Explanations
a. Downgraded by 1 level for serious imprecision, due to wide 95% CI.
b. Downgraded by 1 level for serious ROB, due to some concern.
c. Downgraded by 1 level for serious inconsistency, the I2 is 87%.
Explanations
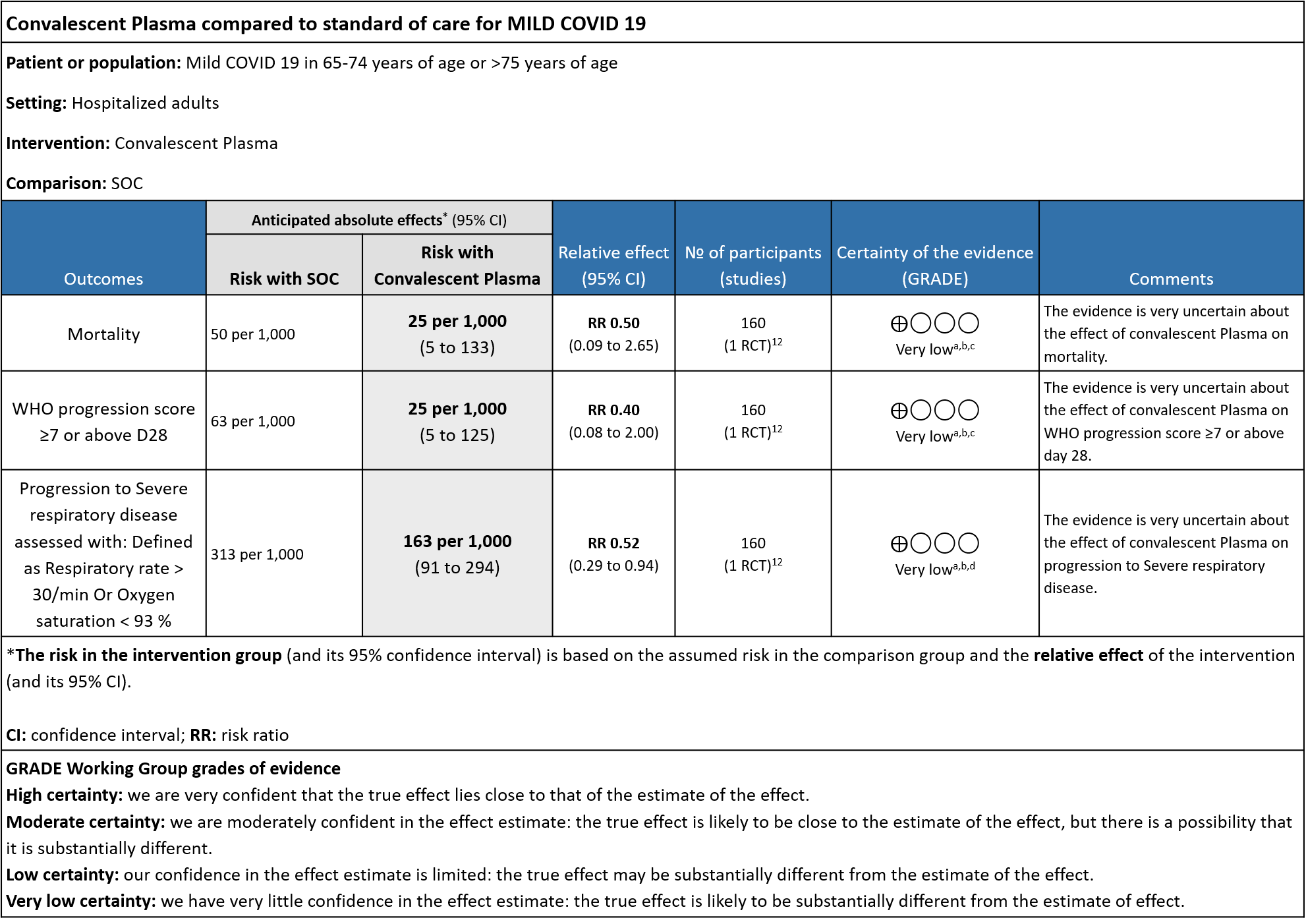
a. Downgrade by one level for risk of bias as the trial stopped early (only recruited 76% of its sample size.
b. Downgrade by on level for indirectness as the study included elderly >65 years of age with co-morbidities and those >75 years of age with or without co-morbidities.
c. Downgrade by 2 levels for very serious imprecision as the numbers were too small, OIS criteria were not met -single small trial. There were 2 deaths in the convalescent plasma group and 4 in the control group.
d. Downgraded by 1 level for serious imprecision as the 95% CI is wide (0.29 to 0.94)
Explanations
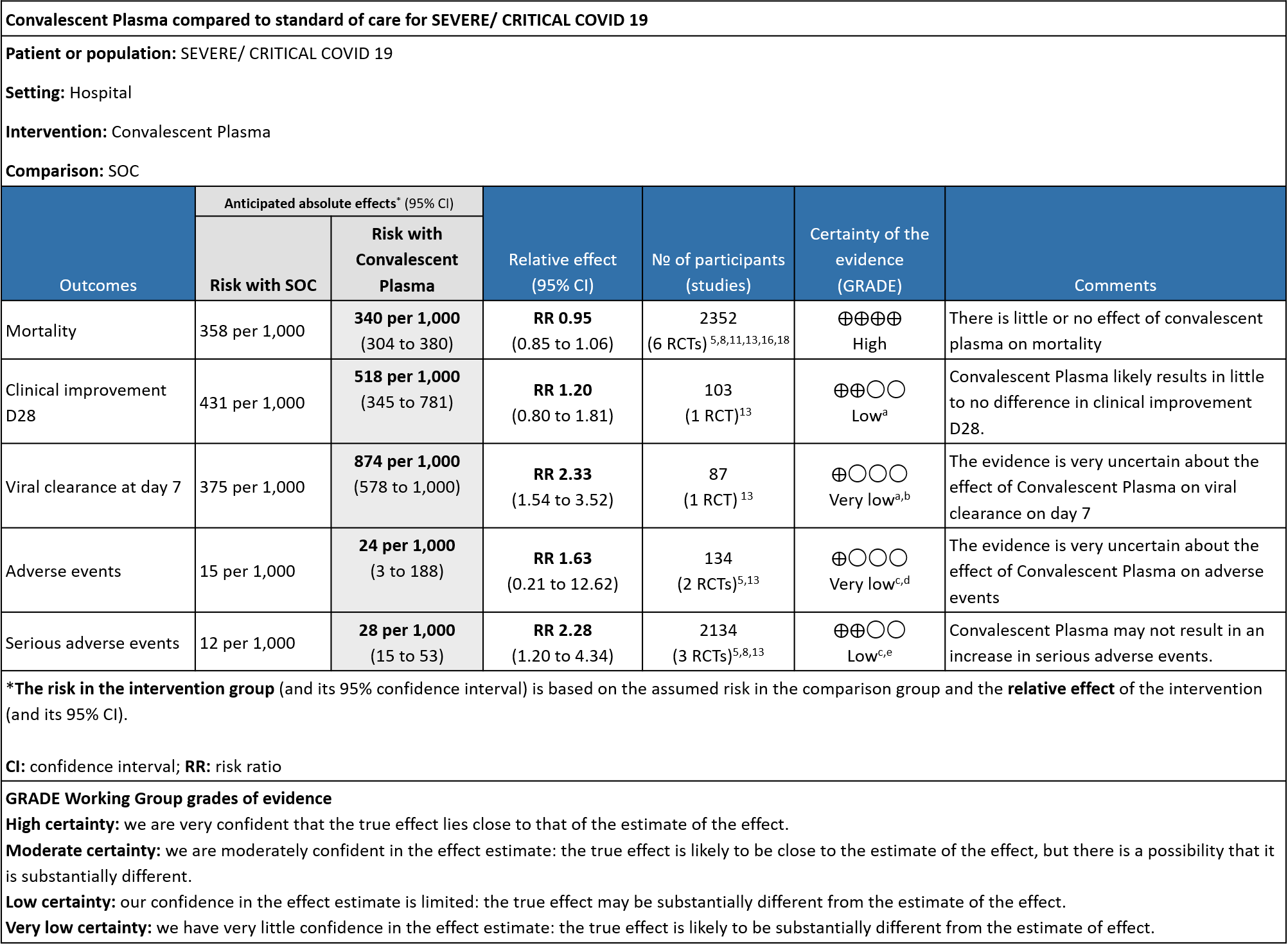
a. Downgrade by 2 levels for very serious imprecision as the numbers were too small, OIS criteria were not met -single small trial in 103 patients
b. Downgraded by 1 level for serious indirectness, due to early stopping at April 2020 and no usage of steroids.
c. Downgraded by 1 level for serious ROB, studies with some concerns.
d. Downgraded by 2 level for very serious imprecision as the 95% CI is wide (0.21 to 12.62)
e. Downgraded by 1 level for serious imprecision as the 95% CI is wide (1.20 to 4.34)
Convalescent plasma providing rapidly acting passive immunity as treatment of infections has been in use for more than a hundred years. Early studies in the COVID 19 pandemic provided weak data on clinical efficacy with limited side effects leading to an emergency use authorization by the FDA. Due to the lack of effective therapeutic options early in the pandemic for COVID 19, thousands of patients across the world received convalescent plasma therapy, many in an unregulated setting. Subsequent trials have shown less promising results.
