Explanations
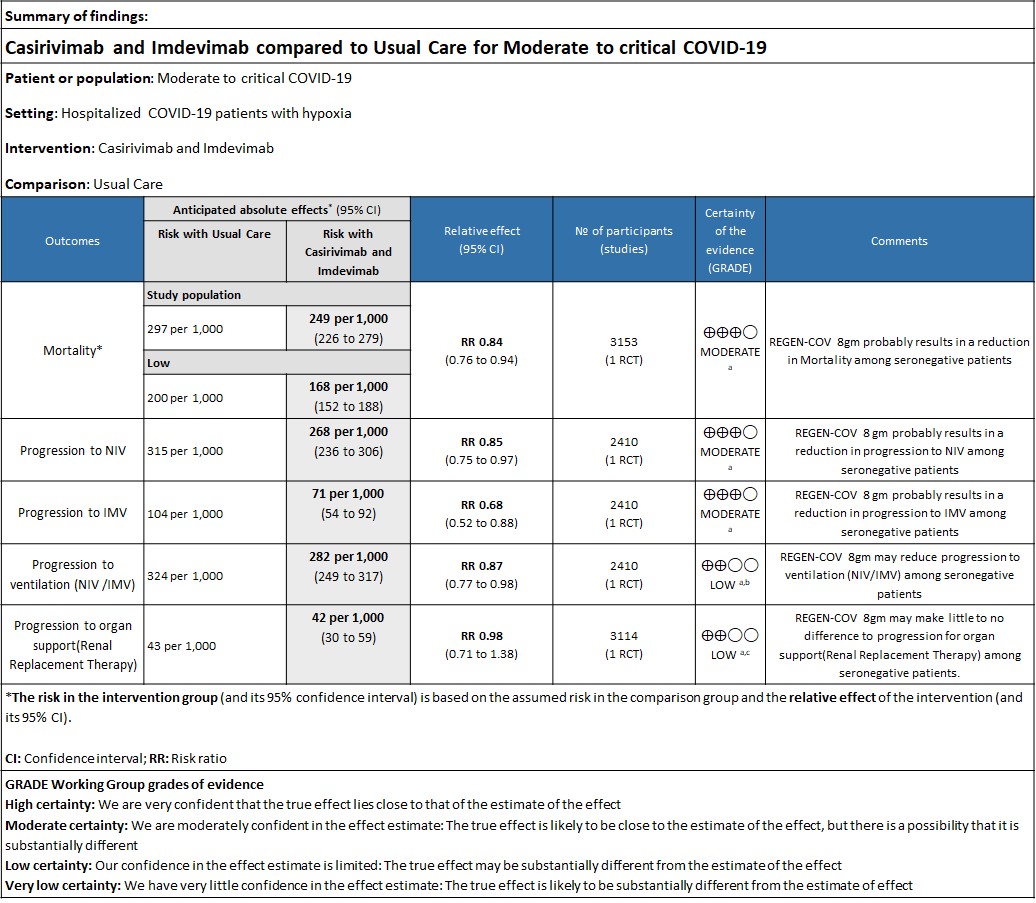
a. Downgraded by one level for serious indirectness due to baseline Risk is different in our setting with is 20%.
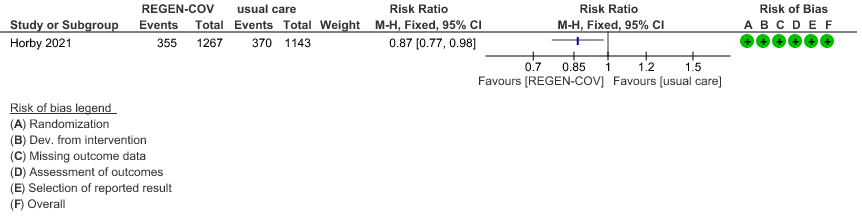
b. Downgraded by one level for serious imprecision as RR is 0.87(95% CI 0.77 to 0.98)
c. Downgraded by one level for serious imprecision as RR is 0.98 (95% CI 0.71 to 1.38)
REGN-COV™ is a cocktail made up of two noncompeting, neutralizing human IgG1 antibodies that target the receptor binding domain of the SARS-CoV-2 spike protein, thereby preventing viral entry into human cells through the angiotensin-converting enzyme 2 (ACE2) receptor(4). The combination is manufactured by Regeneron pharmaceuticals. The drug was approved by the FDA for emergency use on 21 November 2020 in patients ≥12 years of age, weighing at least 40 kilograms with a positive SARS-CoV-2 PCR test and at high risk for progression to severe COVID-19.
