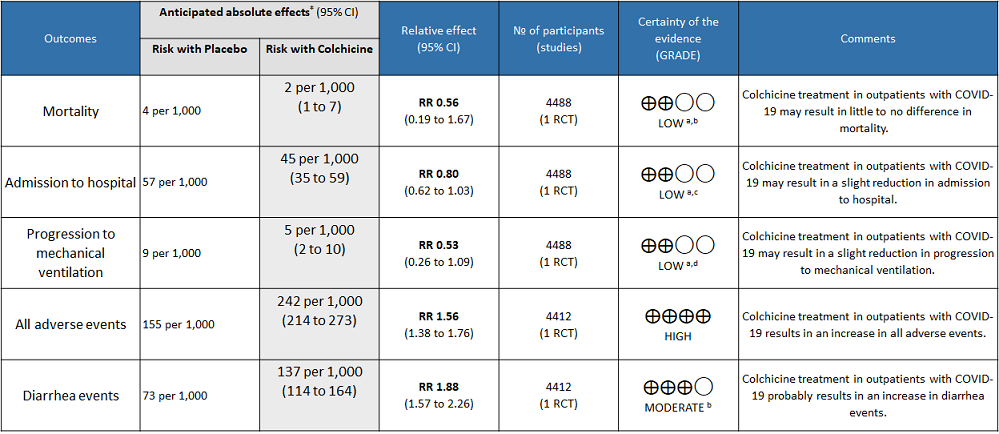
Colchicine compared to Placebo for outpatients with COVID-19
Explanations
a. Downgraded by one level for serious imprecision as the confidence interval crosses 1
b. Downgraded by one level for serious imprecision because of small number of events
c. Downgraded by one level for very small absolute difference(1.2% points) which doesn't cross the clinical decision making threshold
d. Downgraded by one level for serious imprecision because of small number of events
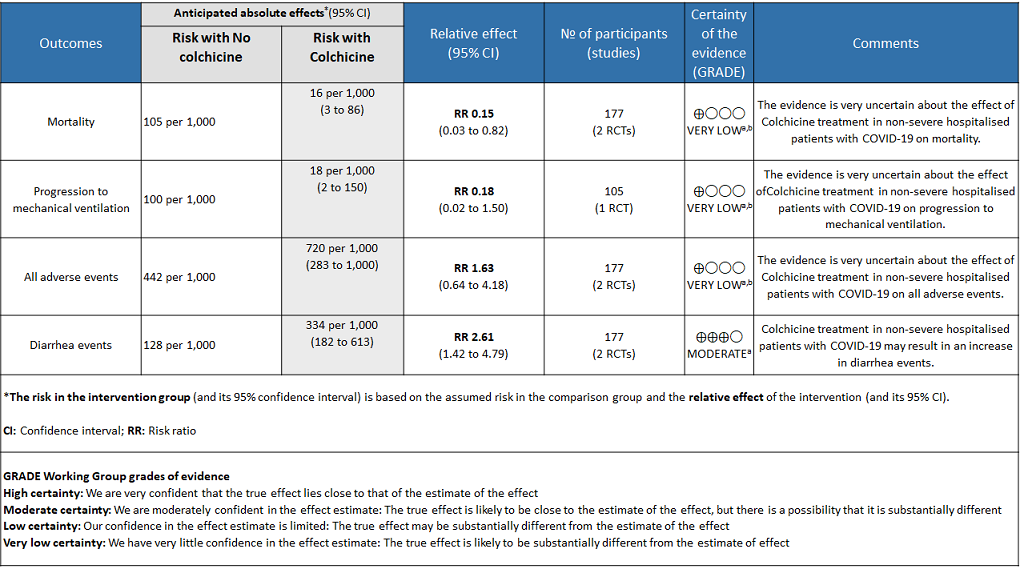
Colchicine compared to no Colchicine for hospitalised non-severe COVID-19 patients
Explanations
a. Downgraded by one level for risk of bias
b. Downgraded by two levels for very serious imprecision as the sample size is small and the confidence interval is large
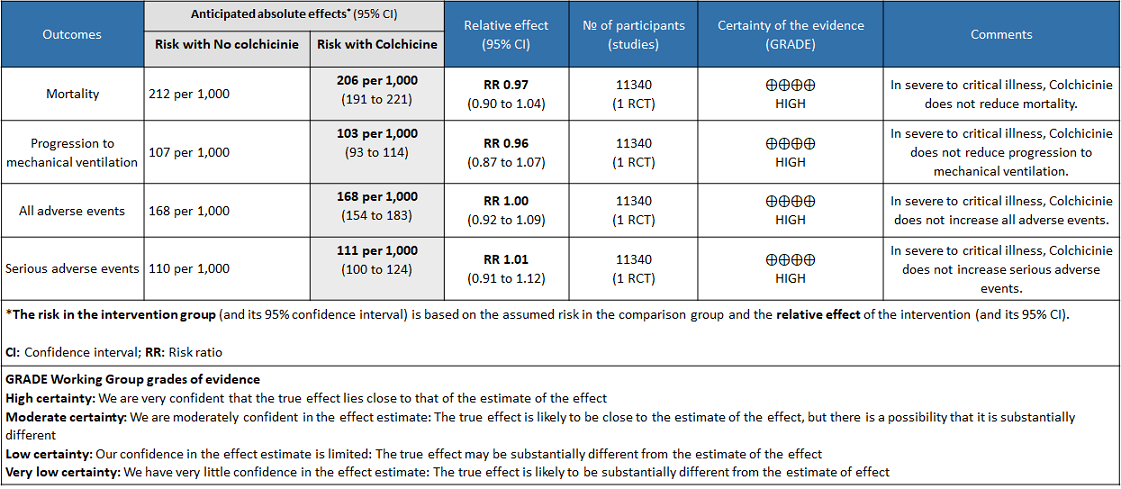
Colchicine compared to No colchicine for hospitalised patients with severe COVID-19
As of July 25, 2021, COVID-19 infection has affected more than 3 crore people and killed more than 4 lakh people in India as per official records1. As the health care systems in India continue to struggle, there is a dire need for cheaper and effective drugs to prevent hospitalization and reduce mortality due to COVID-19. Since inflammation is known to play a major role in pathogenesis of COVID-19, anti-inflammatory drugs are used to treat COVID-192,3. In particular, activation of the NLRP3 inflammasome of the innate immune system is found to increase the inflammatory cytokines IL-1β, IL-6 and TNF which cause tissue damage in COVID-194. It reduces inflammation by multiple mechanisms including inhibiting NLRP3 inflammasome and so was proposed as a potential drug to treat COVID-195. There is extensive experience with the use of Colchicine as an anti-inflammatory drug in Gout. However, adverse effects including multiple debilitating gastrointestinal side effects especially diarrhea have precluded the wide usage of this drug in gout and other rheumatic diseases.