To identify all available systematic reviews pertaining to our PICO question, a systematic search of PubMed, covid-nma.org, Cochrane and the Epistemonikos databases was conducted. The search strategy was designed and validated by the Group’s information specialist.
The most recent Cochrane review had not included several recent studies. All available RCTs therefore, were identified from the above databases and included in this meta-analysis.
For the risk of bias, Cochrane ROB2 tool was used and assessed by at least 2 reviewers with disagreements being settled by consensus.
Data was entered into Review manager version 5.4 for meta-analysis. The results were entered into Grade ProGDT (online), to create the summary of findings (GRADE) table. We used risk ratios (RR) for dichotomous outcomes with 95% confidence intervals (CIs).
Population – Patients in hospital with symptomatic COVID-19
Intervention – Convalescent plasma at any dose or duration
Control – Standard of care/ No convalescent plasma
Outcomes:
Primary:
- All-cause mortality
- Progression to ventilation (invasive or non-invasive) or organ support (dialysis, vasopressors/inotropes, ECMO)
Secondary:
- Time to clinical improvement (WHO ordinal scale or other definition)
- Duration of ventilatory support (invasive or non-invasive)
- Duration of vasopressor support
- Need for oxygen therapy
- Decrease in CRP at 7 days post-CP
- Decrease in IL-6 at 7 days post-CP
Adverse events:
- All
- Serious
- Transfusion-associated events:
- Fever
- Allergic reactions, especially severe
- TRALI
- Circulatory overload
The expert working group was also concerned regarding the impact of high/vs low titer antibody in the efficacy of convalescent plasma as a therapeutic option and also the category of severity that it was deployed in and hence requested a sub group analysis regarding the same. Subgroup analysis conducted were as follows
1. According to the category of severity: Mild. Severe/Critical COVID-19
2. According to high/low titer of antibody as per criteria defined per FDA and IDSA (http://www.idsociety.org)
OUTCOMES: CONVALESCENT PLASMA COMPARED TO STANDARD OF CARE IN COVID-19:
The 19 RCTs differed in the categories of severity in their analysis and we evaluated the following sub group
1. Convalescent plasma compared to standard of care in COVID-19 (across all severity categories)
2. Convalescent plasma compared to standard of care in mild COVID-19
3. Convalescent plasma compared to standard of care in severe - critical COVID-19
I. CONVALESCENT PLASMA COMPARED TO STANDARD OF CARE IN COVID-19(across all severity categories):
1. All-cause mortality at 28 days:
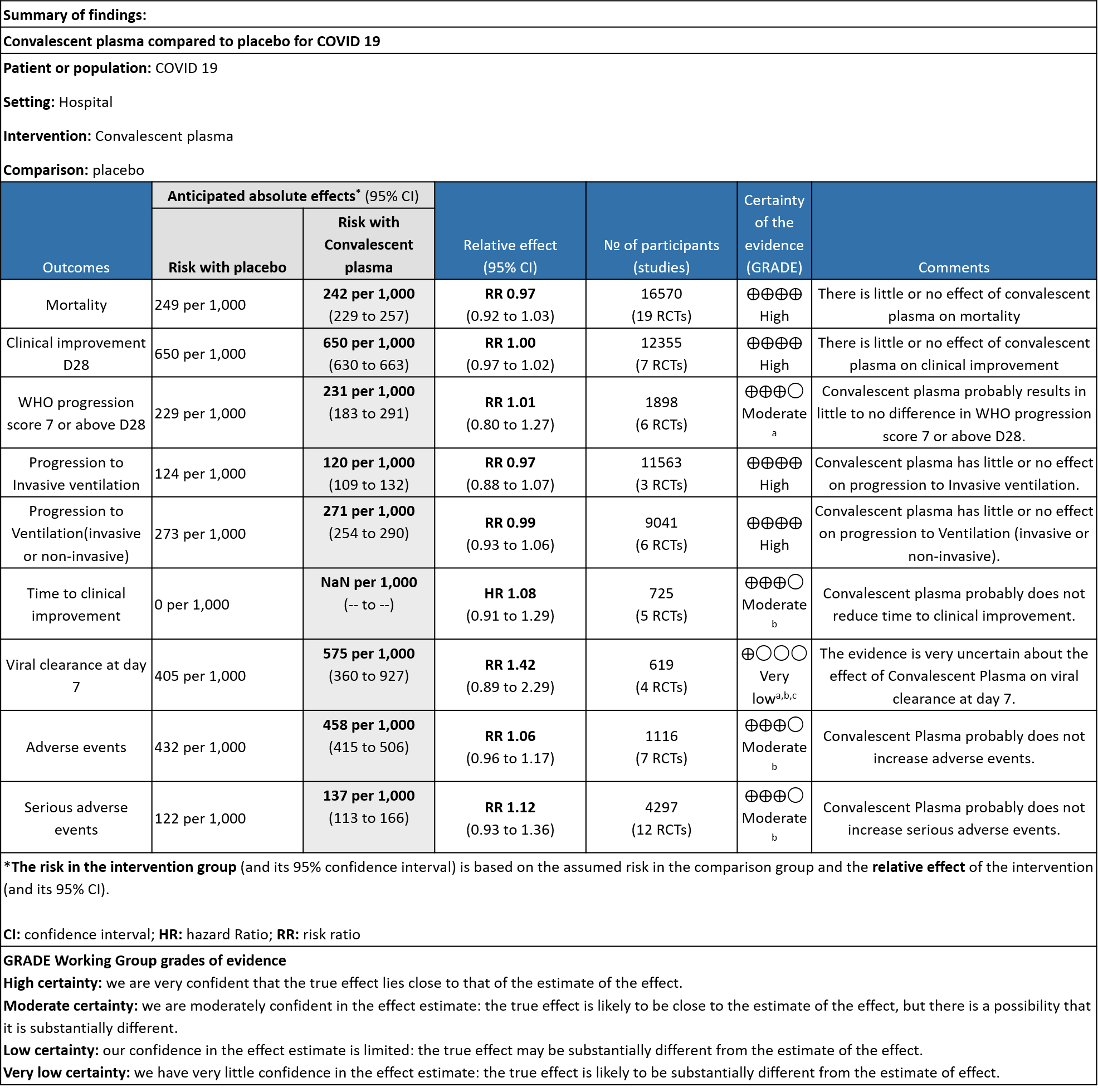
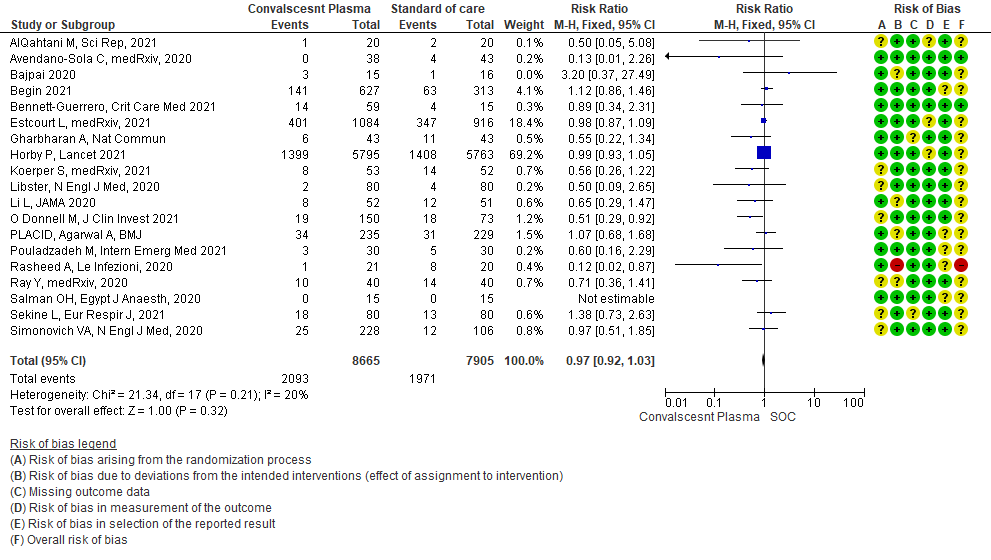
19 RCTs3-21 that included 16570 patients with COVID-19 disease indicated that there is high certainty evidence that Convalescent plasma does not reduce mortality at 28 days RR 0.97 (95% CI 0.92 to 1.03). Based on the data, we anticipate that for 1000 patients with COVID-19 disease treated with standard of care, 249 are likely to die vs 242 if Convalescent plasma is administered for COVID-19. The number needed to treat (NNT) to reduce an additional mortality is 143 patients with COVID-19.
