care
Characteristics of all the trials have been summarized in the Summary of characteristics of included trials table. All the trials have varying risk of bias across all domains. Our expert working group classified all-cause mortality, progression to mechanical ventilation, progression to non-invasive ventilation and participants with serious adverse events as critical outcomes, while outcomes like negative respiratory PCR at Day 14 from randomization, time to clinical improvement, participants with any adverse events and participants with prolonged QTc on ECG were classified as important outcomes.
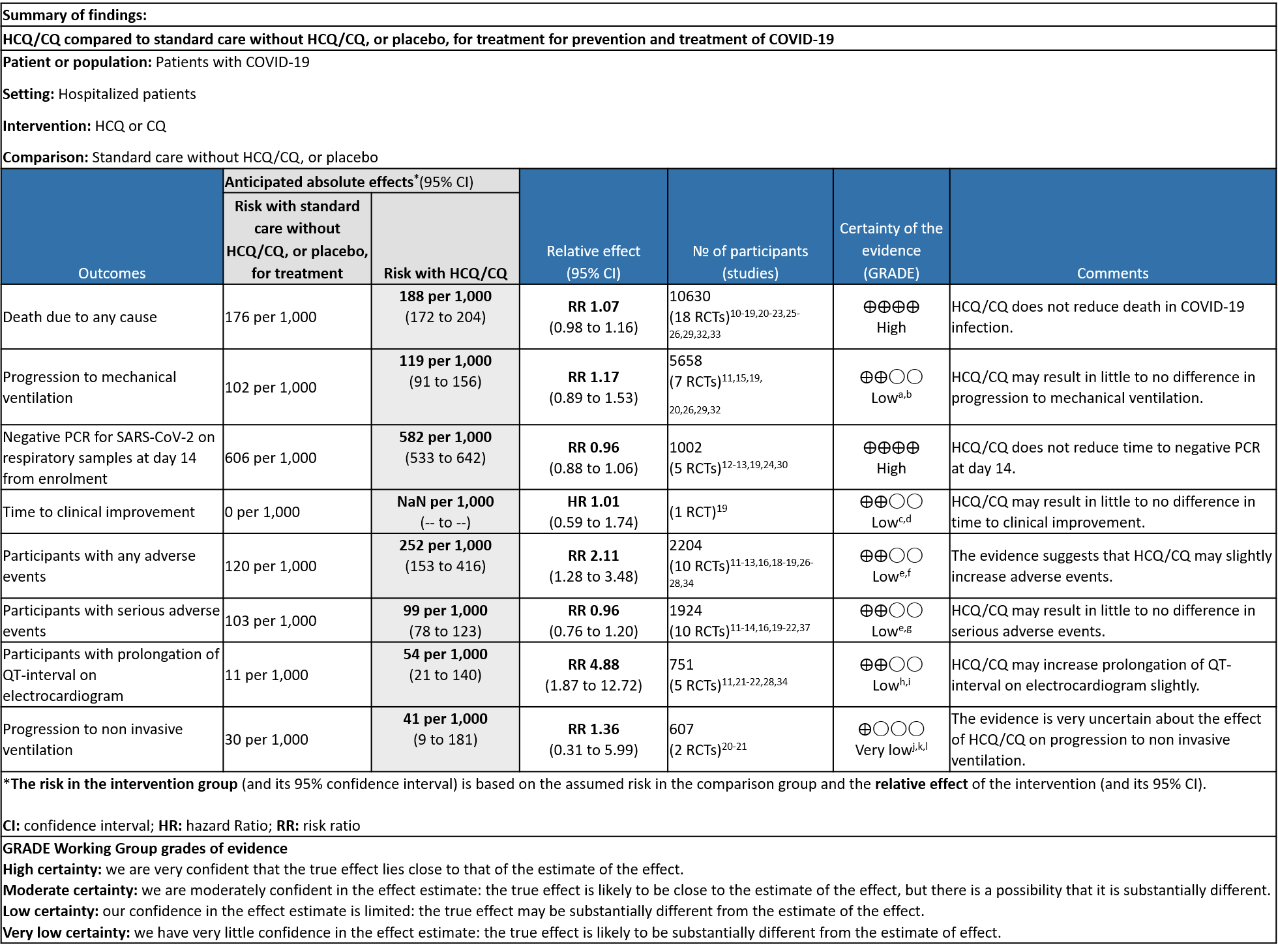
a. All-cause mortality: High certainty of evidence in 10,630 participants from 18 RCTs11-24,26-27,30,33-34 showed that hydroxychloroquine/chloroquine does not reduce mortality in COVID-19 infection RR 1.07 (0.98 to 1.16).
b. Progression to mechanical ventilation: Low certainty of evidence in 5,658 participants from 7 RCTsRCTs12,16,20-21,27,30,33 showed that hydroxychloroquine/chloroquine may result in no difference or even a slight increase in progression to mechanical ventilation in COVID-19 infection RR 1.17 (0.89 to 1.53).
