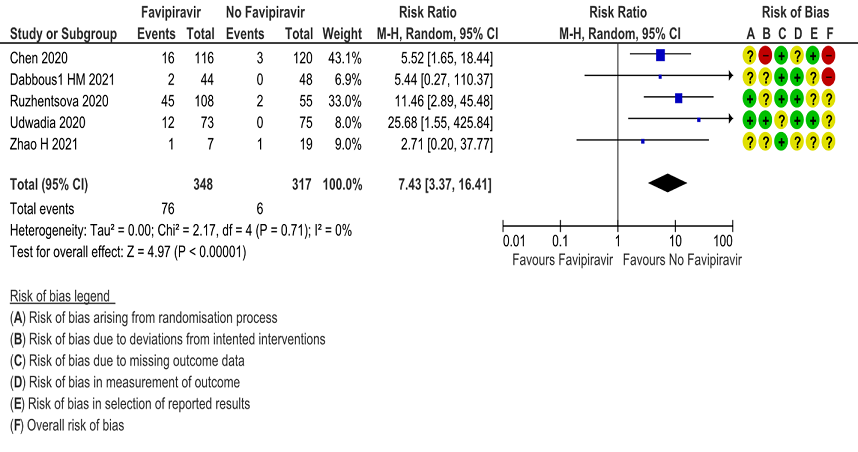
Explanations
a. Downgraded by one level for serious RoB as the larger study with high events contributed to 80% weightage had some concerns
b. Downgraded by one level for serious indirectness as the severity of disease was different across the studies and different comparators used(SOC/placebo/active drug).
c. Downgraded by one level for serious imprecision as 95% CI crosses 1 (0.66 to 1.77). The RR pertains to 5 studies with events. 4 studies did not have events.
d. Downgraded by one level for serious RoB as Chen 2020 and Udwadia 2020 had high/ some concerns in RoB
e. Downgraded by one level for serious imprecision as 95% CI crosses 1 (0.31 to 1.82)
f. Downgraded by one level for high RoB
g. Downgraded by one level for serious indirectness as population had unclear severity in Lou 2020
h. Downgraded by one level for serious imprecision as 95%CI (0.27 to 2.93)
i. Downgraded by one level for serious RoB as two studies that contributed to the 90% of weightage to the results had some concerns for RoB
j. Downgraded by one level for serious imprecision as 95% CI crosses 1 (0.58 to 1.52)
k. Downgraded by one level as both studies had some concerns for RoB
l. Downgraded by one level for serious imprecision as 95% CI crosses 1 (0.73 to 1.89)
m. Downgraded by two-level for very serious RoB as both studies has high RoB
n. Downgraded by one level for serious indirectness as the severity of disease was different across the studies
o. Downgraded by one level for serious imprecision as the decrease was 1.5 days lower but the 95%CI ranged from 0.27 to 3 days which is clinically not interpretable.
p. Downgraded by one level for serious imprecision as the absolute effect is 1 few per 1000 which is clinically insignificant.
Favipiravir (T-705) is a synthetic prodrug, first discovered while assessing the antiviral activity of chemical agents active against the influenza virus. Excellent bioavailability (∼94%), 54% protein binding, and a low volume of distribution (10–20 L). It reaches Cmax within 2 h after a single dose. Favipiravir has a short half-life (2.5–5 h) leading to rapid renal elimination in the hydroxylated form. It was first approved in Japan for the management of emerging pandemic influenza infections in 2014 and later used for Ebola. Favipiravir was first used against SARS-CoV-2 in Wuhan at the very epicenter of the pandemic (1). Shannon et al found that the SARS-CoV-2–RDRp complex is at least 10-fold more active than any other viral RdRp known(2). Favipiravir acts by inhibiting this viral RdRp enzyme, allowing insertion of Favipiravir into viral RNA while sparing human DNA. The optimal dose of Favipiravir is difficult to establish from the limited preclinical, in vitro data. For instance, the higher dosing of favipiravir used in Ebola was based on preclinical studies showing the target concentrations needed to inhibit the Ebola virus were higher than that in influenza(1). Wang et al. found that the high concentrations of Favipiravir were needed to inhibit SARS-CoV-2 infection in Vero cells. Thus, it is difficult to ascertain the basis on which the current dose of this drug has been established in SARS-CoV-2(3). Despite this uncertainty, the dose in clinical use in most countries, including India, is 1800 mg bid on day 1, followed by 800 mg bid on days 2–14. In June 2020, favipiravir received the DCGI approval in India for mild and moderate COVID-19 infections. On June 7th 2021 the health ministry removed the drug from covid-19 treatment guidelines, however many clinicians continue to use in mild to moderate COVID 19 infections. This review aims to provide a summary of the available evidence from randomized clinical trials of Favipiravir for treatment of COVID-19, for any duration, which could guide clinicians and researchers regarding the appropriate use of this drug in the future.
We searched PubMed, Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language. We also reviewed reference lists of systematic reviews and included studies. We performed all searches up to 16 June 2021.
We searched the above databases and found 45 records. After removing duplicates and excluding those that did not match our PICO question, one systematic review was selected and included for the analysis. The AMSTAR score for the systematic review was found to be of low quality of evidence. The systematic review selected had 8 RCTs and 1 non-RCT. The 8 RCTs were further screened and 3 were excluded as they did not match the PICO question. We also went ahead and searched for new RCTs and found further 5 new RCTs which were included in the analysis. Thus a total of 10 RCTs were included for meta-analysis.