2. Clinical improvement D28 (Clinical improvement was defined as patient discharge or a reduction of 2 points on a 6-point disease severity scale)
High certainty evidence from 7 RCTs3,7,9,10,13,20,21 that included 12355 patients with COVID-19 disease indicated that Convalescent plasma does not increase clinical improvement at day28, RR 1.00 (95% CI 0.97 to 1.02). Based on the data, we anticipate that for 1000 patients with COVID-19 disease treated with standard of care or convalescent plasma, 650 are likely to have clinical improvement by 28 days. The number needed to treat (NNT) to have an additional clinical improvement is 68 patients with COVID-19.
3. WHO progression score 7 or above D28:
Moderate certainty evidence from 6 RCTs4,6,12,14,20,21 that included 1898 patients with COVID-19 disease indicated that Convalescent plasma probably has no effect on WHO progression score 7 [mechanical ventilation +/- additional organ support (ECMO, HD or vasopressors) OR death] or discharge on ≥ 28 days RR 1.01 (95% CI 0.80 - 1.27) compared to standard of care. Based on the data, we anticipate that for 1000 patients with COVID-19 disease treated with standard of care 229 are likely to progress according to WHO progression score 7 or discharged on ≥ 28 days vs 231 administered convalescent plasma.
4. Progression to Invasive ventilation:
High certainty evidence from 3 RCTs10,12,15 that included 11563 patients with COVID-19 disease indicates that Convalescent Plasma results in little to no difference in progression to Invasive ventilation compared to standard of care RR 0.97 (95% CI 0.88 - 1.07). Based on the data, we anticipate that for 1000 patients with COVID-19 disease treated with standard of care 124 are likely to progress to invasive ventilation vs 120 per 1000 people if Convalescent plasma is administered for COVID-19.
5. Progression to Ventilation (invasive or non-invasive):
High certainty evidence from 6 RCTs3,4,8,10,12,15 that included 9041 patients with COVID-19 disease indicates that Convalescent Plasma results in little to no difference in progression to ventilation (invasive or non-invasive) compared to standard of care RR 0.99 (95% CI 0.93 - 1.06). Based on the data, we anticipate that for 1000 patients with COVID-19 disease treated with standard of care 273 are likely to progress to ventilation vs 271 i.e., 2 fewer (from 19 fewer to 17 more) per 1000 people if Convalescent plasma is administered for COVID-19.
6. Time to clinical improvement:
Moderate certainty evidence from 5 RCTs4,7,13,14,21 that included 815 patients with COVID-19 disease indicates that Convalescent Plasma probably results in little to no difference in time to clinical improvement, Hazard ratio 1.08 (95% CI 0.91 to 1.29).
7. Viral clearance at day 7
Very low certainty evidence from 4 RCTs13,15,19,20 that included 619 patients with COVID-19 illness indicated that Convalescent Plasma had a very uncertain effect on viral clearance at day 7, RR 1.42 (95% CI 0.79 to 2.29). Based on the data, we anticipate that for 1000 patients with COVID-19 disease treated with standard of care 405 are likely to have viral clearance at day 7 vs 575, if Convalescent plasma is administered for COVID-19. The number needed to treat (NNT) to have an additional patient attaining viral clearance at day 7 is 7.
8. All adverse events:
Moderate certainty evidence from 7 RCTs5, 11,14,20,21that included 1116 patients with COVID-19 illness indicated that Convalescent plasma probably results in little to no difference on all adverse events, RR 1.06 (95% CI 0.96 to 1.17). We anticipate that for 1000 patients with COVID-19 disease treated with standard of care 432 adverse events are likely vs 458 (from 17 fewer to 74 more) in those administered convalescent plasma for COVID-19.
9. Serious adverse events:
Moderate certainty evidence from 12 RCTs4-9, 11,14,20,21 that included 4297 patients with COVID-19 illness indicated that Convalescent Plasma probably results in little to no difference in serious adverse events RR 1.12 (0.93 to 1.36) compared to standard of care. Based on the data, we anticipate that for 1000 patients with COVID-19 treated with standard of care 122 serious adverse events are likely vs 107 (from 9 fewer to 44 more) in those given convalescent plasma.
II. CONVALESCENT PLASMA COMPARED TO STANDARD OF CARE IN MILD COVID-19:
The group noted that there were not too many trials measuring outcomes exclusively in early disease and mild category. Most studies seemed to include all categories of severity and we were unable to tease out disaggregated data for mild or severe infections alone. There was only 1 trial in this category but it included adults 65-74 years of age with comorbidities and those > 75 years of age irrespective of comorbidities. This trial was stopped early after only 76% of sample size was attained.
1. All-cause mortality at 28 days:
Very low certainty evidence from 1 RCT12 that included 160 patients with mild COVID-19 disease indicated that Convalescent Plasma may reduce mortality at 28 days RR 0.50 (95% CI 0.09 - 2.65). Based on the data, we anticipate that for 1000 patients with mild COVID-19 disease treated with standard of care, 50 are likely to die vs 25 (from 45 fewer to 83 more) if Convalescent plasma is administered for mild COVID-19. The number needed to treat (NNT) to reduce an additional mortality is 40 patients with mild COVID-19.
2. WHO progression score 7 or above D28:
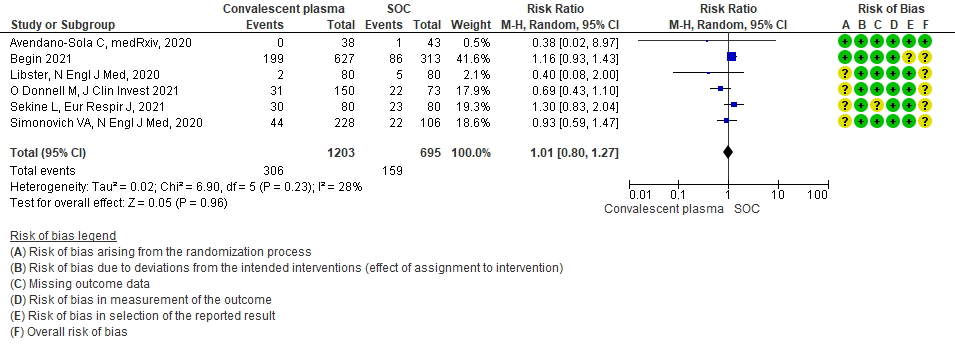
Very low certainty evidence from 1 RCT12 that included 160 patients with mild COVID-19 disease indicated that Convalescent Plasma may reduce progression to WHO progression score 7 and above [mechanical ventilation +/- additional organ support (ECMO, HD or vasopressors) OR death] at day 28, RR 0.40 (95% CI 0.08 to 2.00) compared to standard of care. Based on the data, we anticipate that for 1000 patients with mild COVID-19 disease treated with standard of care 63 are likely to progress beyond WHO progression score 7 or discharge ≥ 28 days vs 25 (from 58 fewer to 62 more) patients per 1000 people if Convalescent plasma is administered for mild COVID-19. The number needed to treat (NNT) to reduce an additional progression to WHO progression score 7 or discharge above day 28 is 26.
