Explanations
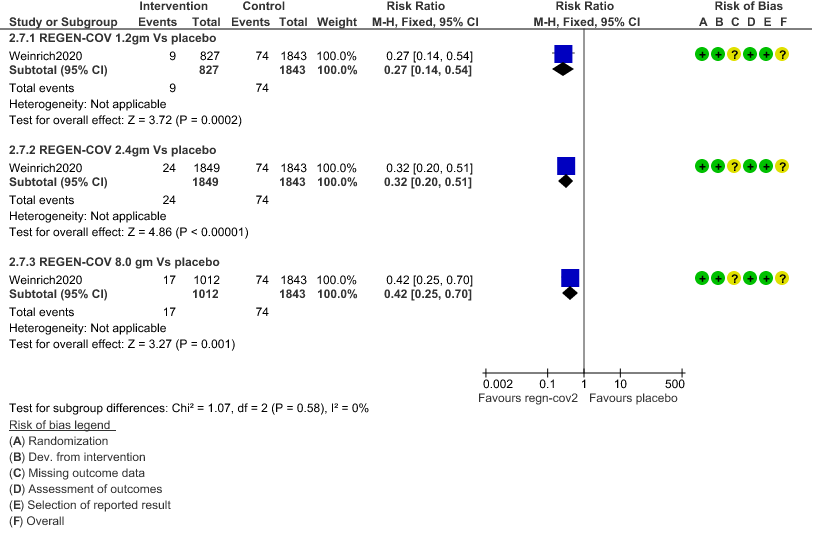
a. Downgraded for one level for serious risk of bias as the study reported some concerns
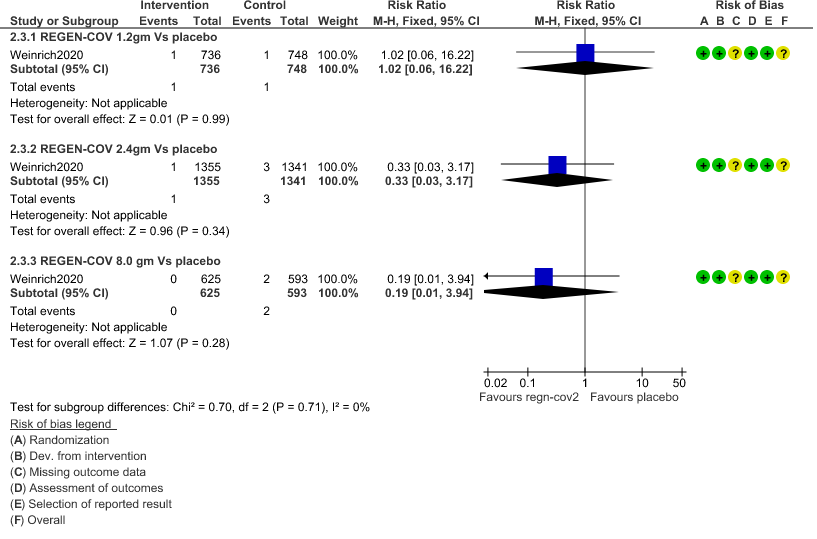
b. Downgraded by two levels for serious imprecision.95% CI ranges from 0.06 to 16.22 and the event rate is very low.(OIS inadequate)
c. Downgraded by two levels for serious imprecision.95% CI ranges from 0.05 to 5.59 and the event rate is very low.(OIS inadequate)
d. Downgraded by two levels for serious imprecision.95% CI ranges from 0.11 to 1.68 and the event rate is very low.(OIS inadequate)
e. Downgraded by one level for serious imprecision 95% CI ranged from 0.64 to 2.16 which is not clinically interpretable.