The authorized dosages are 1,200mg (600mg+600mg) or 2400mg (1200mg+1200mg) of Casirivimab and Imdevimab administered together as a single intravenous (IV) infusion over 60min, within 10 days of symptom onset.
The dose used in this study was 8g (4gm + 4gm), however the optimal dosing for each category of severity has not been established as yet.The CDSCO in India, on 5 May 2021, granted an EUA to Roche (Genentech) and Regeneron for use of REGEN-COV™ in the country. The announcement came in light of the second wave of the COVID-19 pandemic in India(5). This review aims to provide a summary of the available evidence for use of REGEN-COV™ for treatment of COVID-19 in severe category of patients and thus guide clinicians and researchers regarding the appropriate use of this drug.
We searched PubMed, Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language. We also reviewed reference lists of systematic reviews and included studies. We performed all searches up to 03 July 2021.
We searched the above databases and found 11 records. After removing duplicates and excluding that did not match our PICO question, one randomized controlled trial was selected and included for the analysis.
We extracted data for the following outcomes, pre-defined by the Expert Working Group:
- Critical (primary for this review):
- All-cause (in hospital) Mortality at day 28
- Time to clinical recovery
- Progression to severe disease- requiring O2,
- Progression to non-invasive ventilation, mechanical ventilation or other respiratory support
- Time to clinical improvement (WHO Ordinal scale-decrease in 2 scales)
- Discharge from hospital
- Important (secondary):
- Length of hospital stay
- Time to viral clearance (negative PCR)
- Organ support (non-respiratory)
- Length of ICU stay
- Adverse events:
- All
- Serious
- Infusion-related adverse events (if reported, record rate of infusion used)
Two reviewers independently assessed eligibility of search results using the online Rayyan tool. Data extraction was performed by one reviewer, and checked by another, using a piloted data extraction tool on Microsoft Word. Risk of bias (RoB) was assessed using the Cochrane RoB v2.0 tool, with review by a second reviewer and compared with covidnma database. If there was a difference in more than one domain it was assessed by a third independent author.We planned to use risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs) using Review Manager (RevMan) v5.4. We used GRADE methodology to make summary of findings tables on GRADEPro GDT.
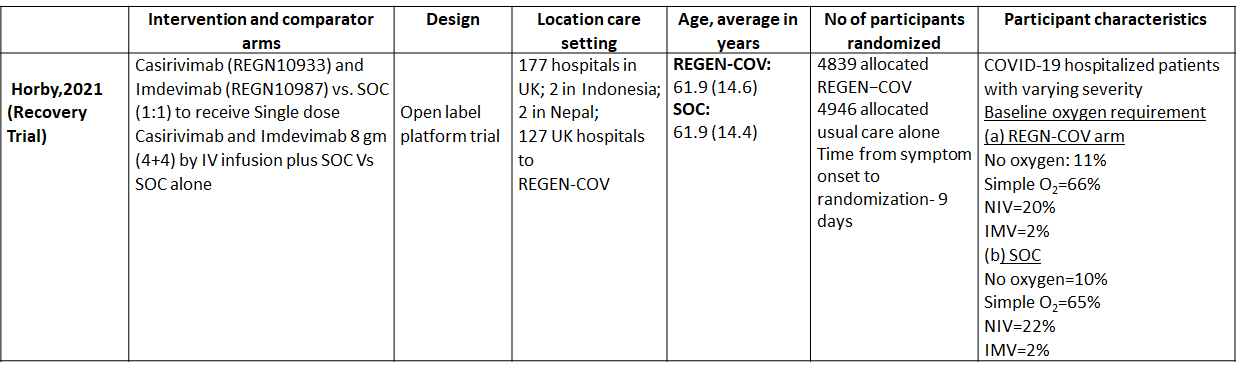
We found one RCT that matched the PICO question as pre-defined by the expert working group. The trial included a total of 9785 participants, all of whom were adult hospitalized patients with varying severity of diseases. This study was done in 177 hospitals in UK; 2 in Indonesia; 2 in Nepal; 127 UK hospitals.