3. Progression to Severe respiratory disease (Defined as Respiratory rate > 30/min Or Oxygen Saturation < 93 %)
Very low certainty evidence from 1 RCT12 that included 160 patients with mild COVID-19 disease indicated that convalescent plasma may reduce progression to severe respiratory disease (defined as respiratory rate >30/min or oxygen saturation <93%), RR 0.52 (95% CI 0.29 to 0.94) compared to standard of care. Based on the data, we anticipated that for 1000 patients with mild COVID-19 disease treated with standard of care, 313 are likely to progress to severe respiratory disease vs 163 patients per 100 patients if convalescent plasma is administered for mild COVID 19.
III. CONVALESCENT PLASMA COMPARED TO STANDARD OF CARE IN SEVERE-CRITICAL COVID-19:
The group noted that there were not too many trials measuring outcomes exclusively in severe/critical category. Most studies seemed to include all categories of severity and we were unable to tease out disaggregated data for mild or severe infections alone.
1. All-cause mortality at 28 days:
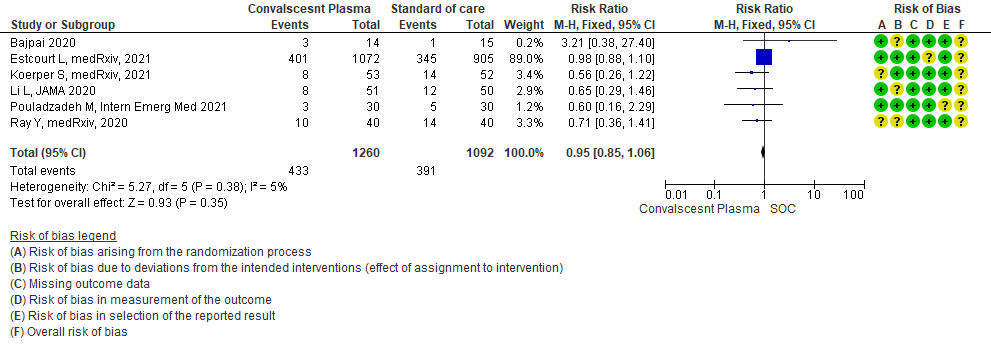
High certainty evidence from 6 RCTs5,8,11,13,16,18 that included 2352 patients with severe-critical COVID-19 disease indicated that Convalescent Plasma results in little to no difference in mortality at 28 days, RR 0.95 (95% CI 0.85 to 1.06). We anticipate that for 1000 patients with severe-critical COVID-19 disease treated with standard of care, 358 are likely to die vs 340 people if Convalescent plasma is administered for severe-critical COVID-19. The number needed to treat (NNT) to reduce an additional mortality is 71 patients with severe-critical COVID-19.
2. Clinical improvement D28 (Clinical improvement was defined as patient discharge or a reduction of 2 points on a 6-point disease severity scale):
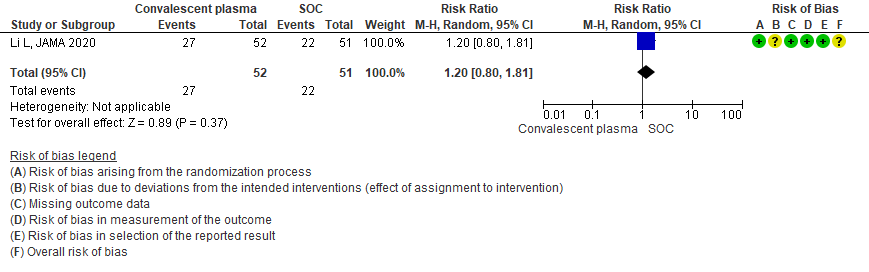
Low certainty evidence from 1 RCT13 that included 103 patients with severe-critical COVID-19 disease indicated that Convalescent Plasma may result in a slight increase in clinical improvement by 28 days RR 1.20 (95% CI 0.80 to 1.81). We anticipate that for 1000 patients with severe-critical COVID-19 disease treated with standard of care, 431 are likely to have clinical improvement by 28 days vs 518 (from 86 fewer to 350 more) if Convalescent plasma is administered.
3. Time to clinical improvement:
Very low certainty evidence from 1 RCT13 that included patients with severe-critical COVID-19 disease revealed a very uncertain effect of Convalescent Plasma on time to clinical improvement, HR 2.56 (95% CI 1.80 to 3.63)
4. Viral clearance at day 7:
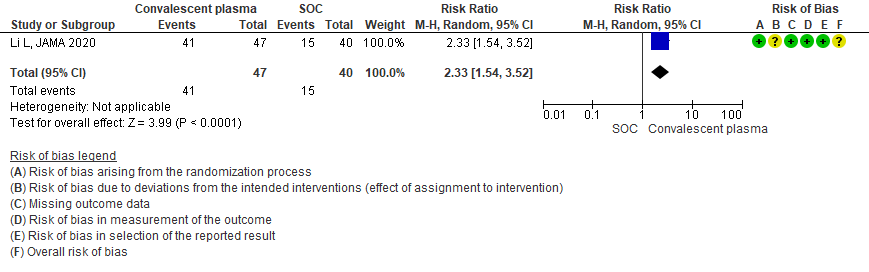
Very low certainty evidence from 1 RCT13 that included 87 patients with severe-critical COVID-19 disease revealed that the effect of Convalescent Plasma on viral clearance at day 7 is uncertain RR 2.33 (95% CI 1.54 to 3.52).
5. All adverse events:
Very low certainty evidence from 2 RCTs5,13 that included 134 patients with severe-critical COVID-19 disease revealed an uncertain effect of Convalescent plasma on all adverse events RR 1.63 (95% CI 0.21 to 12.62).
6. Serious adverse events:
Low certainty evidence from 3 RCTs5,8,13 that included 2134 patients with COVID-19 disease revealed that Convalescent plasma may increase serious adverse events RR 2.28 (95%CI 1.20 to 4.34) compared to standard of care. Based on the data, we anticipate that for 1000 patients with severe-critical COVID-19 treated with standard of care 12 serious adverse events are likely vs 28 if convalescent plasma is administered for patients with severe-critical COVID-19. The number needed to harm (NNH) to result in one additional serious adverse event is 53 patients with severe-critical COVID-19.