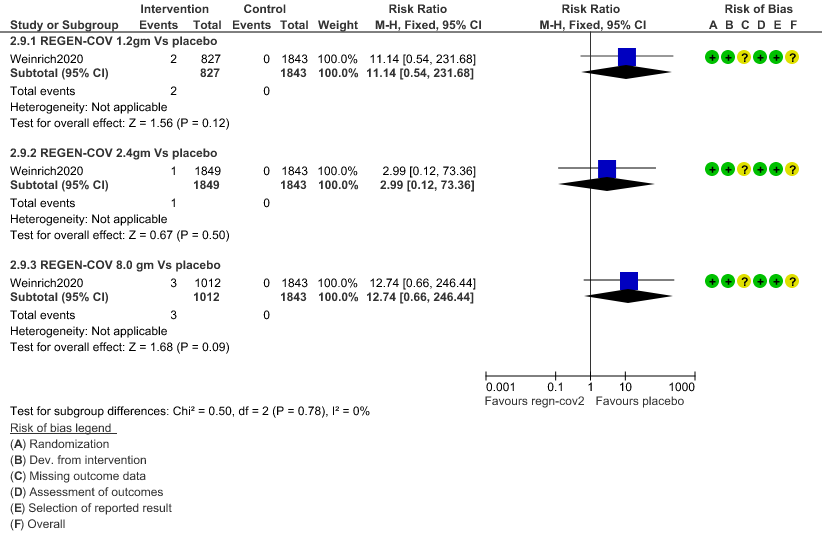
f. Downgraded by two levels for serious imprecision.95% CI ranges from 0.54 to 231.68 and the event rate is very low.(OIS inadequate)
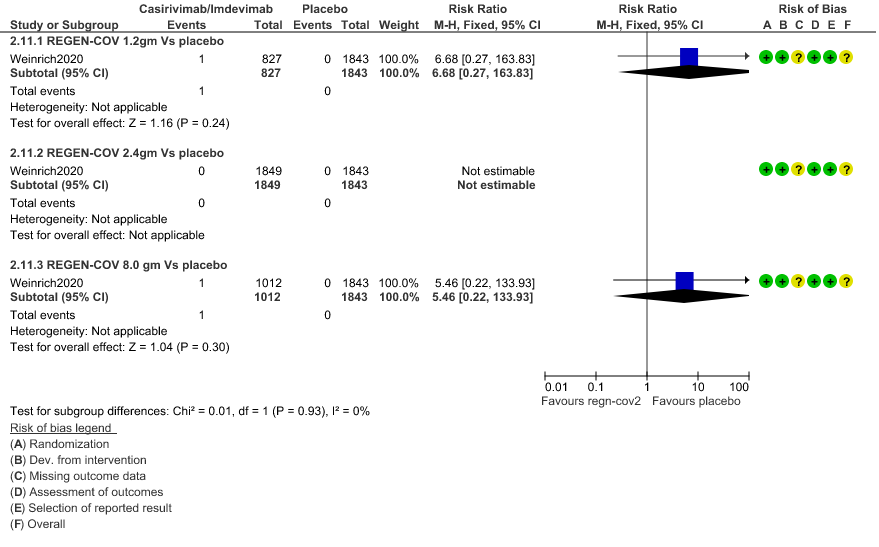
g. Downgraded by two levels for serious imprecision.95% CI ranges from 0.27 to 163.83 and the event rate is very low.(OIS inadequate
Explanations
a. Downgraded for one level for serious risk of bias as the study reported some concerns
b. Downgraded by two levels for serious imprecision.95% CI ranges from 0.03 to 3.17 and the event rate is very low.(OIS inadequate)
c. Downgraded by two levels for serious imprecision.95% CI ranges from 0.02 to 1.37 and the event rate is very low.(OIS inadequate)
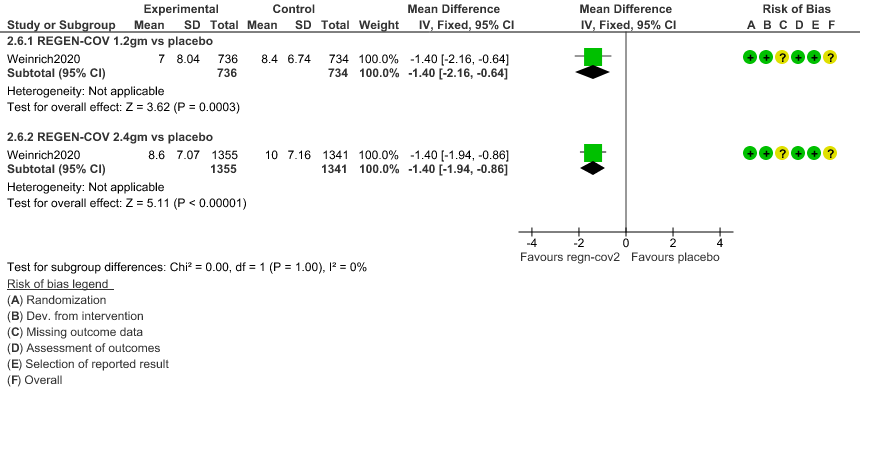
d. Downgraded by one level for serious imprecision as the decrease was 1.4 days lower but 95% CI ranged from 0.86 to 1.94 which is not clinically relevant
e. Downgraded by two levels for serious imprecision.95% CI ranges from 0.12 to 73.36 and the event rate is very low.(OIS inadequate)
Explanations
a. Downgraded for one level for serious risk of bias as the study reported some concerns
b. Downgraded by two levels for serious imprecision.95% CI ranges from 0.01 to 3.94 and the event rate is very low.(OIS inadequate)
c. Downgraded by two levels for serious imprecision.95% CI ranges from 0.66 to 246.44 and the event rate is very low.(OIS inadequate)
d. Downgraded by two levels for serious imprecision.95% CI ranges from 0.22 to 133.93 and the event rate is very low.(OIS inadequate)
REGEN-COV™ is a cocktail made up of two noncompeting, neutralizing human IgG1 antibodies that target the receptor binding domain of the SARS-CoV-2 spike protein, thereby preventing viral entry into human cells through the angiotensin-converting enzyme 2 (ACE2) receptor(1). The combination is manufactured by Regeneron pharmaceuticals. The drug was approved by the FDA for emergency use on 21 November 2020 in patients ≥12 yrs. of age, weighing at least 40 kilograms with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progressing to severe COVID-19. The authorized dosage is 1,200 mg of Casirivimab and 1,200 mg of Imdevimab administered together as a single intravenous (IV) infusion over 60min as soon as possible after positive viral test for SARS-CoV-2 and within 10 days of symptom onset. (Optimal dosing regimen is not established yet)(2).The CDSCO in India, on 5 May 2021, granted an Emergency use authorization (EUA) to Roche (Genentech) and Regeneron for use of REGEN-C0V™ in the country. The announcement came in light of the second wave of the COVID-19 pandemic in India(3). This review aims to provide a summary of the available evidence from randomized clinical trials of REGEN-COV™ for treatment of COVID-19 which could guide clinicians and researchers regarding the appropriate use of this drug in the future