The results are described below; characteristics of the trials are summarised in the Summary of characteristics of included trials table.
The following comparisons were investigated in the trial (we compared outcomes for arms randomized to Casirivimab and Imdevimab combination (REGEN-COV™) vs usual care.
Outcomes
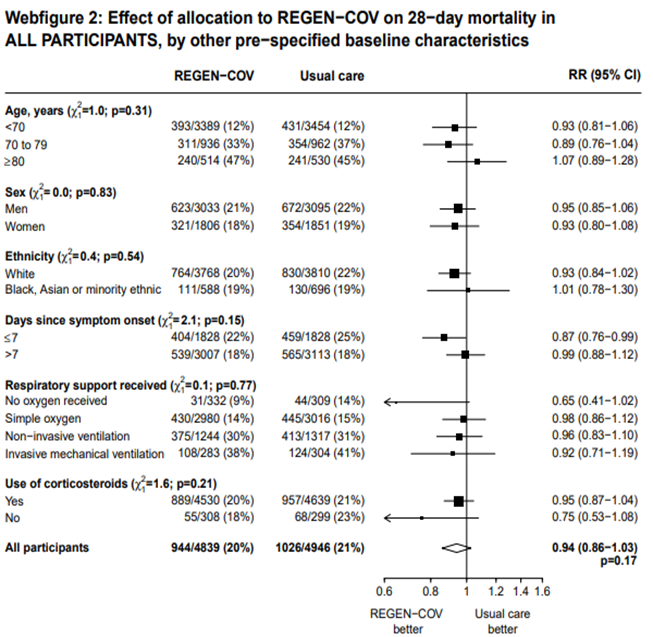
As presented in the ‘Summary of findings’ tables, the evidence is of moderate certainty about the effect of Casirivimab and Imdevimab combination (REGEN-COV™) 8 gm as a single dose intravenous infusion on mortality, progression to Invasive mechanical ventilation, progression to non-invasive mechanical ventilation and progression to organ replacement therapy (Renal replacement therapy) in all patients (seronegative or seropositive). Then a separate analysis for all outcomes was done in all the seronegative patients as well. The intervention was delivered at a median of 9(6-12) days in both the groups.
a. Mortality:
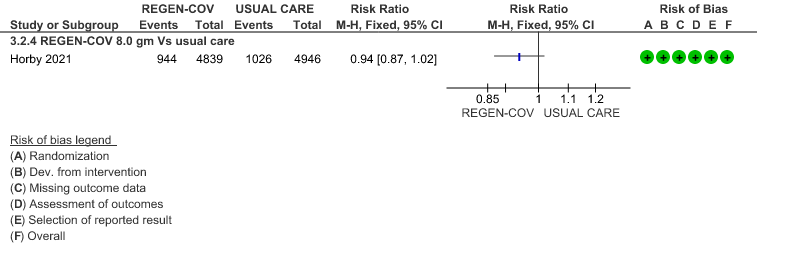
All patients: Mortality in all (seropositive and seronegative) revealed that REGEN-COV™ resulted in a mild reduction in mortality with a RR=0.94 (95% CI 0.87-1.02) suggesting that this mild benefit may be due to the effect on REGEN-COV™ on seronegative patients rather than in all patients.