Subgroup analysis
1. High vs low titer antibody levels
It has been postulated that convalescent plasma with high antibody titer levels is likely to be more effective as a therapeutic option vs low antibody titer. Hence a subgroup analysis was done. The cut off was based on a cut-off of a high neutralizing antibody titer of >1:250 as per Federal Drug Administration (FDA) or Infectious Diseases Society of America (IDSA) 24, 25
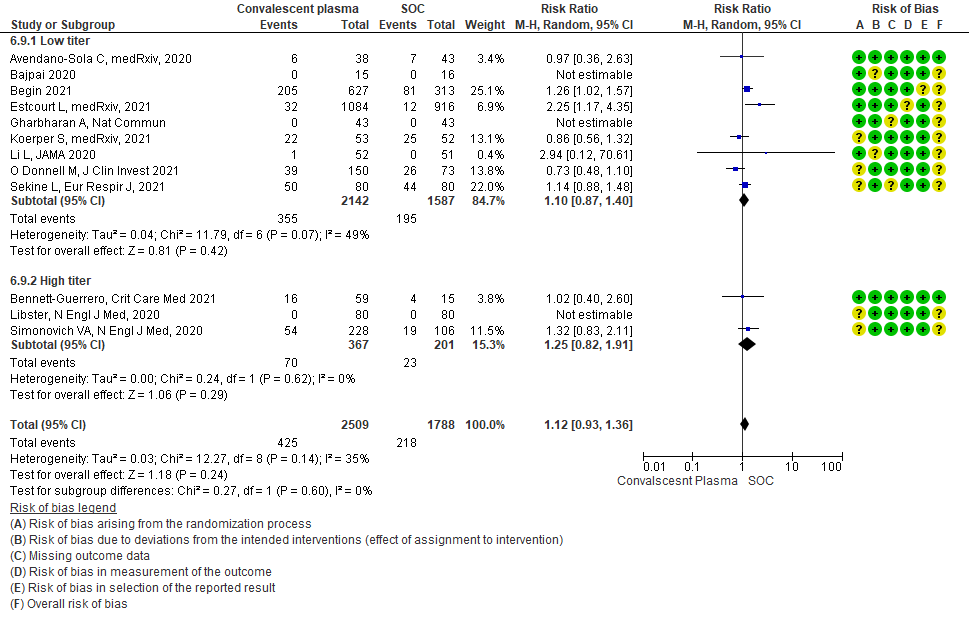
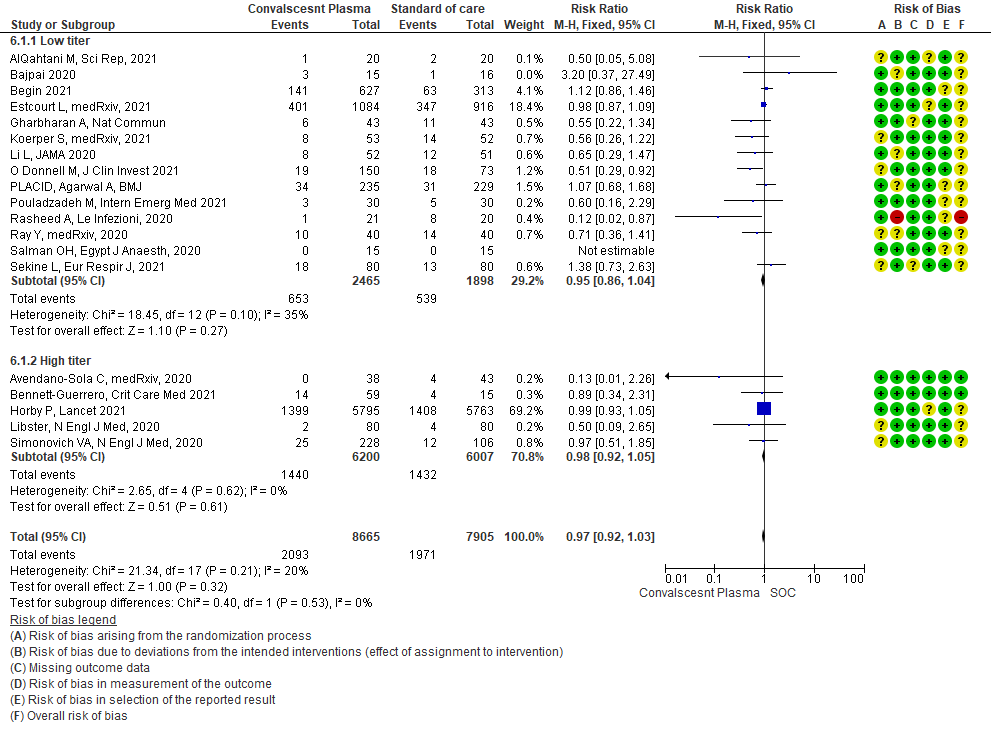
• Mortality in high vs low titer antibody group: 14 studies3,5,6,8,9,11,13-20 where low titer antibody donor convalescent plasma was administered, did not show any impact on mortality RR 0.95 (95% CI 0.86-1.04). 5 studies4, 7,10,12,21 with high titer antibody suggested similarly that convalescent plasma does not impact mortality RR 0.98 (95% CI 0.92-1.05). A test of subgroup differences revealed an I2 =0% suggesting that there is no difference between the subgroups.
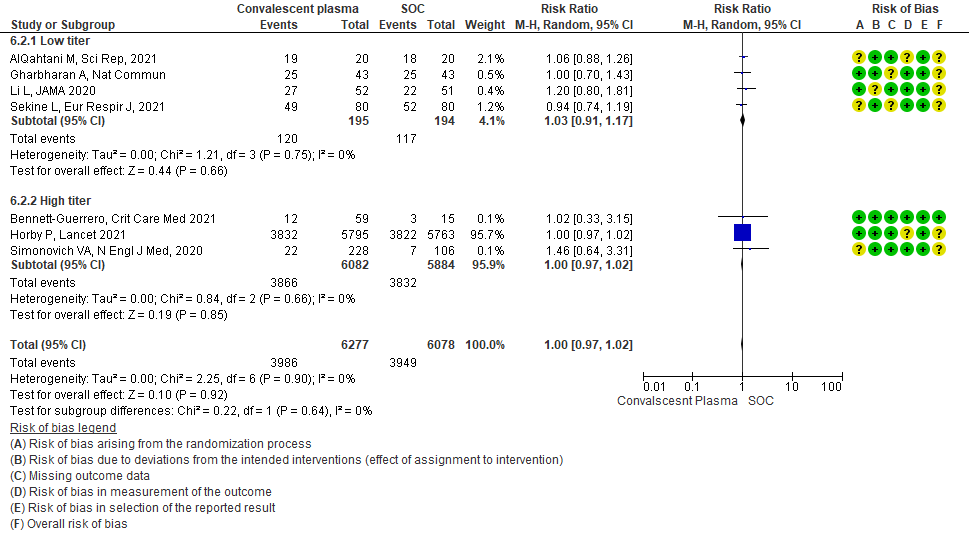
• Clinical improvement: 4 studies3, 9, 13, 20 where a low titer antibody donor convalescent plasma was administered did not impact clinical improvement RR 1.03 (95% CI 0.91-1.17). 3 studies7,10,21 with high titer donor convalescent plasma also showed similar results RR 1.00 (95% CI 0.97-1.02). Test for subgroup differences revealed an I2 = 0% suggesting there was no difference between the two groups.
• Progression to WHO progression score ≥ 7 or discharge at day 28 or later: 3 studies6,14,20 where a low titer antibody donor convalescent plasma was administered did not reduce progression to WHO progression score >7 or lead to early discharge RR 1.04 (95% CI 0.75-1.44). 3 studies4,12,21 where a high titer donor convalescent plasma was administered showed similar results RR 0.86 (95% CI 0.56-1.33). Test for subgroup differences revealed an I2 = 0% suggesting there was no difference between the two groups.
• Progression to invasive ventilation: 1 study15 where a low titer antibody donor convalescent plasma was administered did not reduce progression to invasive ventilation RR 1.03 (95% CI 0.55-1.91). 2 studies10,12 where a high titer donor convalescent plasma was administered showed similar results RR 0.97 (95% CI 0.88-1.07). Test for subgroup differences revealed an I2 = 0% suggesting there was no difference between the two groups.
• Progression to non-invasive (NIV) or invasive mechanical ventilation (IMV): 3 studies3,8,15 where a low titer antibody donor convalescent plasma was administered did not reduce progression to NIV or IMV RR 1.05 (95% CI 0.94-1.18). 3 studies4,10,12 where a high titer donor convalescent plasma was administered showed similar results RR 0.96 (95% CI 0.89-1.04). Test for subgroup differences revealed an I2 = 36.3 % suggesting there was no difference between the two groups.
• Time to clinical improvement: 2 studies13,14 where a low titer antibody donor convalescent plasma was administered did not reduce time to clinical improvement RR 1.25 (95% CI 0.95-1.64). 3 studies4,7,21 where a high titer antibody donor convalescent plasma was administered showed similar results RR 0.98 (95% CI 0.77-1.23). Test for subgroup differences revealed an I2 = 46.3%.
• Viral clearance at day 7: 4 studies12,15,19,20 where a low titer antibody donor convalescent plasma was administered did not reduce viral clearance at day 7; RR 1.42 (95% CI 0.89-2.29). There were no studies with high titer convalescent plasma which measured this outcome.
• Adverse events: 5 studies5,11,13,14,20 where a low titer antibody donor convalescent plasma was administered did not seem to cause an increase in adverse events RR 1.05 (95% CI 0.92-1.19). Similarly 2 studies12,21 with high titer antibody donor convalescent plasma also did not seem to cause an increase in adverse events RR 1.08 (95% CI 0.96-1.17). Test for subgroup differences revealed an I2 = 0% suggesting there was no difference between the two groups.
• Serious adverse events: 9 studies4-6,8,9,11,13,14,20 where a low titer antibody donor convalescent plasma was administered did not seem to cause a major increase in serious adverse events RR 1.10 (95% CI 0.87-1.40). Similarly in 3 studies7,12,21 with high titer antibody donor convalescent plasma there was no increase in serious adverse events RR 1.25 (95% CI 0.82-1.91). Test for subgroup differences revealed an I2 = 0% suggesting there was no difference between the two groups.
We included 19 RCTs4-21 from the COVID NMA data base in our analysis.