We extracted data for the following outcomes, pre-defined by the Expert Working Group:
- Critical (primary for this review):
- Progression to:
- Oxygen therapy
- Ventilation: non-invasive or invasive
- Critical or Intensive care (any reason)
- Duration of hospitalisation
- Progression to:
- Important (secondary):
- Mortality (all-cause) – at 28-30 days, or in-hospital
- Time to clinical improvement
- Time to negative PCR for sars-cov-2
- Complications of COVID-19:
- Thrombotic events
- Pulmonary function/fibrosis
- Long covid/post-acute sequelae
- Secondary infections
- Adverse events:
- All and serious, hyperuricemia
Two reviewers independently assessed eligibility of search results using the online Rayyan tool. Data extraction was performed by one reviewer, and checked by another, using a piloted data extraction tool on Microsoft Word. Risk of bias (RoB) was assessed using the Cochrane RoB v2.0 tool, with review by a second reviewer and compared with covidnma database. If there was a difference in more than one domain it was assessed by a third independent author. We planned to use risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). We planned to meta-analyze if appropriate, i.e., if outcomes were measured and reported in a similar way for similar populations in each trial, using random-effects models with the assumption of substantial clinical heterogeneity (the appropriateness of which would be checked by the Methodology Committee), and the I2 test to calculate residual statistical heterogeneity, using Review Manager (RevMan) v5.4. We used GRADE methodology to make summary of findings tables on GradeProGDT.
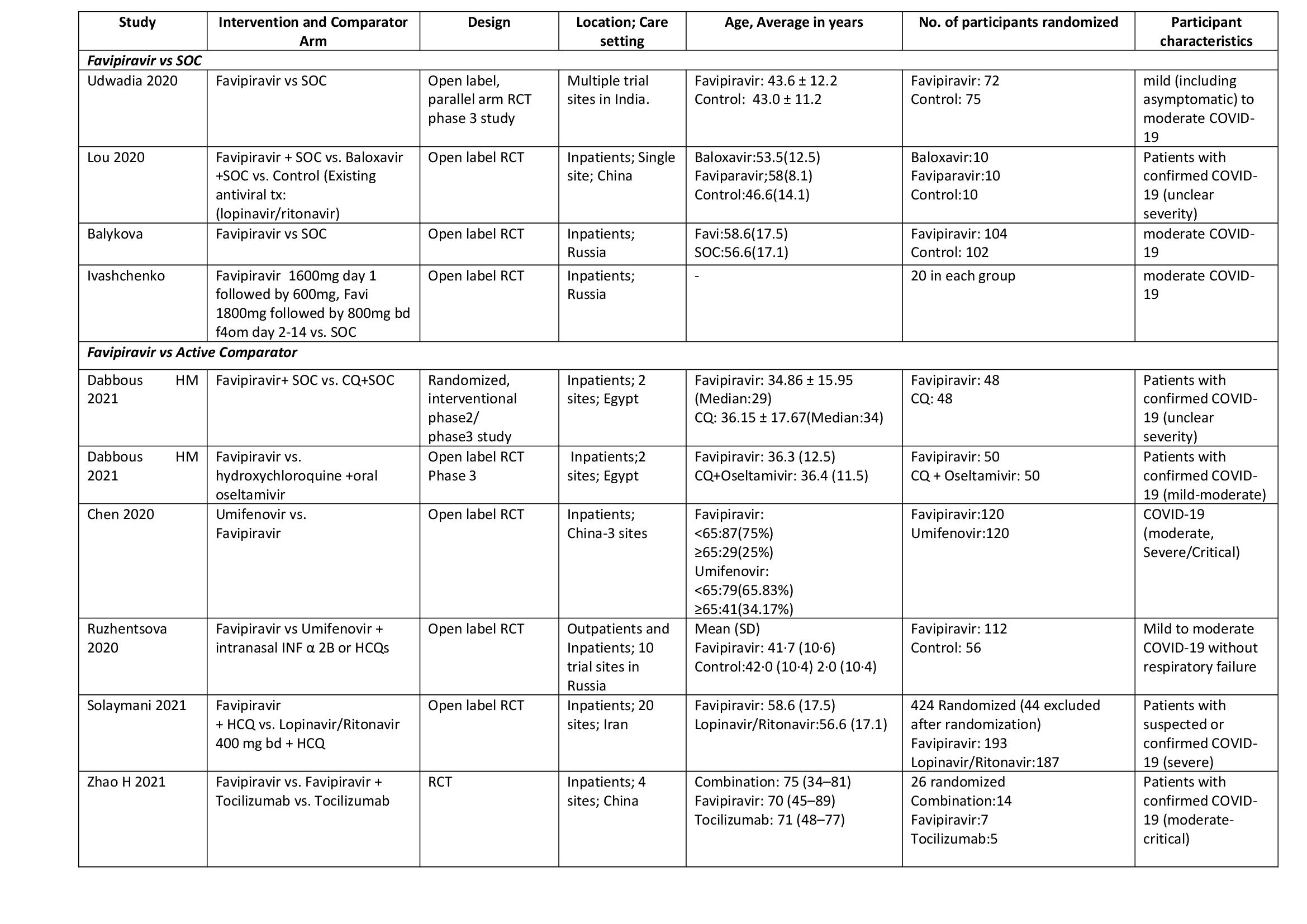
We found 10 RCTs that matched the PICO question as pre-defined by the expert working group. The trials included a total of 1494 participants, all of whom were adults. One trial reported from India(4), three each from China(5–7) and Russia(8–10), two from Egypt(11,12) and one from Iran(13) all of whom were of varying disease severity ranging from mild to critical. Also, the trials recruited participants to be enrolled to varying interventions. i.e. some studies compared Favipiravir with standard of care or placebo whereas others with active comparators. 8 trials (4–6, 8, 9, 11–13) were done purely on hospitalized patients where one study was done both inpatients and outpatients(10) and one study on outpatients alone(7).