We searched PubMed, Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language. We also reviewed reference lists of systematic reviews and included studies. We performed all searches up to 28 June 2021.
We searched the above databases and found 11 records. After removing duplicates and excluding studies that did not match our PICO question, only one randomized controlled trial was found and included for the analysis.
We extracted data for the following outcomes, pre-defined by the Expert Working Group:
- Critical (primary for this review):
- Admission into hospital (all cause)
- Time to clinical recovery
- Progression to severe disease- requiring O2, mechanical ventilation or ICU care
- Important (secondary):
- Time to viral clearance (negative PCR)
- Death
- Adverse events:
- All
- Serious
- Infusion-related adverse events (if reported, record rate of infusion used)
Two reviewers independently evaluated and validated search results using the online Rayyan tool. Data extraction was performed by one reviewer, and checked by another, using a piloted data extraction tool on Microsoft Word. Risk of bias (RoB) was assessed using the Cochrane RoB v2.0 tool, by one reviewer and checked by a second reviewer and compared with covid NMA database. If there was a difference in more than one domain it was assessed by a third independent reviewer and a consensus obtained.We planned to use risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs) using Review Manager (RevMan) v5.4. We used GRADE methodology to make summary of findings tables on GRADEPro GDT.
We found one RCT that matched the PICO question as pre-defined by the expert working group. The trial included a total of 4057 participants, all of whom were adult out patients. This study was done in 82 centers in United States and Mexico. The results are described below; characteristics of the trials are summarized in the Summary of characteristics of included trials table.
The following comparisons were investigated in the trial (we compared outcomes for arms randomized to Casirivimab and Imdevimab combination (REGEN-COV™) vs Placebo in out patients with mild COVID illness with >1 risk factor for disease progression.
Outcomes
As presented in the ‘Summary of findings’ tables, the evidence ranged from low to very low certainty for the effect of Casirivimab and Imdevimab combination (REGEN-COV) 1.2 gm on admission to critical care, length of stay, mortality, progression to Invasive mechanical ventilation, infusion related adverse events and adverse reactions leading to drug interruption. Moderate certainty of evidence for hospital admission and serious adverse events.