SOC=standard of care
1. CONVALESCENT PLASMA COMPARED TO PLACEBO IN PATIENTS WITH COVID 19
1.1 All-cause Mortality at 28 days in patients treated with Convalescent plasma as compared to standard of care in COVID 19 patients
1.2 Clinical improvement by day 28in patients treated with Convalescent plasma as compared to standard of care in COVID 19 patients
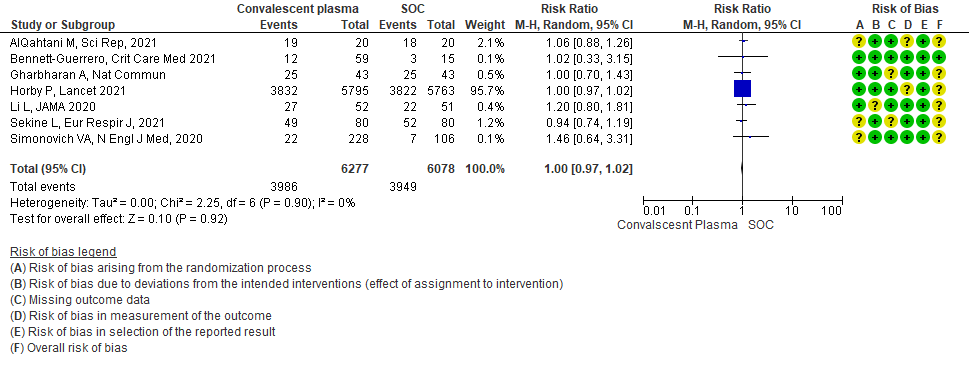
Clinical improvement was defined as patient discharge or a reduction of 2 points on a 6-point disease severity scale.
Scale was defined as follows: 6 points, death; 5 points, hospitalization plus ECMO or invasive mechanical ventilation;
4 points, hospitalization plus noninvasive ventilation or high-flow supplemental O2;
3 points, hospitalization plus supplemental O2 (not high-flow or noninvasive ventilation);
2 points, hospitalization with no supplemental O2;
1 point, hospital discharge
1.3 WHO progression score7 or above Day 28 [mechanical ventilation +/- additional organ support (ECMO, HD or vasopressors) OR death- or discharge beyond 28 days]28in patients treated with Convalescent plasma as compared to standard of care in COVID 19 patients
1.4 Progression to Invasive ventilation in patients treated with Convalescent plasma as compared to standard of care in COVID 19 patients
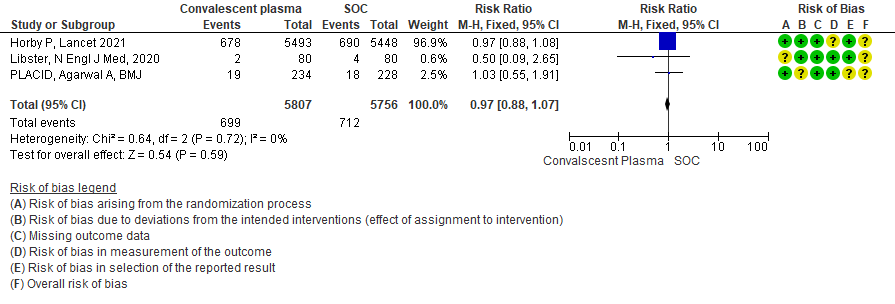
1.5 Progression to Ventilation (invasive or non-invasive) in patients treated with Convalescent plasma as compared to standard of care in COVID 19 patients
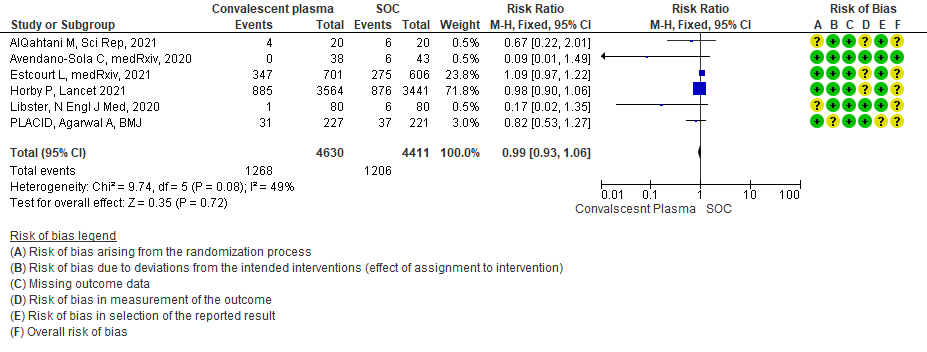
1.6 Time to clinical improvement in patients treated with Convalescent plasma as compared to standard of care in COVID 19 patients
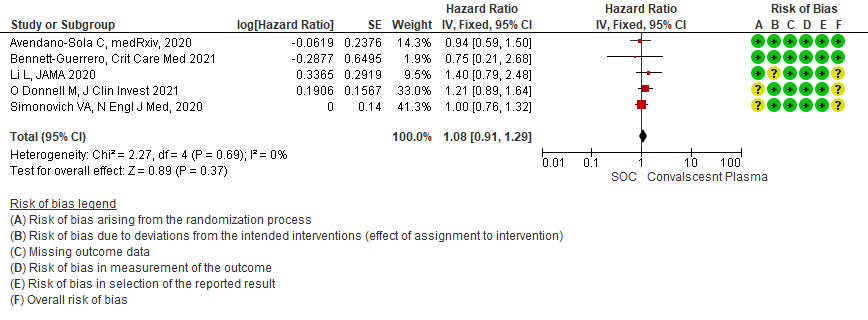
1.7 Viral clearance at day 7 in patients treated with Convalescent plasma as compared to standard of care in COVID 19 patients