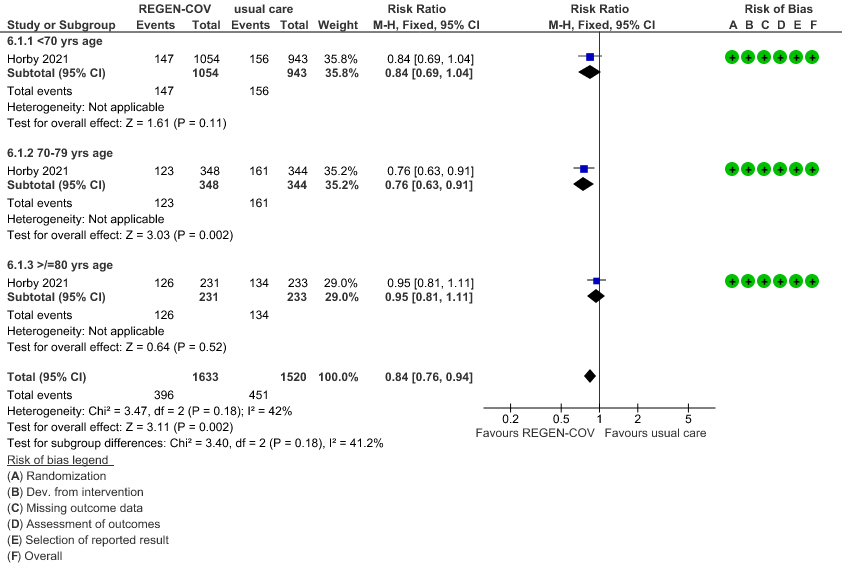
Seronegative: Moderate certainty evidence from 1 trial in 3153 patients, revealed that REGEN-COV™ 8gm probably reduces mortality in seronegative patients; RR 0.84; (95% CI 0.76 to 0.94). The mortality reduction was 20% with a NNT of 19 to prevent 1 death.
The group also felt that the projected mortality in the control group was very high and felt that the baseline risk in the Indian population was around 20% rather than what was projected in the trial as 29% and hence the NNT was baseline risk was modified was 25.
b. Progression to non-invasive ventilation (NIV):
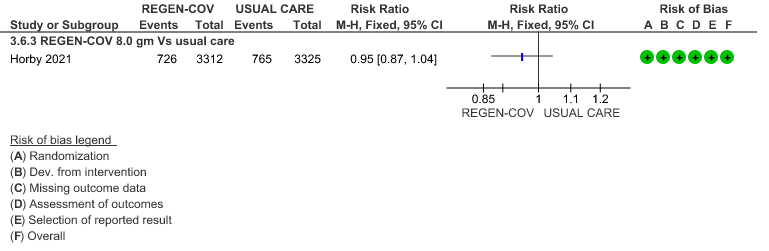
All patients: Progression to non-invasive ventilation was mildly reduced by REGEN-COV™ with a RR=0.95(95% CI 0.87-1.04) suggesting that this mild benefit may be due to the effect on REGEN-COV™ on seronegative patients rather than in all patients.
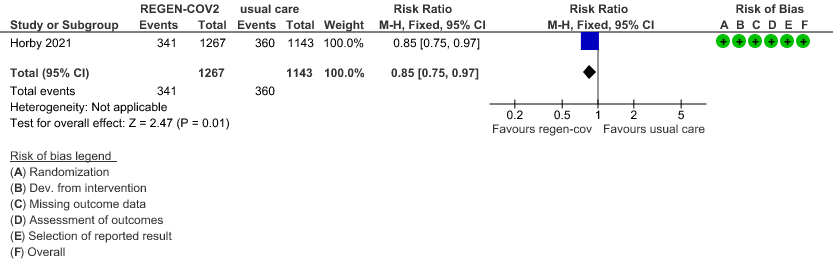
Seronegative: Moderate certainty of evidence in 1 trial with 2,410 patients revealed that REGEN-COV™ 8 gm reduces the progression to non-invasive mechanical ventilation in seronegative patients with (RR 0.85; 95 percent CI 0.75 to 0.97).
c. Progression to invasive mechanical ventilation (IMV):
All patients: Progression to invasive mechanical ventilation was mildly reduced by REGEN- COV™ with a RR=0.86 (95% CI 0.71-1.04) suggesting that this mild benefit may be due to the effect on REGN-COV™ on seronegative patients rather than in all patients.