Since this was an adaptive phase 3 randomized controlled trial it compared patients in the doses of 1.2g, 2.4g and 8.00g
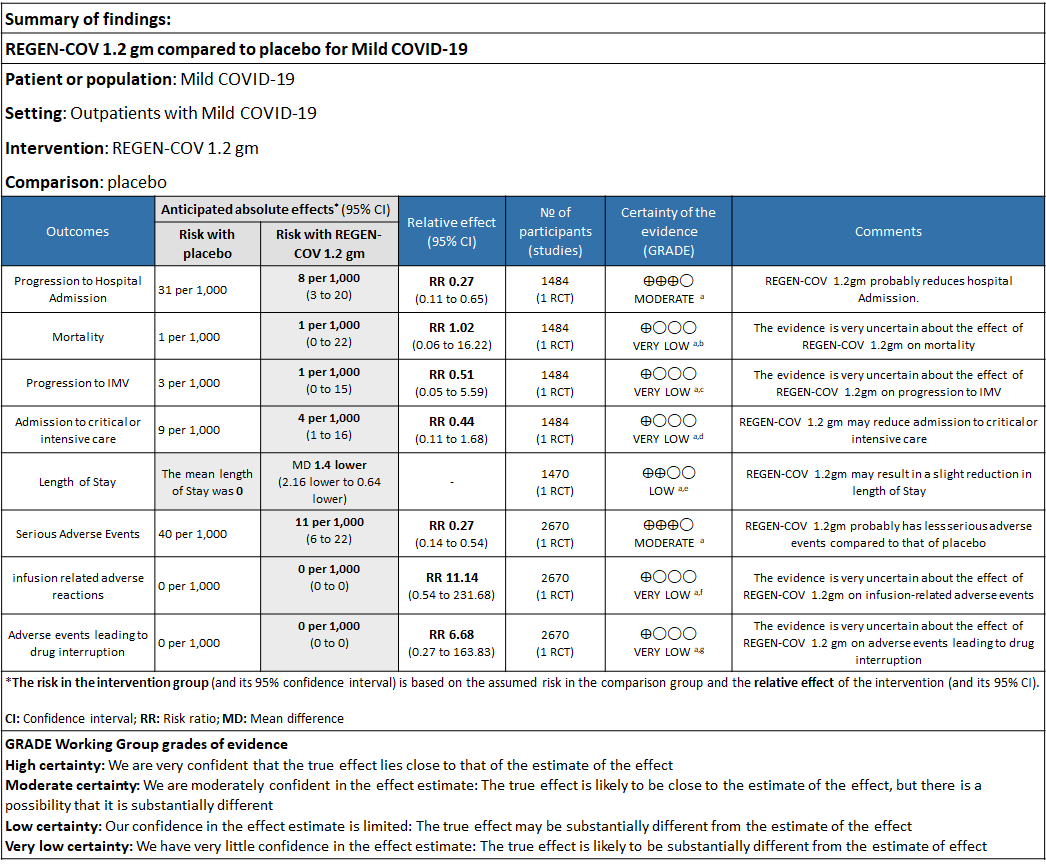
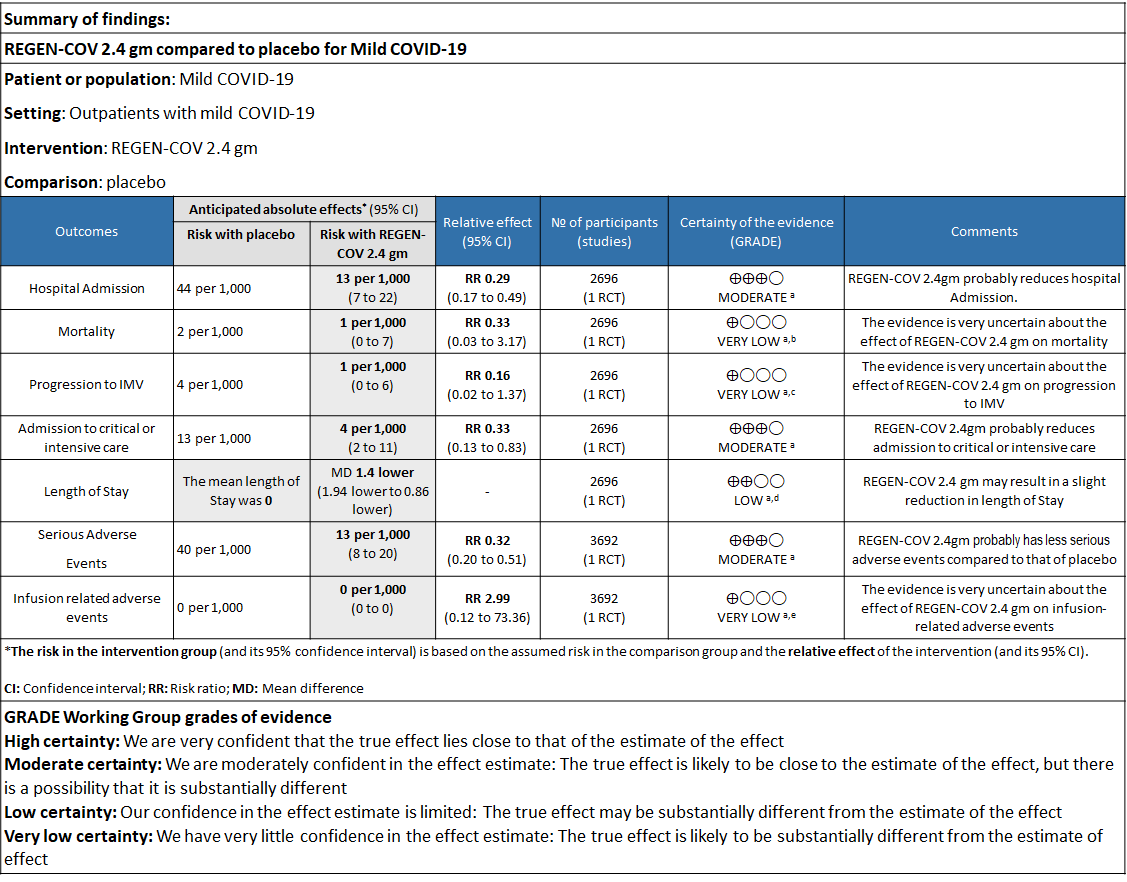
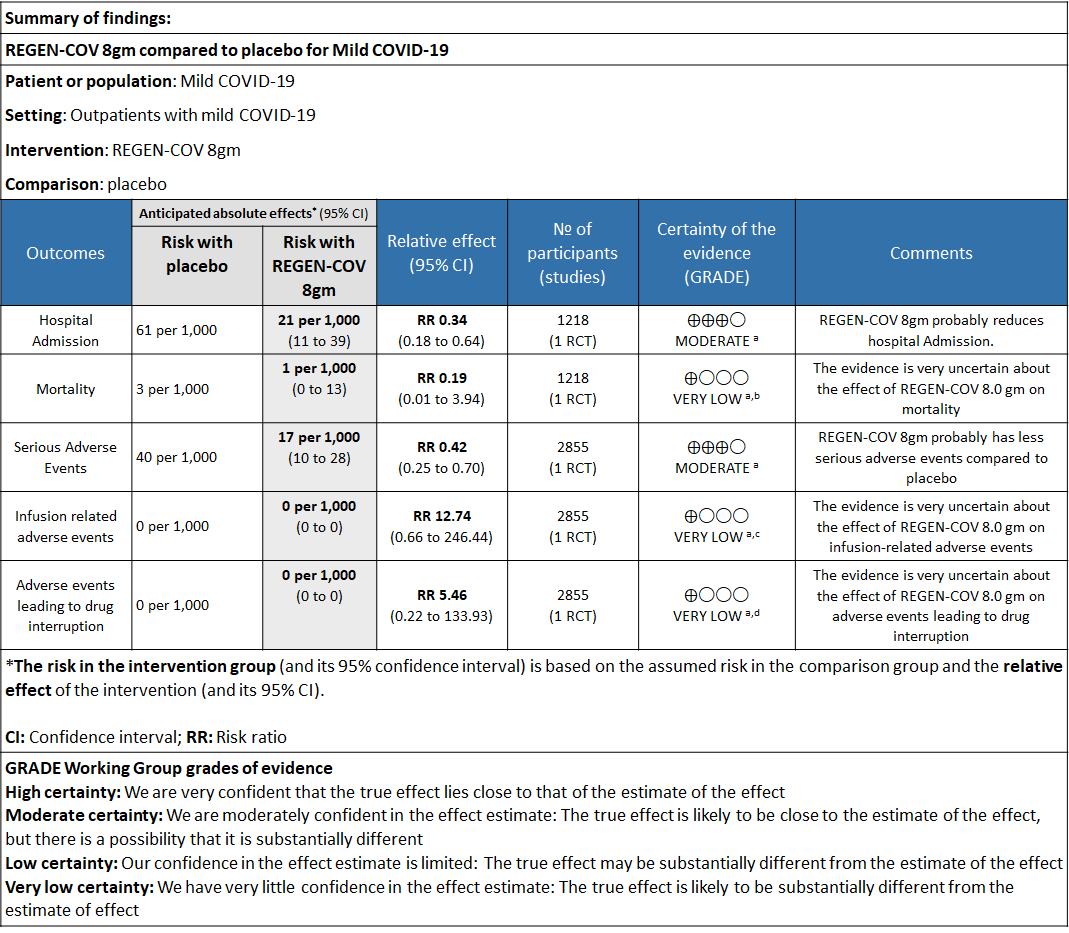
a. Admission to Hospital: Moderate certainty of evidence in 1484 patients belonging to 1.2gm arm (RR 0.27; 95% CI 0.11 to 0.65), 2696 patients in 2.4gm arm (RR 0.29; 95% CI 0.17 to 0.49) and 1218 patients in 8gm arm (RR 0.34; 95% CI 0.18 to 0.64) of Casirivimab-Imdevimabin the selected trial revealed that it reduced admission to hospital in out patients with mild illness (1) .The number needed to treat to prevent one hospital admission included 44, 36 and 26 with doses of 1.2g,2.4g and 8.0g respectively.
b. Progression to Invasive mechanical ventilation: Very low certainty of evidence in 1484 patients 1.2gm arm (RR 0.51; 95% CI 0.05 to 5.59), 2696 patients in 