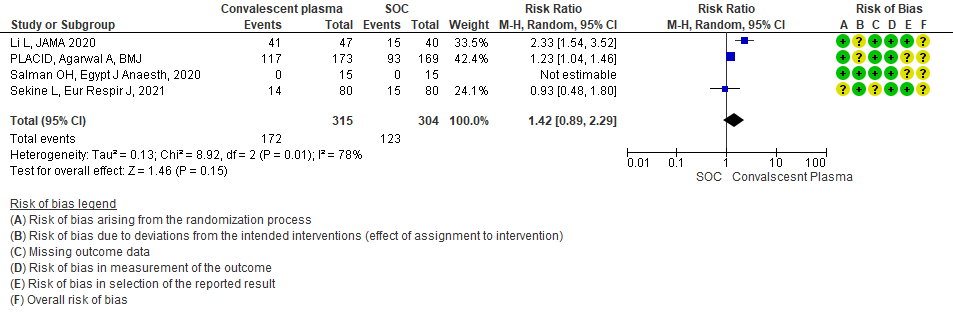
1.8 Adverse events in patients treated with Convalescent plasma as compared to standard of care in COVID 19 patients
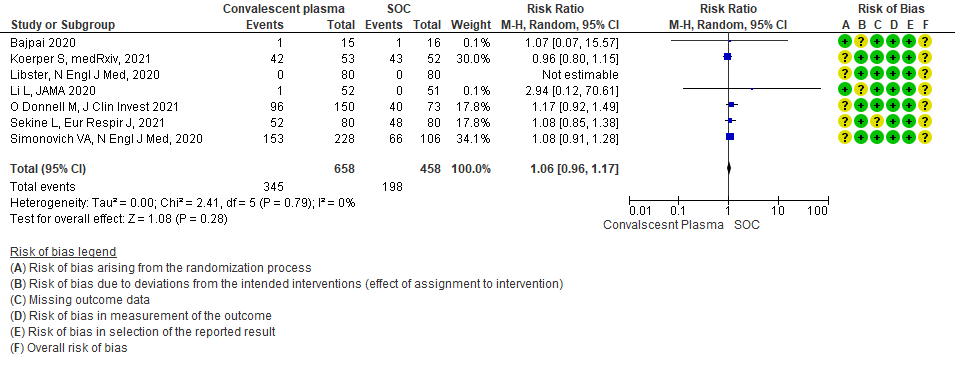
1.9 Serious adverse events in patients treated with Convalescent plasma as compared to standard of care in COVID 19 patients
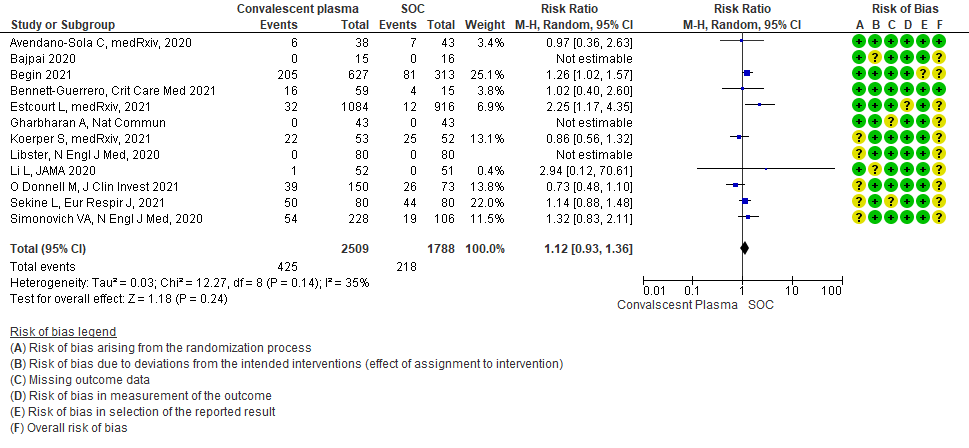
2. MILD CATEGORY – OUTCOMES
2.1 All-cause Mortality at 28 days in patients treated with Convalescent plasma as compared to standard of care in mild COVID 19 patients
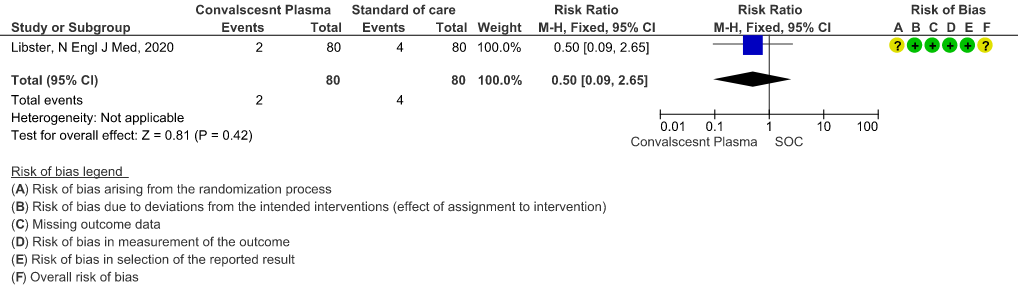
2.2 Mild - WHO progression score 7 or above D28 [mechanical ventilation +/- additional organ support (ECMO, HD or vasopressors) OR death- or discharge beyond 28 days]in patients treated with Convalescent plasma as compared to standard of care in mild COVID 19 patients
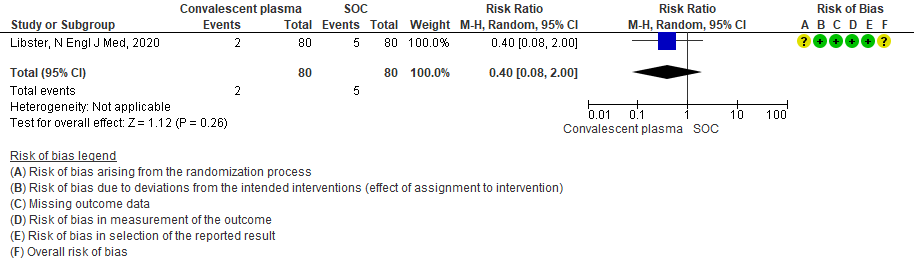
2.3 Progression to “Severe respiratory disease”in patients treated with Convalescent plasma as compared to standard of care in mild COVID 19 patients [Severe respiratory disease defined as Respiratory rate > 30/min Or Oxygen saturation < 93%]
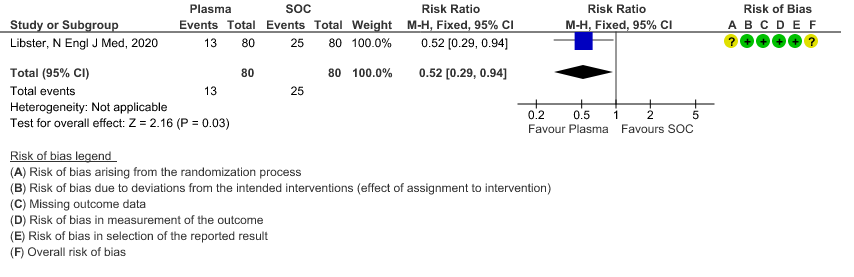
3. SEVERE/CRITICAL CATEGORY-OUTCOMES
3.1 All-cause Mortality at 28 days in patients treated with Convalescent plasma as compared to standard of care in severe-critical COVID 19 patients
3.2 Clinical improvement by 28 days in patients treated with Convalescent plasma as compared to standard of care in severe-critical COVID 19 patients
3.3 Viral clearance at day 7in patients treated with Convalescent plasma as compared to standard of care in severe-critical COVID 19 patients
3.4 Adverse events in patients treated with Convalescent plasma as compared to standard of care in severe-critical COVID 19 patients