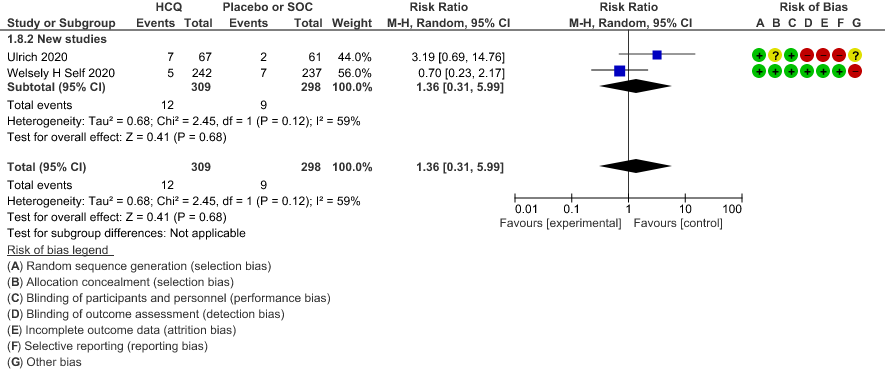
c. Progression to non-invasive ventilation: Very low certainty of evidence in 607 patients from 5 RCTs21-22 showed that the evidence was very uncertain regarding the effect of hydroxychloroquine/chloroquine on progression to non-invasive ventilation RR 1.36 (0.31 to 5.99).
d. Time to clinical improvement: Low certainty of evidence from 1 RCT20 showed that hydroxychloroquine/chloroquine may result in no difference in time to clinical improvement in COVID-19; HR 1.01 (0.59 to 1.74).
e. Negative PCR at Day 14: High certainty of evidence in 1002 patients from 5 RCTs13-14,20,25,31 showed that hydroxychloroquine/chloroquine does not reduce time to negative PCR for SARS-CoV-2 at day 14; RR 0.96 (0.88 to 1.96).
f. Any adverse events: Low certainty of evidence in 2204 patients from 10 RCTs12-14,17,19-20,27-29,35 showed that hydroxychloroquine/chloroquine may increase adverse events RR 2.11 (1.28 to 3.48).
g. Serious adverse events: Low certainty of evidence in 1924 patients from 10 RCTs12-15,17,20-23,38 showed that hydroxychloroquine/chloroquine may result in no difference in serious adverse events in COVID-19 infection RR 0.96 (0.76 to 1.20).
h. Prolonged QTc: Low certainty of evidence in 751 patients from 5 RCTs12,22-23,29,35 showed that hydroxychloroquine/chloroquine may increase prolongation of QT interval on electrocardiogram RR 4.88 (1.87 to 2.72).