A scoping search for use of Colchicine in COVID-19 was done. The criteria for selection of this systematic review were based on the following:
Included and only meta-analysed data from randomized controlled trials
Had a recent latest search date (within the last three months)
Included all available outcomes
Matched the pre-defined PICO question closely
Scored a high or moderate score on the AMSTAR 2 quality assessment tool
To identify all available systematic reviews pertaining to our PICO question, a systematic search of PubMed, Cochrane and the Epistemonikos databases was conducted. The search strategy was designed and validated by the Group’s information specialist. The search results were screened independently by two reviewers against the pre-defined criteria set out above, using Rayyan. 17 articles were found, and 8 articles were identified as systematic reviews on use of Colchicine in COVID-19. But none of the 8 articles exclusively included and only meta-analysed data from randomized controlled trials. So, we decided to conduct our own meta-analysis of randomised controlled trials of Colchicine use in COVID-19.
Population: Outpatients, Non-severe hospitalised patients, severe hospitalised patients.
Following were the outcomes we wanted to extract data for:
1. Primary outcome:
a. Time to clinical recovery
2. Secondary outcome:
a. Inpatients:
-
-
- Need for mechanical ventilation
- All-cause mortality
- Length of stay in hospital
- Admission to critical care
-
b. Outpatients:
-
-
- Admission to hospital
- Time to symptom resolution
-
3. Adverse events:
-
-
- All
- Serious
- Neutropaenia
- Renal impairment
- GI intolerance
-
Cochrane RoB2 tool was used to assess the risk of bias in the randomised control trials. For all outcomes, one review author performed the assessment, and cross-checked it against the assessment by another review author who compared it with the COVID NMA platform. If there was a difference in more than one domain, it was assessed by a third independent author.