Seronegative: Moderate certainty of evidence in 1 trial with 2412 patients (RR 0.68; 95 percent CI 0.52 to 0.88), revealed that REGEN-COV™ 8 gm reduces the progression to invasive mechanical ventilationin seronegative individuals.
d. Progression to invasive mechanical ventilation or non-invasive ventilation:
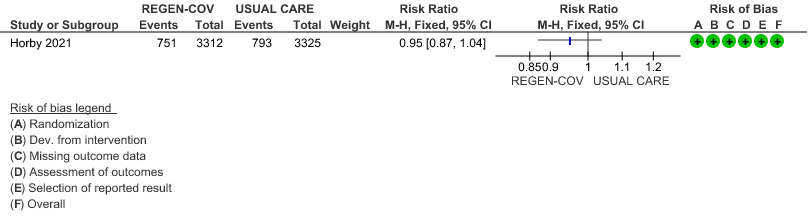
All patients: Progression to respiratory support (non-invasive ventilation or invasive mechanical ventilation) was mildly reduced by REGEN-COV™ with a RR=0.95(95% CI 0.87-1.04) suggesting that this mild benefit may be due to the effect on REGEN-COV™ on seronegative patients rather than in all patients.
Seronegative: Moderate certainty evidence from 1 trial with 6,646 patients revealed that REGEN-COV™ 8 gm probably results in a reduction in progression to invasive and non-invasive mechanical ventilation in seronegative patients (RR 0.87; 95 percent CI 0.77 to 0.98).
e. Progression to organ replacement therapy (Renal replacement therapy):
All patients: Progression to organ replacement therapy was not reduced by REGEN-COV™ with a RR=1.03 (95% CI 0.85-1.25).
Seronegative: Moderate certainty of evidence in 1 trial with 9670 patients found that REGEN-COV™ 8 gm may make little to no difference in reducing the progression to organ replacement therapy (Renal replacement therapy) in seronegative patients(RR 0.98; 95% CI 0.71 to 1.38).
f. Length of hospital stay: The median length of stay in seronegative individuals given REGEN-COV™ was a median 13 (7->28) days vs 17 (7->28) days given usual care.
g. Hospital discharge by day 28: About 1046/1633 seronegative individuals given REGEN-COV™ vs878/1520 given usual care were discharged alive at day 28.
Subgroup analysis
This trial included pregnant, lactating mothers, adolescents 12-18 years, those with co-morbidities >1 and as also those on chronic oxygen therapy. However, disaggregated data could not be obtained for these categories. Though, immunocompromised and those with renal or liver failure were not excluded from this trial, data was not available separately for this group.
The other subgroup analyses that was requested included high vs low dose, past infection, interaction and efficacy with variants, vaccination status, however these outcome variables were not available in the journal article or supplementary data.
Baseline antibody status was available in both the groups and we did a separate analysis for the outcomes of interest.
a. Mortality: In the seronegative group 396/1633 vs 411/2696 in the seropositive died by 28 days; RR=1.56 (1.37-1.76)
b. Progression to invasive mechanical ventilation or death: In the seronegative group 487/1599 vs 456 out of 2449 in the seropositive group progressed to invasive mechanical ventilation or death; RR=1.64(1.46-1.83).
c. Discharged alive from hospital: In the seronegative group 1046/1633 vs 1970/2636 in the seropositive group were discharged alive from hospital; RR=0.86(0.82-0.89).