10 Studies from Singh et al7
16 new RCTS
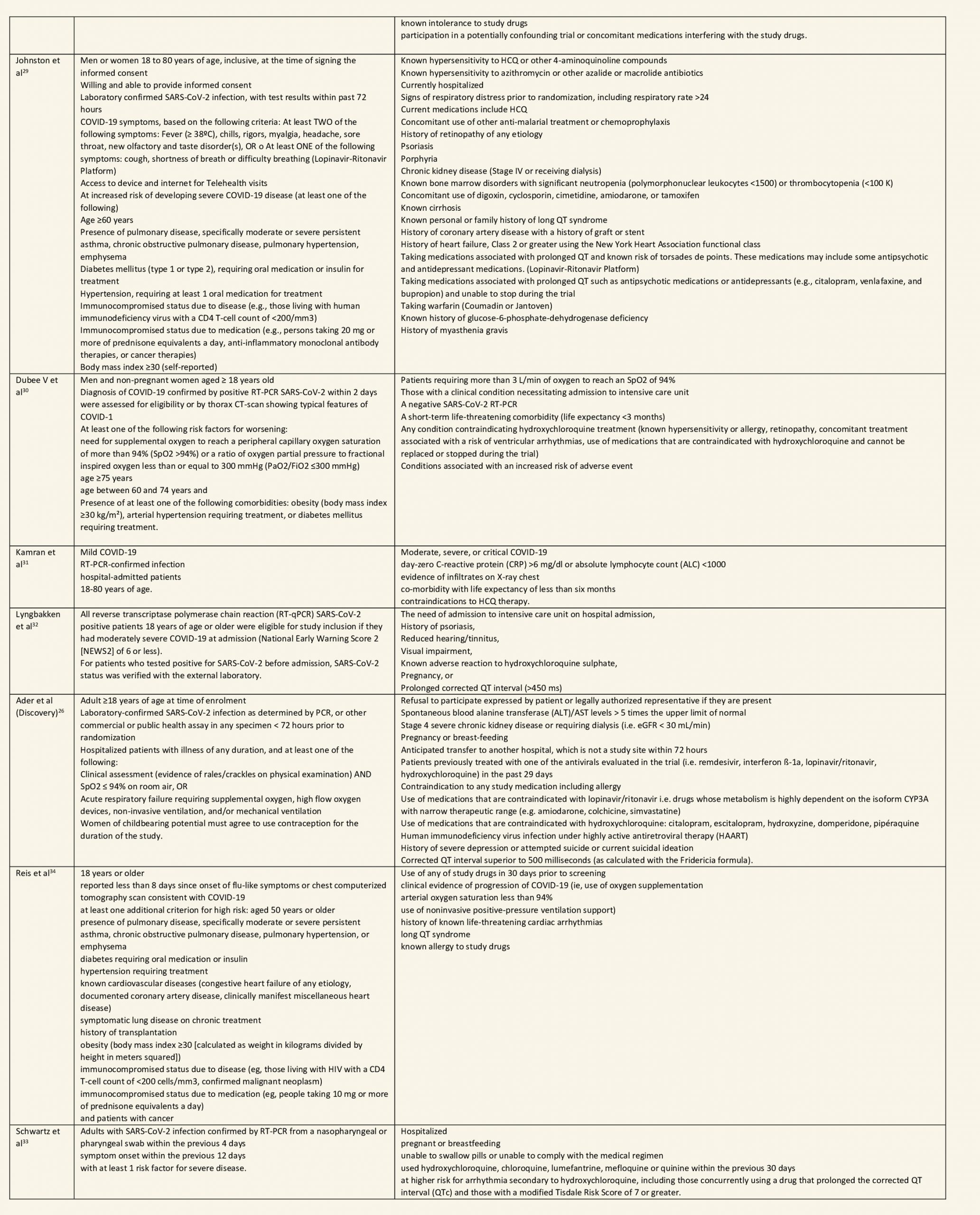
HCQ/CQ compared to standard care without HCQ/CQ, or placebo
1. Mortality
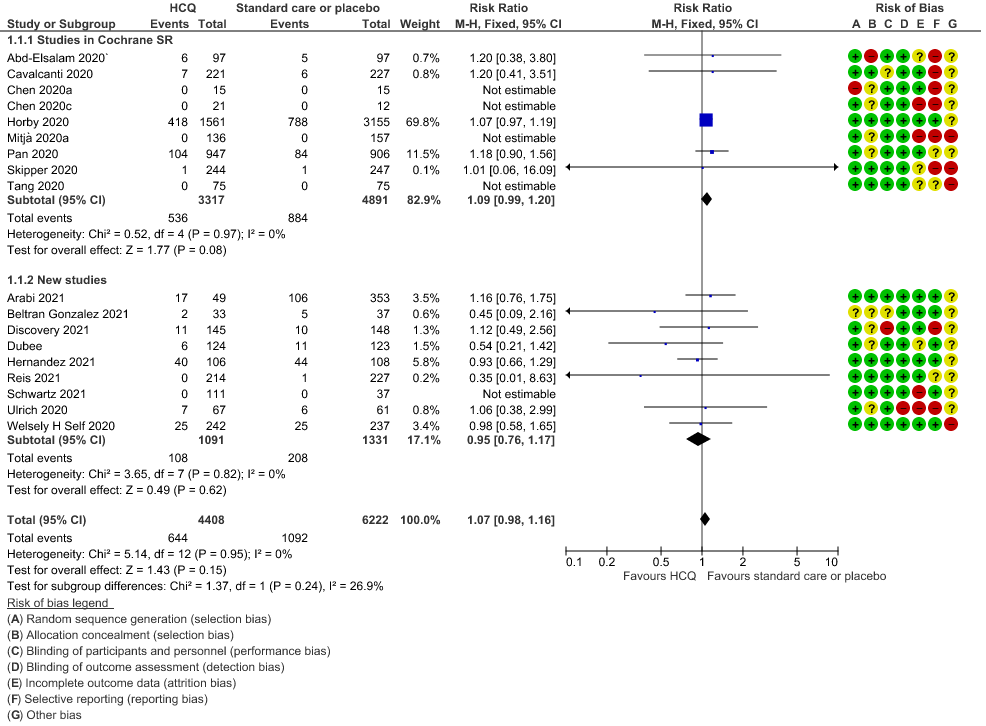