We performed our own meta-analysis using Review Manager (RevMan) 5.4 using a fixed‐effects model for each of the critical and important outcomes. We used risk ratios (RR) for dichotomous outcomes with 95% confidence intervals (CIs). We used the I2 statistic to measure residual heterogeneity. We used GRADE methodology to assess the certainty in the evidence and documented this in a ‘Summary of findings’ table using GradeProGDT.
We found 5 randomised controlled trials that met our search criteria6–10.
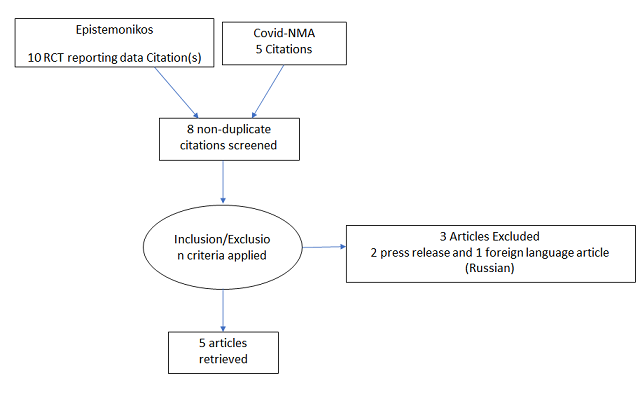
The five RCTs differed in their patient population and so we divided our analysis into three groups:
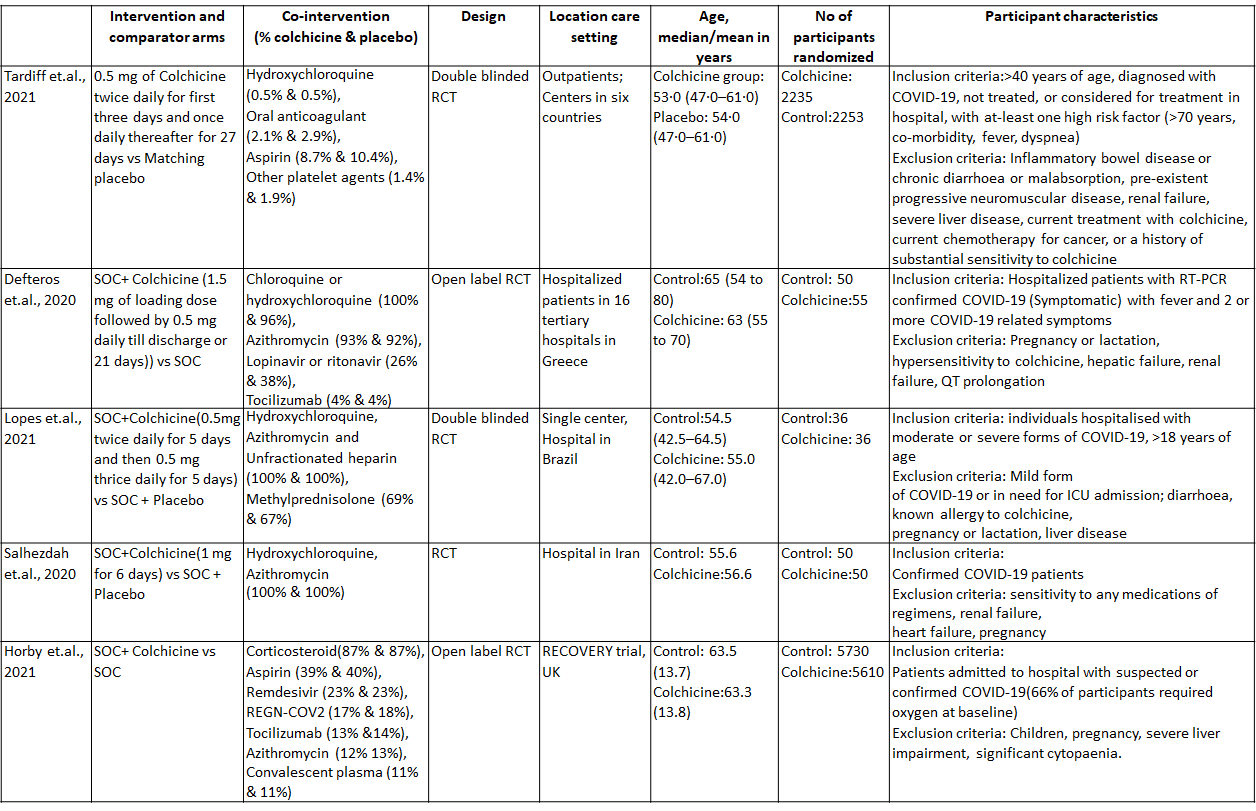
1. Colchicine compared to Placebo for outpatients with COVID-19 (Tardiff et.al., 2021)
2. Colchicine compared to No colchicine for hospitalised non-severe COVID-19 patients (Defteros et.al., 2020, Lopes et.al., 2021, Salhezdah et.al., 2020)
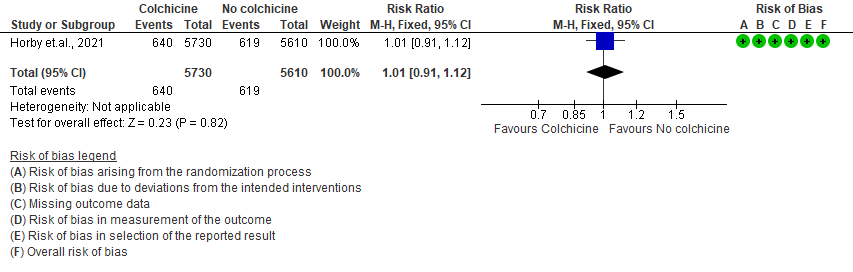
3. Colchicine compared to No colchicine for hospitalised patients with severe COVID-19 (Horby et.al., 2021)
Colchicine compared to Placebo for outpatients with COVID-19:
- All-cause mortality: Low certainty evidence from 1 RCT6 that included 4488 outpatients with COVID-19 disease indicated that Colchicine makes little to no difference in mortality in outpatients with COVID-19 infection RR=0.56(95% CI; 0.19-1.67).
- Admission to hospital: Low certainty evidence from 1 RCT6 that included 4488 outpatients with COVID-19 disease indicates that colchicine reduces hospital admission by 20% (RR = 0.80;95% CI 0.62-1.03) compared to no colchicine.The number needed to treat (NNT) to reduce one additional admission to hospital is 83.33 outpatients with COVID-19 infection.