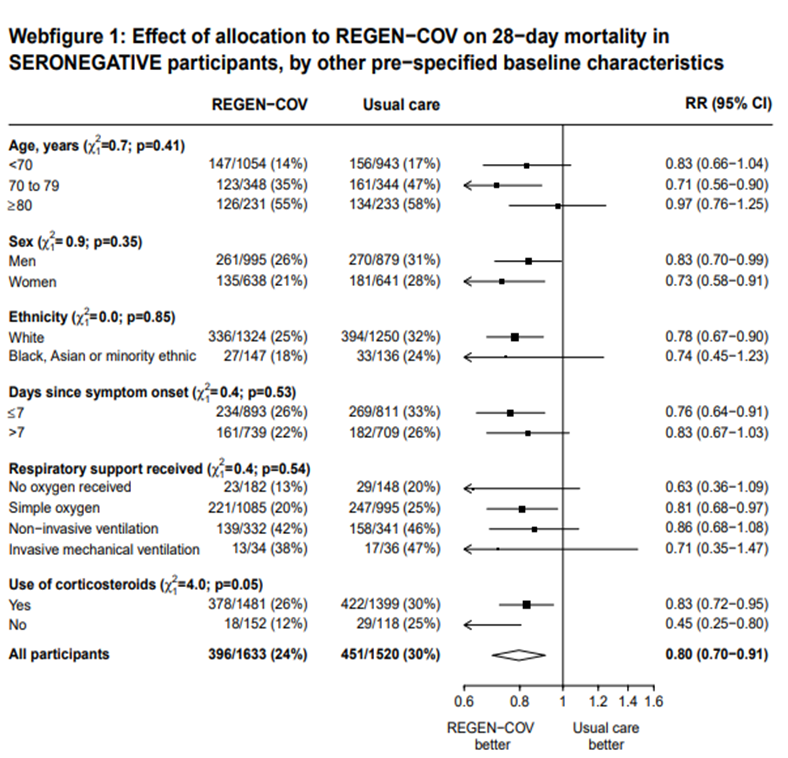
In another pre-specified analysis according to baseline characteristics in seronegative patients, mortality was stratified by
a. Age and the risk of dying in those 80 years of age, RR=0.97(95% CI 0.76-1.25).
b. In those who had symptoms 7 days; RR = 0.83 (95% CI 0.67-1.03)
c. Respiratory support: In those with no oxygen at baseline RR=0.63 (0.36-1.09); on simple oxygen at baseline RR=0.81 (95% CI 0.68-0.97); NIV RR=0.86 (95% CI 0.68-1.08); IMV RR=0.71 (95% CI 0.35-1.47)
1.
(a) Mortality in all (seronegative and seropositive patients)
(b) Mortality in seronegative patients stratified by age
2.
(a) Progression to non-invasive ventilation in all (seronegative and seropositive):
(b) Progression to NIV (seronegative)
3.
(a) Progression to invasive mechanical ventilation (IMV) in all (seronegative and seropositive):
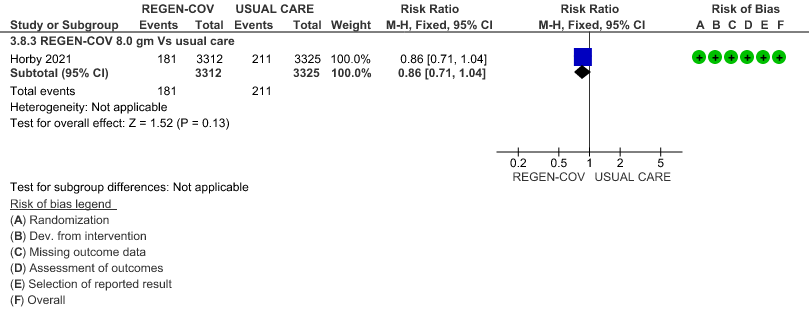
(b) Progressive to IMV in seronegative
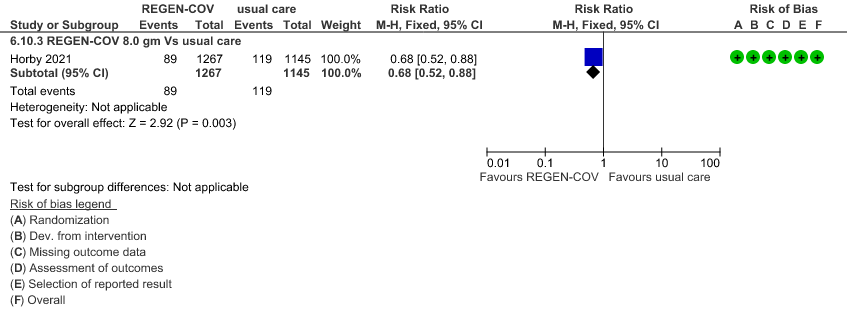
4.
(a) Progression to ventilatory support (IMV or NIV) in all(seronegative and seropositive)
(b) Progression to ventilatory support (IMV or NIV) in seronegative
5.