Each trial and its results are described below; characteristics of the trials are summarised in the Summary of characteristics of included trials table. All the trials have varying risk of bias across all domains. Risk of bias for each domain per trial is displayed alongside the forest plots below.
The following comparisons were investigated in the trials (we compared outcomes for arms randomized to Favipiravir vs. standard of care or active comparators).
- Four trials(4,5,8,9) compared Favipiravir with standard of care(440 participants)
- Six trials(6,7,10–13) compared Favipiravir with an active comparator (e.g.: Chloroquine, Hydroxychloroqine+oseltamivir, Umifenovir, Umifenovir + Interferonα2B, Lopinavir/Ritonavir, Tocilizumab)
Since Favipiravir was compared with Standard of care, placebo and active comparators we did a sensitivity analysis to assess the robustness of the pooled estimate by stratifying the comparators separately from standard of care or placebo. We did this for critical outcomes of mortality, time to clinical improvement, time to negative PCR and duration of hospitalization. We concluded that there was no difference between the pooled estimate and the analysis done separately for Favipiravir vs SOC/placebo and Favipiravir vs Active comparator and hence decided to use the pooled estimate for representing the meta-analysis and summary of findings tables.
Our expert working group classified progression to oxygen, non-invasive ventilation (NIV) and invasive mechanical ventilation (IMV) as critical outcomes and mortality, time to clinical improvement and thrombotic or secondary infections as important outcomes. However, as the situation in the country evolved, the guidelines group upgraded mortality, progression to respiratory failure, oxygen requirement and critical and intensive care admission as critical outcomes and others as important outcomes.
Critical (primary) Outcomes
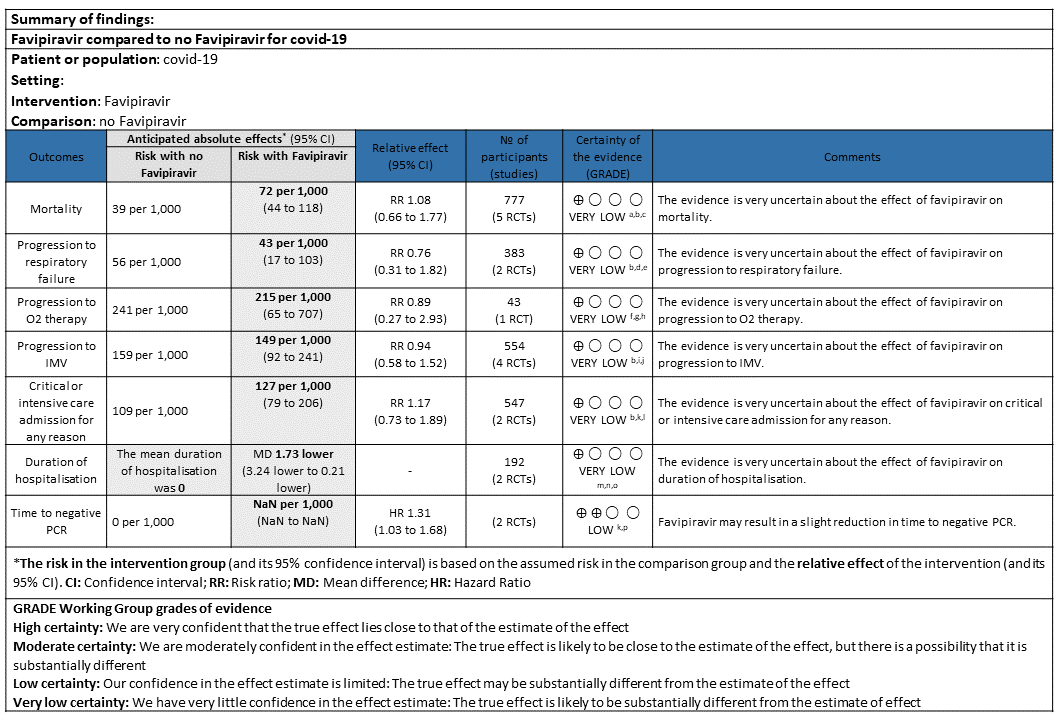
As presented in the ‘Summary of findings’ table, the evidence is of low or very low certainty about the effect of Favipiravir on mortality, progression to non-invasive ventilation or high flow oxygen, progression to invasive mechanical ventilation, time to clinical recovery (not requiring oxygen or hospitalised care) and adverse events-all and serious, those leading to drug discontinuation or hyperuricemia. Whereas the summary of findings table represented the outcome, Time to negative PCR to have moderate quality evidence.