- Progression to mechanical ventilation: Low certainty evidence from 1 RCT6 that included 4488 outpatients with COVID-19 disease indicates that colchicine may have reduced progression to mechanical ventilation by 47% (RR=0.53;95% CI 0.26-1.09) compared to no colchicine. The number needed to treat (NNT) to reduce one additional progression to mechanical ventilation is 250 outpatients with COVID-19.
- All adverse events: High certainty evidence from 1 RCT6 that included 4488 outpatients with COVID-19 disease indicates that colchicine increases adverse events by 56% (RR=1.56; 95%CI 1.38-1.76 ) compared to placebo. The number needed to harm (NNH) for one additional adverse event is 11.5 outpatients with COVID-19 infection given Colchicine.
- Diarrhea events: High certainty evidence from 1 RCT6 that included 4488 outpatients with COVID-19 disease indicates that colchicine increases diarrhea events by 88% (RR=1.88; 95%CI 1.57-2.26) compared to placebo. We anticipate that there would be 64 more (from 41 more to 91 more) diarrhea events per 1000 people if colchicine is administered for outpatients with COVID-19. The number needed to harm (NNH) resulting in one additional diarrhea event for 6 outpatients with COVID-19 given Colchicine.
Colchicine compared to No colchicine for hospitalised non-severe COVID-19 patients:
- All-cause mortality: Very low certainty evidence from 2 RCTs7,8 that included 177 hospitalized patients with non-severe COVID-19 disease indicates that the evidence is very uncertain about the effect of Colchicine treatment in hospitalized non-severe COVID-19 patients on mortality [RR= 0.15; 95%CI 0.03-0.82].