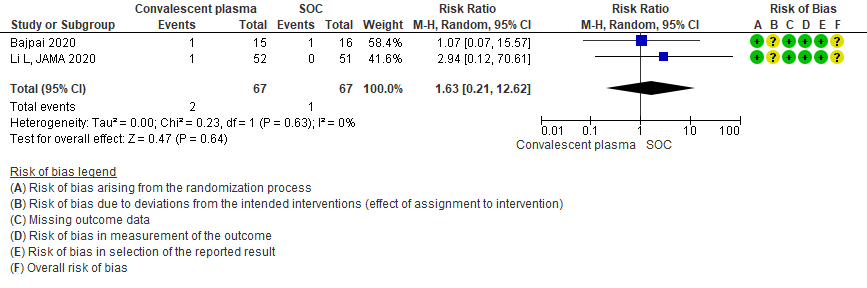
3.5 Serious adverse events in patients treated with Convalescent plasma as compared to standard of care in severe-critical COVID 19 patients
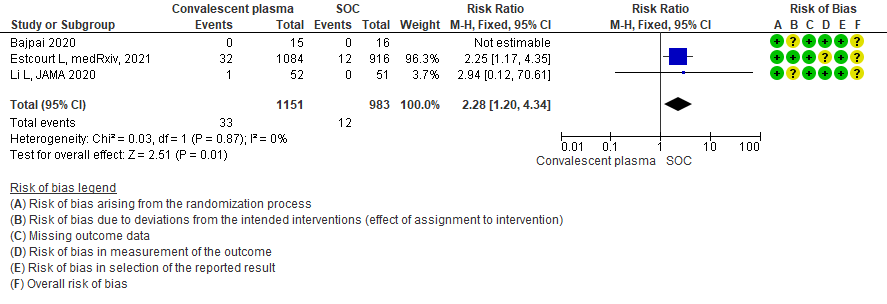
4. Subgroup-based on Antibody Titer of donor plasma
4.1 All-cause Mortality at 28 days in subgroup of patients treated with Convalescent plasma based on Antibody Titer of donor plasma
4.2 Clinical improvement at 28 days in subgroup of patients treated with Convalescent plasma based on Antibody Titer of donor plasma
4.3 WHO progression score 7 or above D28[mechanical ventilation +/- additional organ support (ECMO, HD or vasopressors) OR death- or discharge beyond 28 days]in subgroup of patients treated with Convalescent plasma based on Antibody Titer of donor plasma
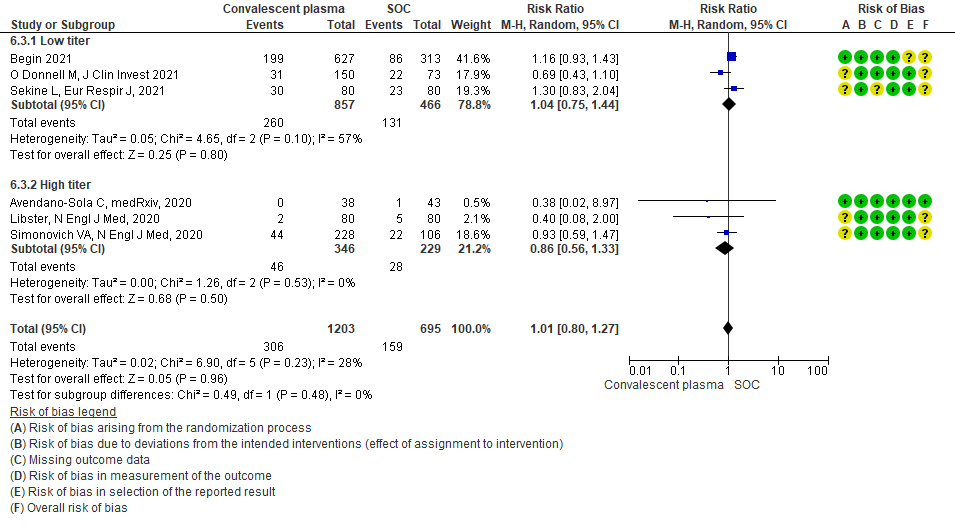
4.4 Progression to invasive ventilationin subgroup of patients treated with Convalescent plasma based on Antibody Titer of donor plasma
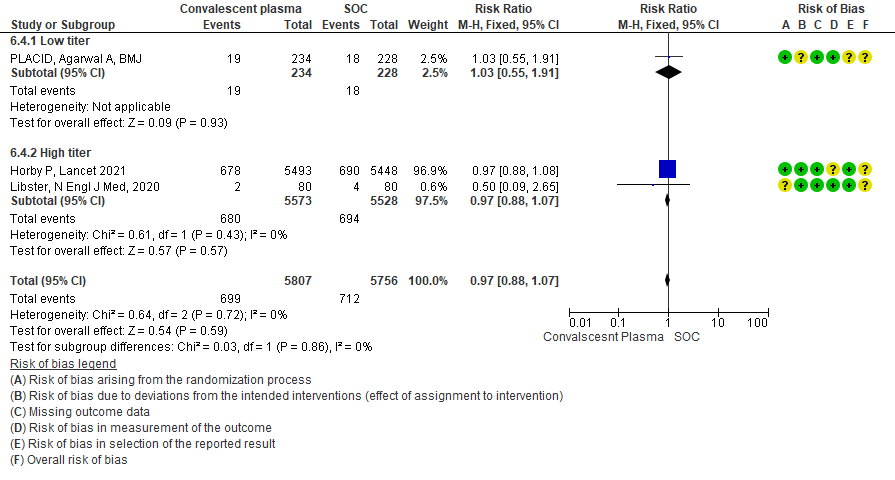
4.5 Progression to ventilation[invasive or non-invasive]in subgroup of patients treated with Convalescent plasma based on Antibody Titer of donor plasma
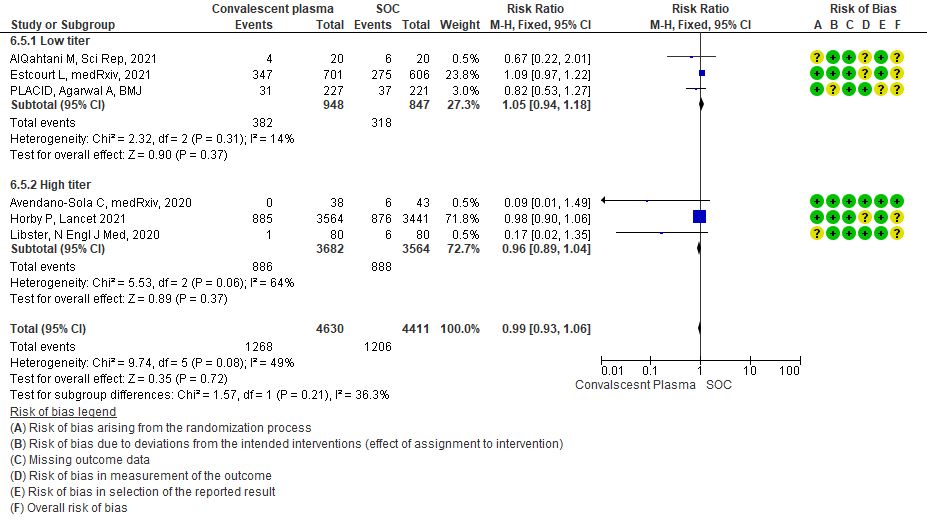
4.6 Time to clinical improvementin subgroup of patients treated with Convalescent plasma based on Antibody Titer of donor plasma
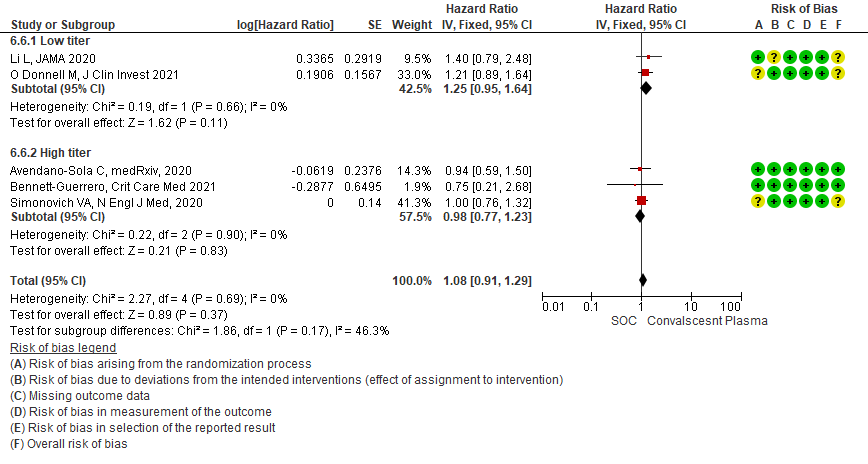
4.7 Viral clearance at day 7in subgroup of patients treated with Convalescent plasma based on Antibody Titer of donor plasma
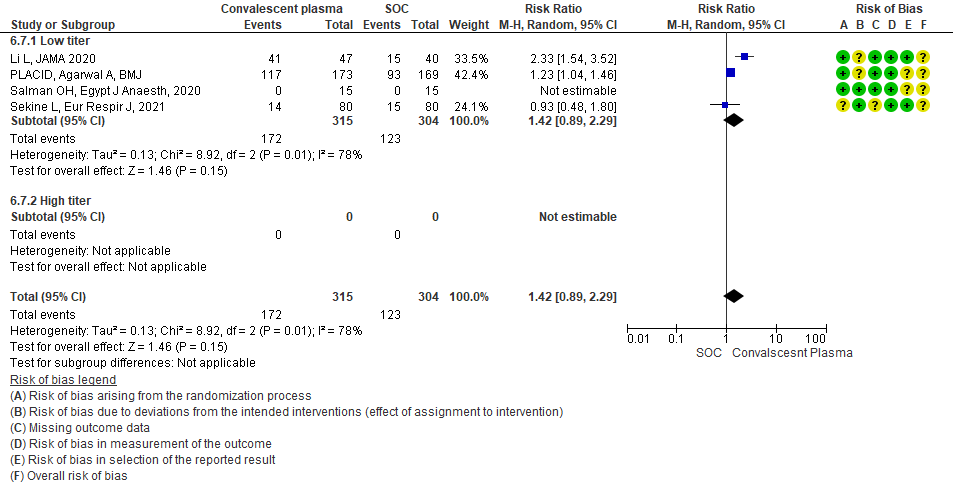
4.8 Adverse eventsin subgroup of patients treated with Convalescent plasma based on Antibody Titer of donor plasma
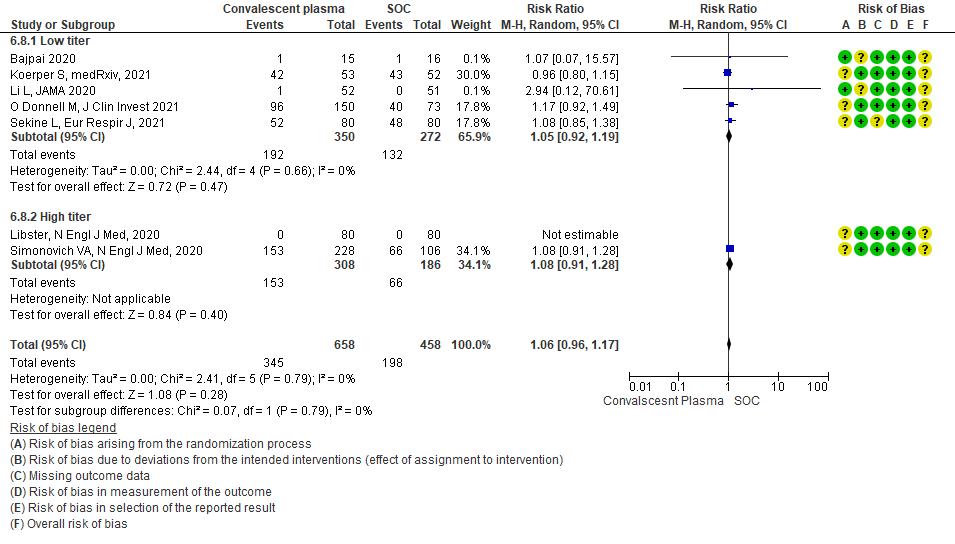
4.9 Serious adverse eventsin subgroup of patients treated with Convalescent plasma based on Antibody Titer of donor plasma