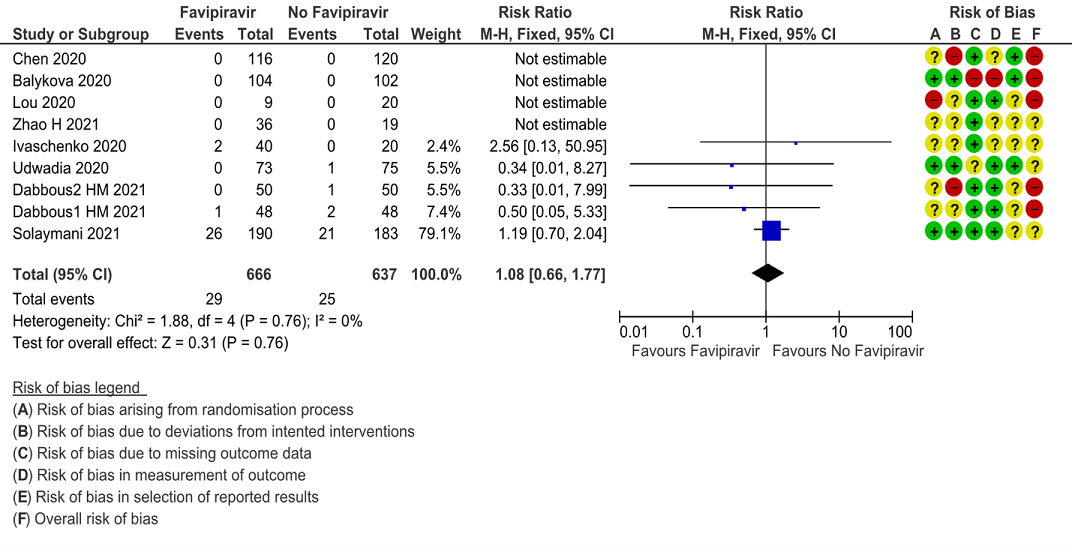
a. All-cause mortality: Very low certainty of evidence in 777 patients in five RCTs (4, 9, 11–13) found little or no difference between Favipiravir vs. standard of care or active comparator (RR 1.08; 95% CI 0.66 to 1.77). There were no significant differences observed even when trials were stratified by severity, risk of bias or comparators.
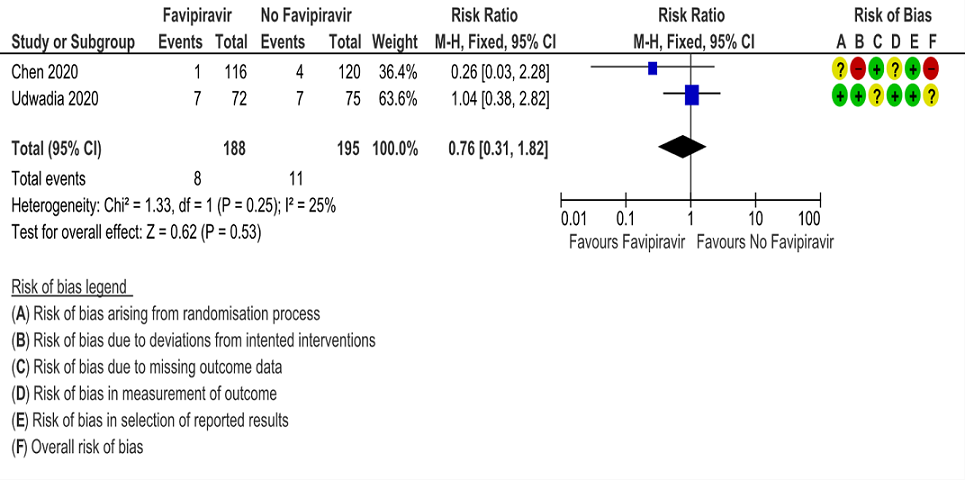
b. Progression to respiratory failure: Very Low certainty evidence in 383 patients from two RCT (4, 6), found that the evidence is uncertain about the effect of Favipiravir vs. standard of care or active comparator (RR 0.76; 95% CI 0.31 to 1.81).
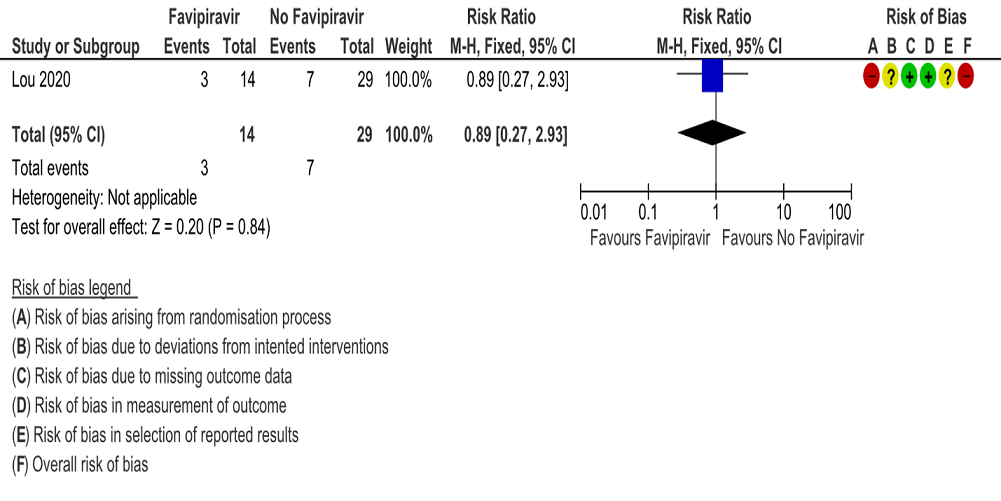
c. Progression to oxygen therapy: Very low certainty evidence in 43 patients from one RCT (5), found that Favipiravir make little or no difference in progression to oxygen therapy when compared to standard of care or active comparator (RR 0.89; 95% CI 0.27 to 2.93).