- Progression to mechanical ventilation: Very low certainty evidence from 1 RCT7 that included 105 hospitalised patients with non-severe COVID-19 disease indicates that the evidence is very uncertain about the effect of Colchicine treatment in hospitalized non-severe COVID-19 patients on progression to mechanical ventilation [RR=0.18;95% CI 0.02-1.50].
- All adverse events: Very low certainty evidence from 2 RCTs7,8 that included 177 hospitalized patients with non-severe COVID-19 disease indicates that the evidence is very uncertain about the effect of Colchicine treatment in hospitalized non-severe COVID-19 patients on all adverse events [RR= 1.63; 95% CI 0.64-4.18].
- Diarrhea events: Moderate certainty evidence from 2 RCTs7,8 that included 177 hospitalized patients with non-severe COVID-19 disease indicates that colchicine may increase in diarrhea events by 161% [RR = 2.61; 95% CI 1.42-4.79] compared to placebo. The number needed to harm (NNH) to result in one additional diarrhea event is 5.7 hospitalized non-severe COVID-19 patients.
Colchicine compared to No colchicine for hospitalised patients with severe COVID-19:
- All-cause mortality: Moderate certainty evidence from 1 RCT10 that included 11340 people with severe COVID-19 disease indicates that colchicine probably does not reduce mortality [RR = 0.97; 95% CI 0.90-1.04].
- Progression to mechanical ventilation: Moderate certainty evidence from 1 RCT10 that included 11340 people with severe COVID-19 disease indicates that colchicine probably does not reduce progression to mechanical ventilation [RR=0.96; 95% CI 0.87-1.07].
- All adverse events: Moderate certainty evidence from 1 RCT10 that included 11340 people with severe COVID-19 disease indicates that colchicine probably does not increase/reduce all adverse events [RR=1.00; 95% CI 0.92-1.09].
- Serious adverse events: Moderate certainty evidence from 1 RCT10 that included 11340 people with severe COVID-19 disease indicates that colchicine probably does not increase serious adverse events [RR=1.01;95% CI 0.91-1.12].
I. Colchicine compared to Placebo for outpatients with COVID-19 (Tardiff et.al., 2021)
1. All-cause mortality