2. Progression to Mechanical Ventilation
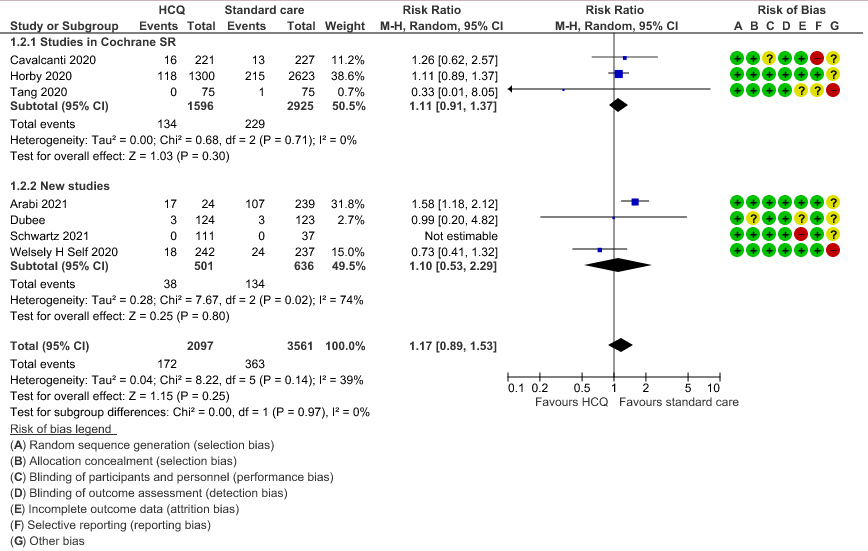
3. Negative PCR at day 14
4. Time to clinical improvement
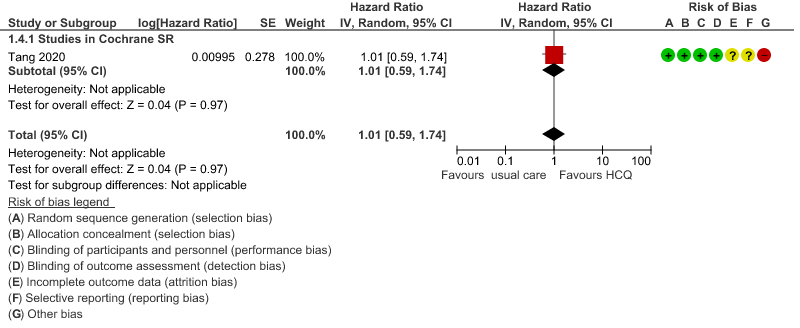
5. Adverse events