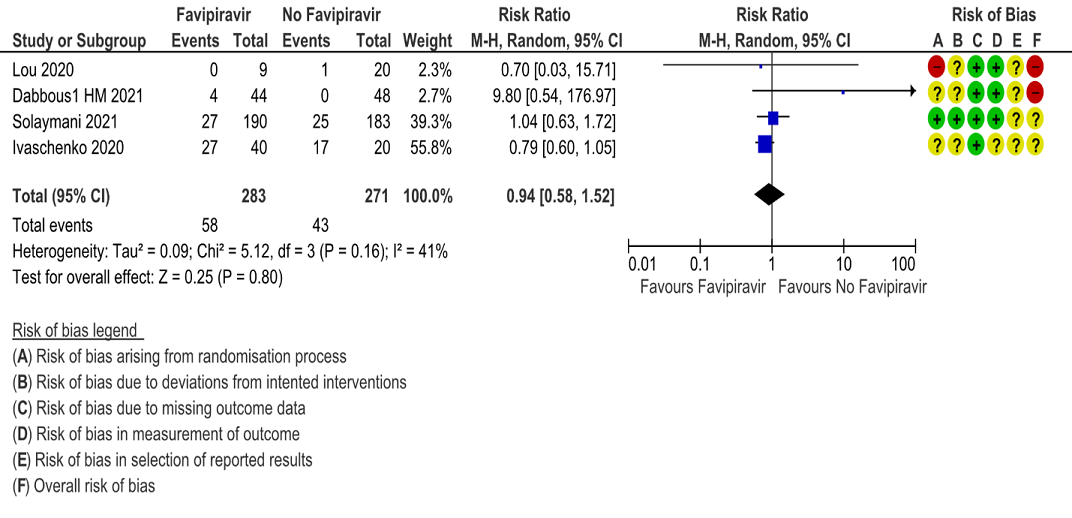
d. Progression to Invasive mechanical ventilation: Very low certainty evidence in 554 patients from four RCTs (5, 9, 11, 13), found that Favipiravir makes little or no difference in progression to invasive mechanical ventilation when compared to standard of care or active comparator (RR 0.94; 95% CI 0.58 to 1.52).
e. Progression to critical or intensive care for any reason: Low certainty evidence in 547 patients from two RCTs (10, 13), found that Favipiravir makes little or no difference in progression to invasive mechanical ventilation when compared to standard of care or active comparator (RR 1.17; 95% CI 0.73 to 1.89).
f. Duration of hospitalisation: Very low certainty of evidence in 192 patients from two RCTs (11, 12) that reported the mean duration of hospitalisation was 1.73 days lower in patients receiving Favipiravir when compared to standard of care or active comparator. One study reported a median interquartile range of 7(4-9) in Favipiravir vs. 6 (4–10) in the non Favipiravir group (13) which was not pooled with the other studies into the meta-analysis.
g. Time to negative PCR: Low certainty of evidence from 2 RCTs (4,10) found that Favipiravir may result in slight reduction in time to negative PCR when compared to standard of care or active comparator (HR 1.31; 95% CI 1.03 to 1.68). Two other RCTs (8,9) expressed the proportion of individuals becoming negative by PCR by day 5 and day 7 as 24/40(60%) for Favipiravir vs 6/20(30%) control and 98/100(98%) for Favipiravir and 79/100(79%) for control respectively.
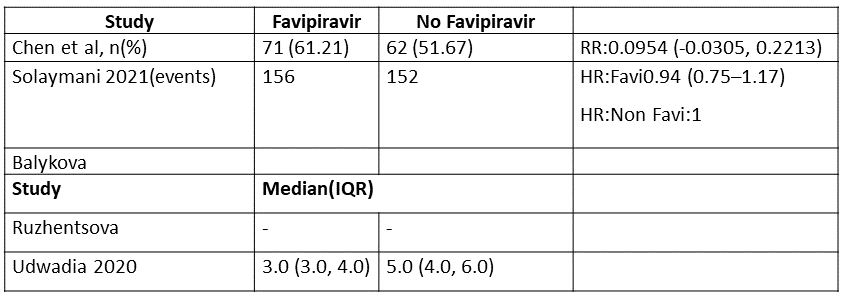
h. Time to clinical recovery (time to achieve WHO score 1,2,3 or not requiring hospitalization for oxygen or medical care): This was variably expressed in 3 trials and hence data could not be pooled. Proportion of people who recovered clinically in 1 trial (6,13) was greater in Favipiravir group as compared to the no Favipiravir group. In another study (4) this was expressed as median (IQR)and was found to be a median of 3.0(3.0-4.0) days in the Favipiravir group vs 5.0(4.0,6.0) in the control group
i. Time to clinical improvement (>2 point reduction in the WHO ordinal score): This was evaluated in 2 studies (5,10) and the time to clinical improvement was 6-9.25 days for Favipiravir and 5-21 days for standard of care or placebo. However, this data could not pooled as studies reported this outcome as median (IQR) and Hazard ratios.