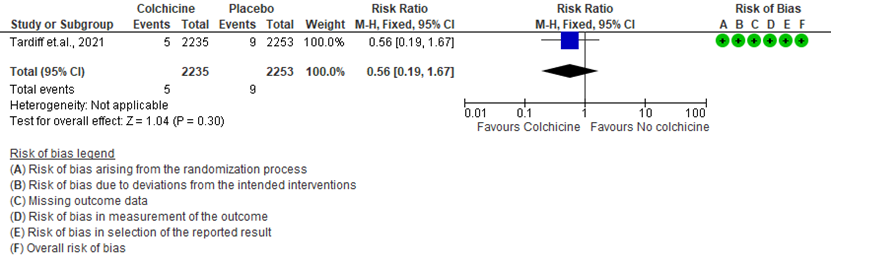
2. Admission to hospital
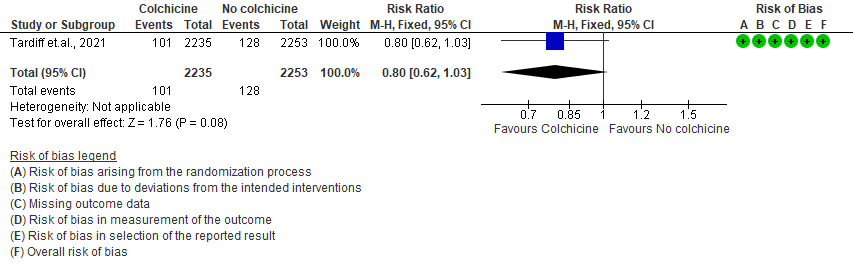
3. Progression to mechanical ventilation
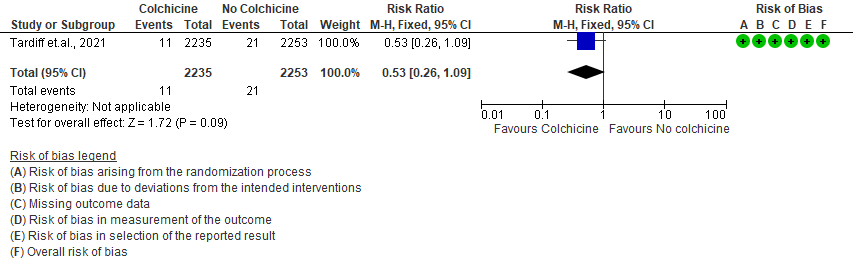
4. All adverse events
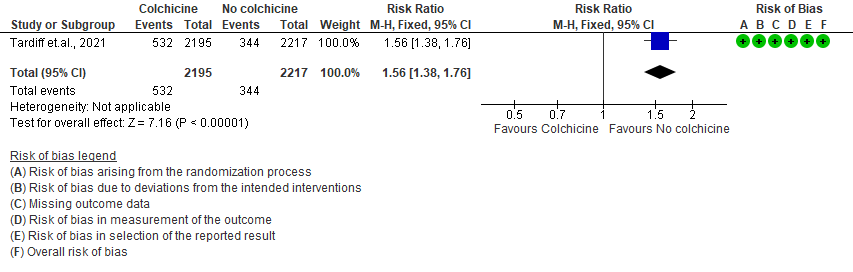
5. Diarrhea events
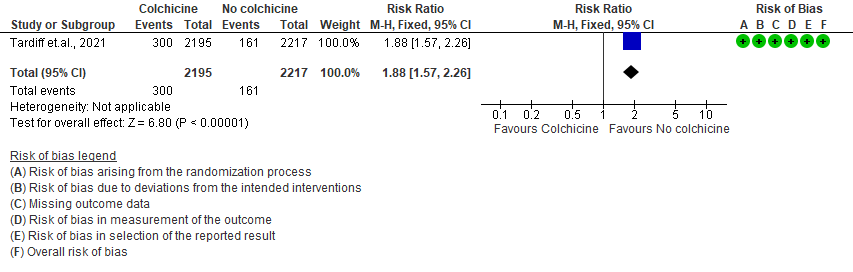
II. Colchicine compared to No colchicine for hospitalised non-severe COVID-19 patients (Defteros et.al., 2020, Lopes et.al., 2021, Salhezdah et.al., 2020)
1. All-cause mortality
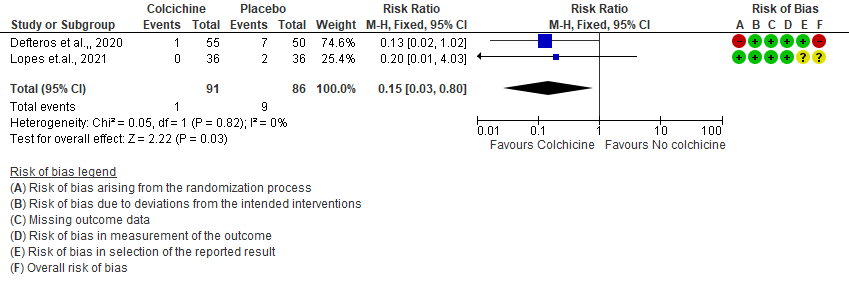
2. Progression to mechanical ventilation
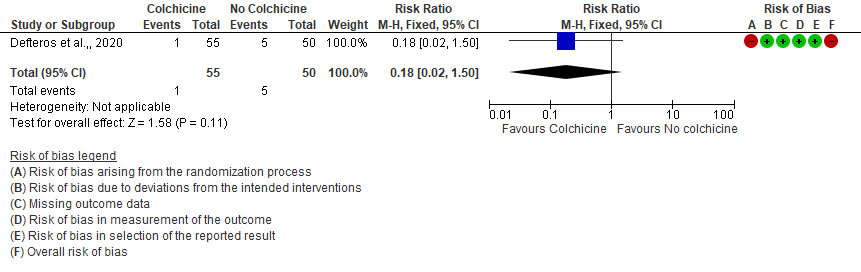
3. All adverse events
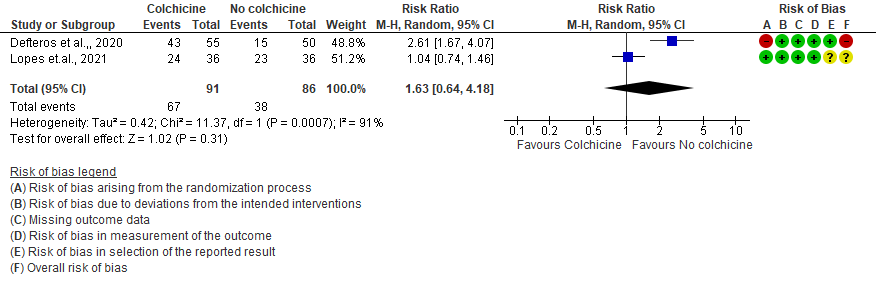
4. Diarrhea
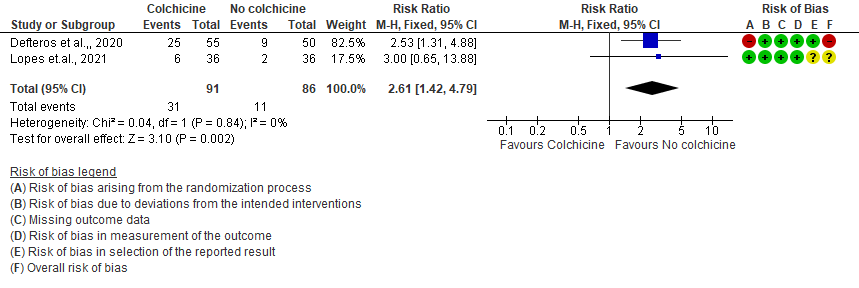
III. Colchicine compared to No colchicine for hospitalized patients with severe COVID-19 (Horby et.al., 2021)
1. All-cause mortality
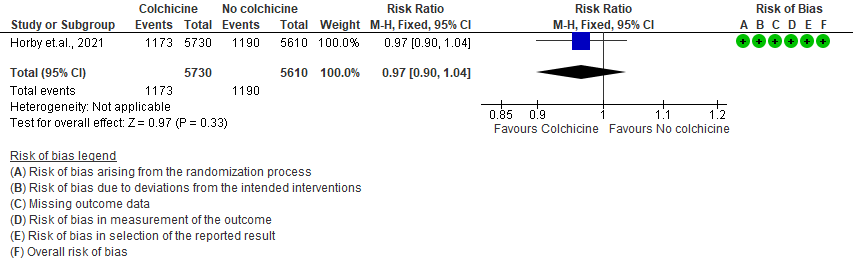
2. Progression to mechanical ventilation
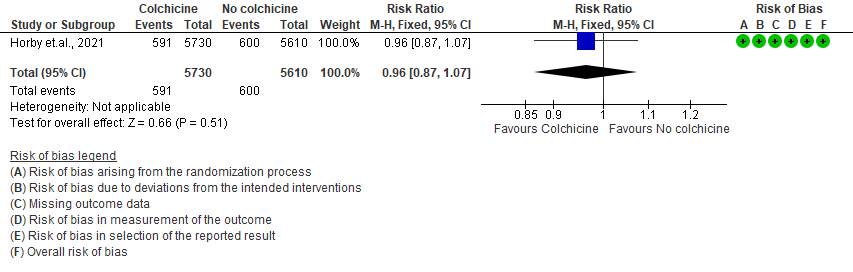
3. All adverse events
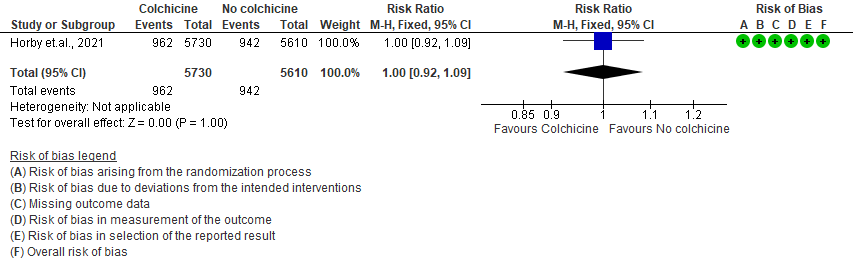
4. Serious adverse events