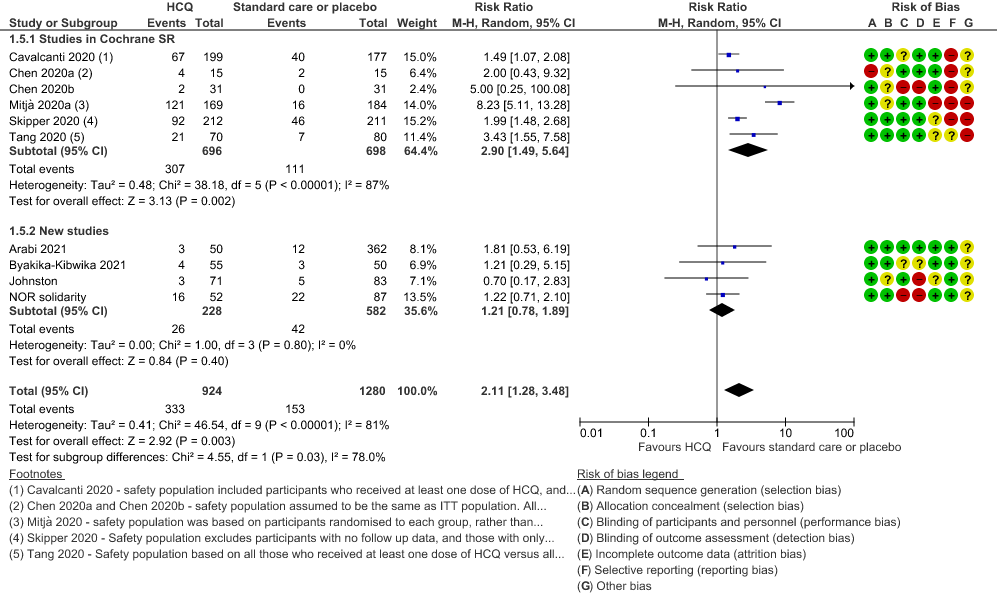
6. Serious adverse events
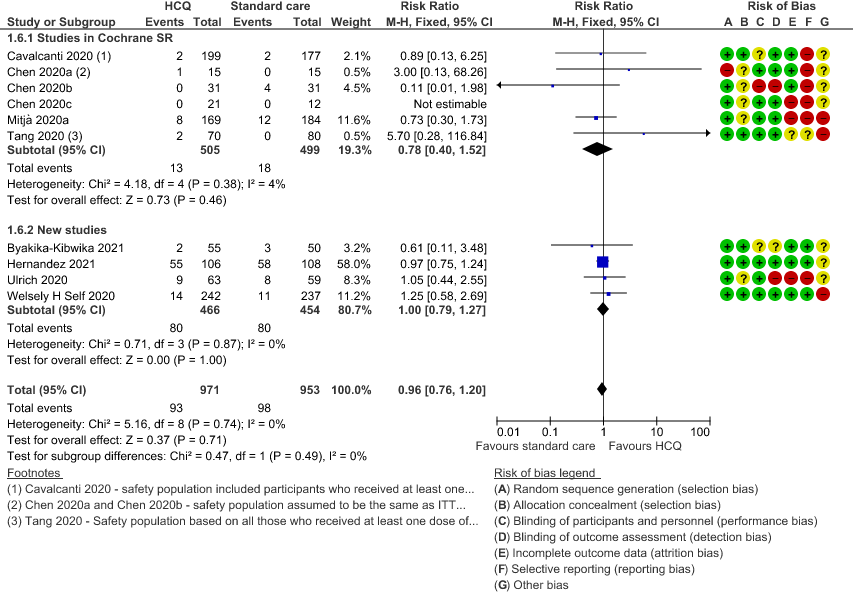
7. Prolonged QTc interval
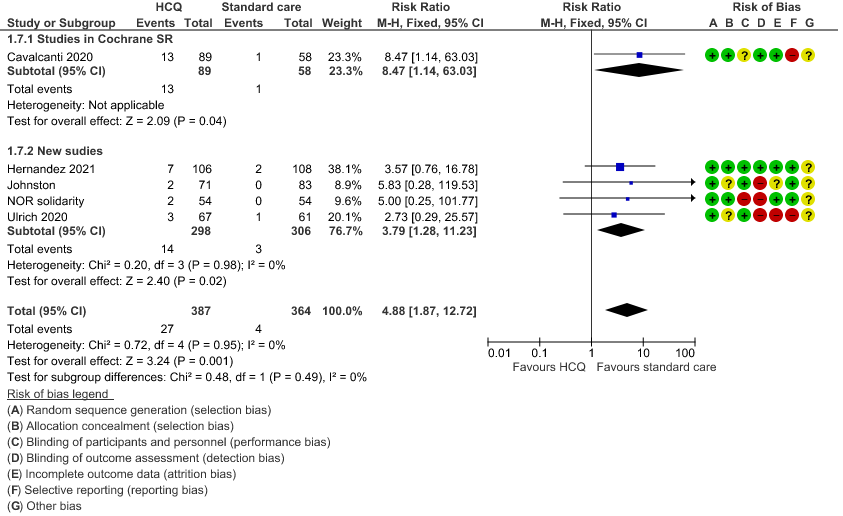
8. Progression to non-invasive mechanical ventilation