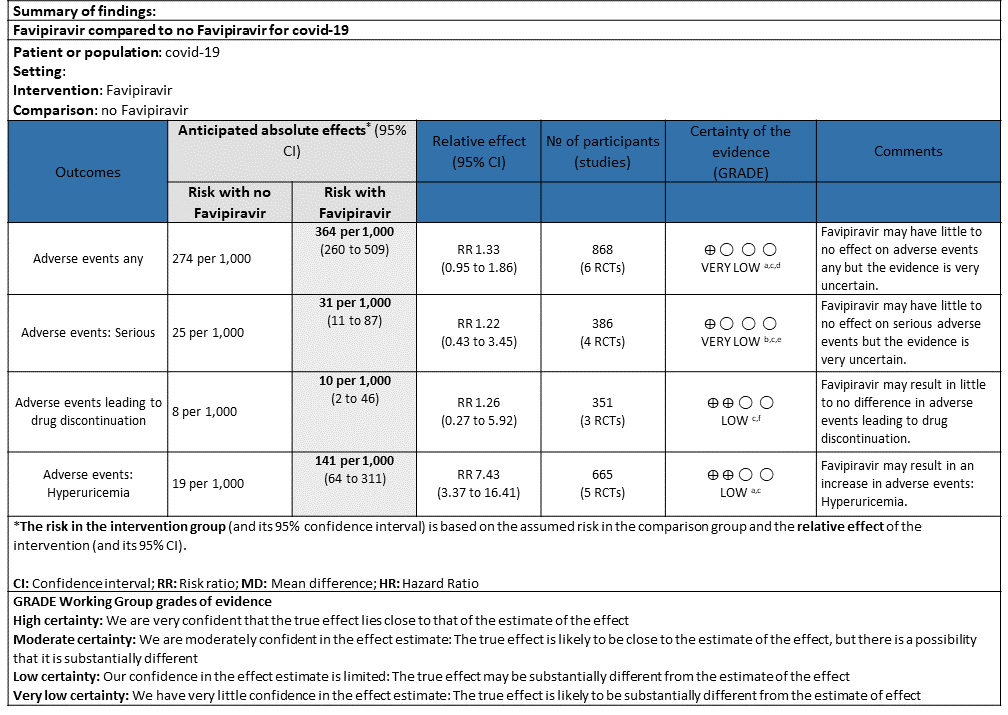
j. Adverse events any: Very low certainty of evidence in 888 patients from 6 RCTs(4,6,7,8,9,10) found that Favipiravir may have little to no effect on adverse events any but the evidence is very uncertain when compared to standard of care or active comparator (RR 1.33; 95%CI 0.95 to 1.86).
k. Adverse events Serious: Very low certainty of evidence in 386 patients from 4 RCTs(4,5,7,10) found that Favipiravir may have little to no effect on serious adverse events but the evidence is very uncertain when compared to standard of care or active comparator (RR 1.22; 95%CI 0.43 to 3.45).
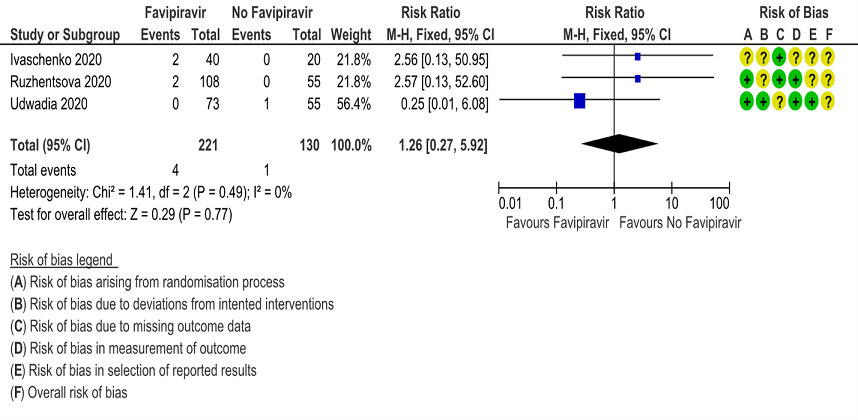
l. Adverse events leading to drug discontinuation: Low certainty of evidence in 351 patients from 3 RCTs(4,9,10) found that Favipiravir may have little to no effect on adverse events leading to drug discontinuation (RR 1.26; 95%CI 0.27 to 5.92).
m. Adverse Events – Hyperuricemia: Low certainty of evidence in 665 patients from 5 RCTs(4,6,7,10,11) found that Favipiravir may result in an increase in hyperuricemia (RR 7.43; 95%CI 3.37 to 16.41).
Subgroup analysis
Disaggregated data could not be obtained for co-morbidities, inflammatory markers or different age groups. Subgroups of pregnancy, lactation, renal failure, liver failure were excluded from most trials. No data were available separately for immunocompromised individuals in any of the trials.
We also evaluated as per category of severity i.e., mild to moderate, moderate, severe and critical.
Mild-Moderate: Mortality [RR= 0.34(95% CI 0.04-3.20)]; Progression to respiratory failure [RR=1.04(95% CI 0.38-2.82)]; Critical or intensive care admission [RR = 1.5 (95% CI 0.16-14.09)]; any adverse event [RR= 2.23 (95% CI 0.55-8.97)]; adverse event leading to discontinuation [RR=0.9(95% CI 0.14-5.840] were not found to favor Favipiravir. We found that in the mild to moderate category there was a reduction in the time to negative PCR [RR=1.31 (95% CI 1.03-1.68)] and duration of hospitalization by 1-3 days [Mean difference -0.9(95% CI -3.02-1.22)].