(a) Progression to organ replacement therapy (Renal replacement therapy) in all:
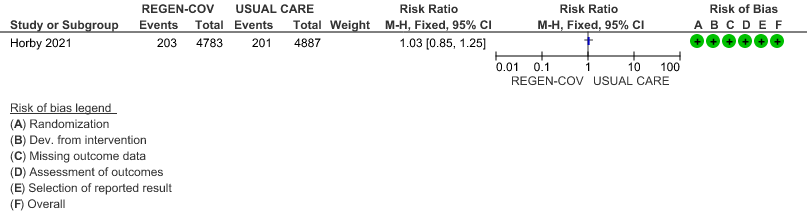
(b) Progression to organ replacement therapy (Renal replacement therapy) in seronegative:
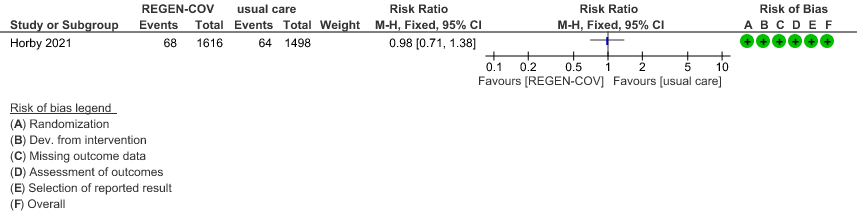
6.
(a) Outcomes of interest in the seropositive vs seronegative individuals:
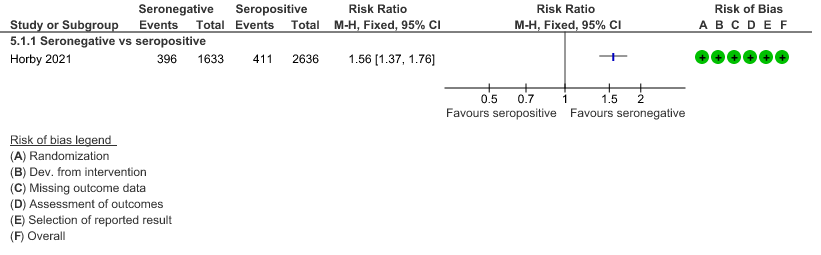
(b) Progression to IMV or death
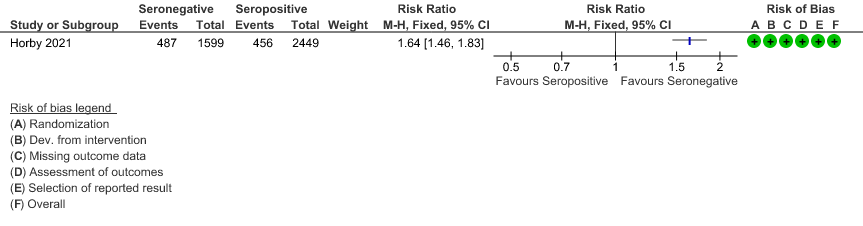
(c) Discharged alive from hospital
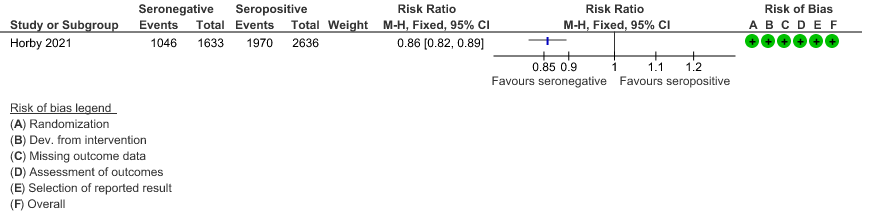
7. Outcomes of interest as per baseline characteristics [web figures of tables taken from Supplementary material Recovery trial for REGEN-COV™ – (Ref 6)]
Horby PW, Mafham M, Peto L, et al. Casirivimab and imdevimab in patients admitted to hospital with covid-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv 2021.06.15.21258542v1[Preprint].2021. https://www.medrxiv.org/content/10.1101/2021.06.15.21258542v1.supplementary-material)