Moderate: Mortality [RR=2.56(95% CI 0.13-50.95)]; Progression to invasive mechanical ventilation [RR=0.79(95% CI 0.60-1.05)]; any adverse event [RR=1.01(95% CI 0.63-1.61)]; adverse event leading to drug discontinuation [RR = 2.56(95% CI 0.13-50.95)] were not found to favor Favipiravir or the comparison.
Severe: Data available only for Progression to invasive mechanical ventilation [RR=1.04 (95% CI 0.63-1.72)] or Critical or intensive care admission [RR=1.16 (95% CI 0.71-1.88)] were also not to favor either Favipiravir or the comparison.
In some of the studies the severity was unclear and some others it ranged from moderate to critical severity. The outcomes when analyzed in these categories of severity were not found to favor Favipiravir or the comparison.
1. All-cause Mortality:
2. Progression to Respiratory Failure
3. Progression to Oxygen Therapy
4. Progression to Invasive Mechanical Ventilation
5. Critical or Intensive Care admission for any reason
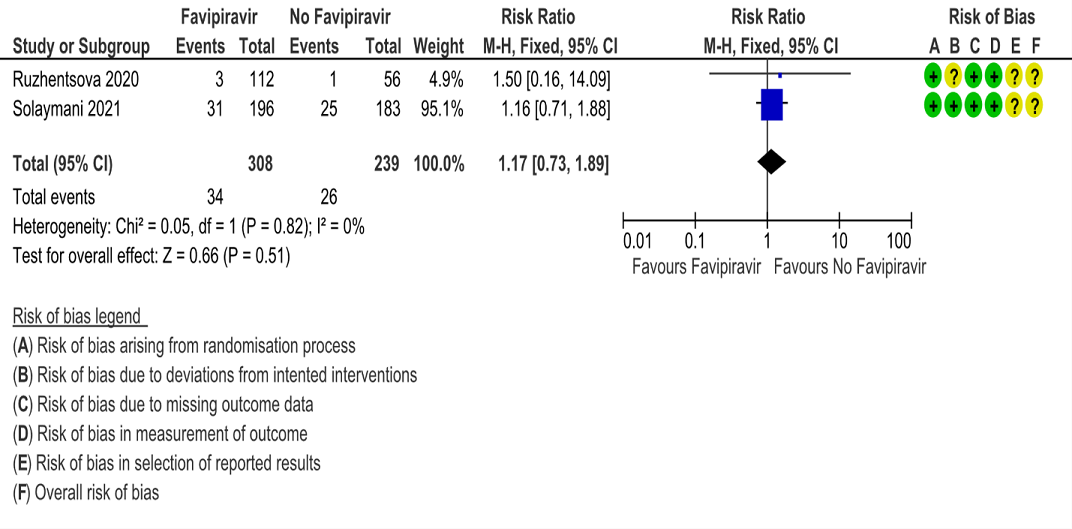
6. Duration of Hospitalization
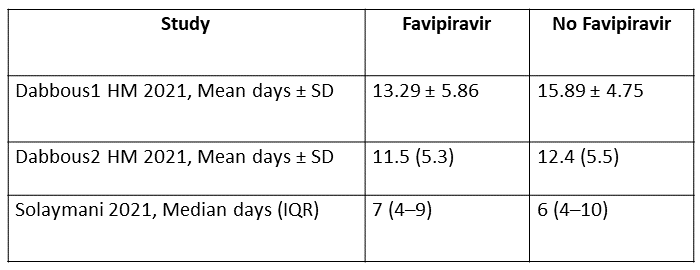
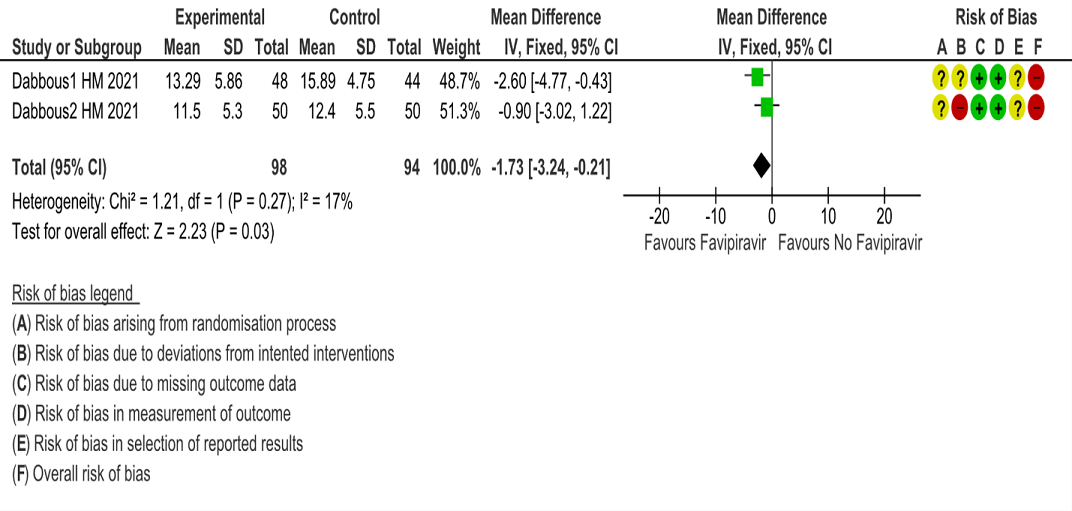
7. Time to negative PCR
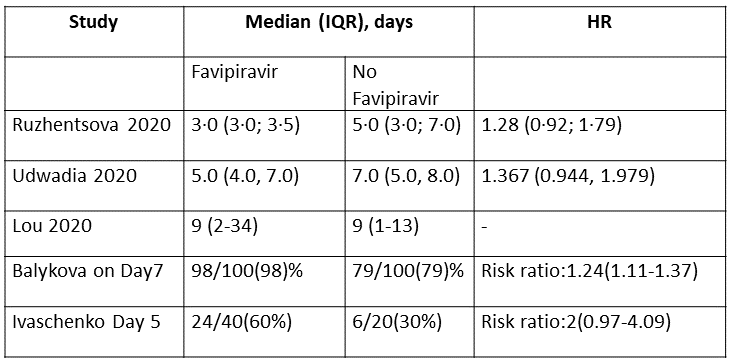
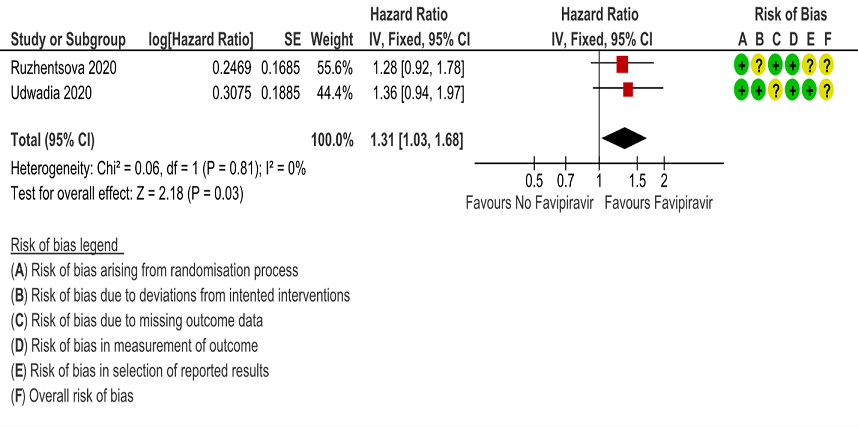
8. Time to Clinical Improvement
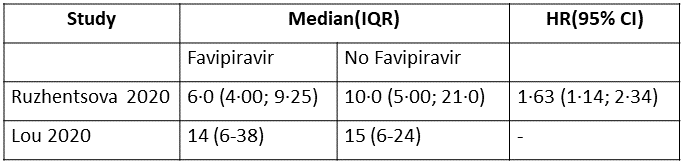
9. Time to Clinical Recovery
10. Adverse Events any
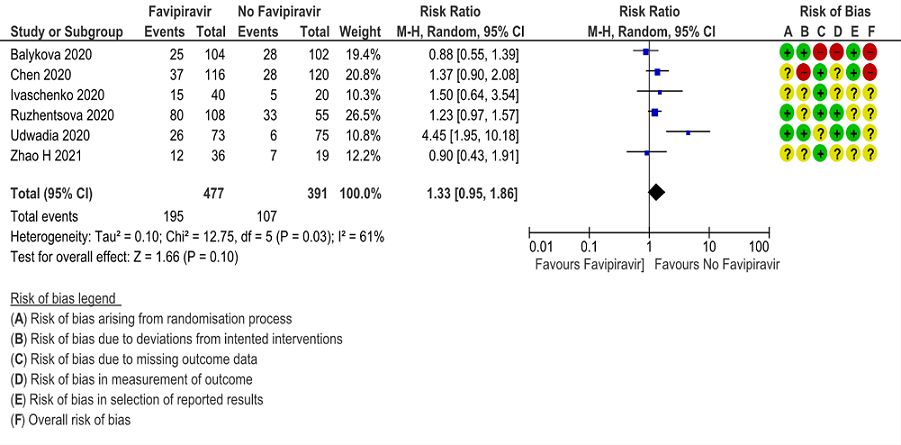
11. Adverse Events Serious
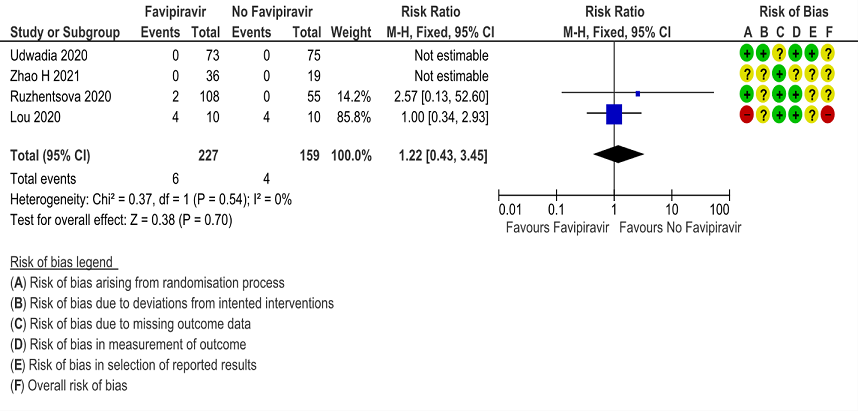
12. Adverse Events leading to drug discontinuation
13. Adverse events - Hyperuricemia