2.4gm arm (RR 0.16; 95% CI 0.02 to 1.37) and revealed that there was a very uncertain impact on progression to invasive mechanical ventilation.
c. Admission to critical or intensive care for any reason:Low certainty of evidence in 1484 patients (RR 0.44; 95% CI 0.11 to 1.68) revealed that 1.2g of REGN-COV did not prevent admission to critical or intensive care.
However, moderate certainty of evidence in 2696 patients in 2.4gm arm (RR 0.33; 95% CI 0.13 to 0.83) showed that REGN-COV at this doseprobably reduces admission to critical or Intensive care.
d. Duration of hospitalization: Low certainty of evidence in 1,470 patients in the 1.2g dose and 2696 patients in the 2.4 gm dose of REGEN-COV may reduce duration of hospitalization by a mean difference of 1.4 days when compared to placebo. The median difference in the 1.2g dose arm was 1.5 days and in the 2.4g dose arm was 1 day.
e. All-cause mortality: Very low certainty of evidence in 1484 patients in 1.2gm arm (RR 1.02; 95% CI 0.06 to 16.22), 2696 patients in 2.4gm arm (RR 0.33; 95% CI 0.03 to 3.17), 1218 patients in 8gm arm (RR 0.19; 95% CI 0.01 to 3.94), found that REGEN-COV in doses of 1.2gm and 2.4gm, 8gm has a very uncertain effect on reducing mortality.
f. Adverse events:
1. Serious Adverse events: Moderate certainty of evidence in 2670 patients in the 1.2gm arm (RR 0.27; 95% CI 0.14 to 0.54), 3692 patients in 2.4gm arm (RR 0.32; 95% CI 0.2 to 0.51) and 2855 patients in 8gm arm (RR 0.42; 95% CI 0.25 to 0.7) found that REGEN-COV in doses of 1.2gm, 2.4gm and 8 gm probably has less serious adverse events compared to placebo.
2. Infusion related adverse events: Very low certainty of evidence in 2670 patients 1.2gm arm (RR 11.14; 95% CI 0.54 to 231.68), 3692 patients in 2.4gm arm (RR 2.99; 95% CI 0.12 to 73.36) and 2855 patients in 8gm arm (RR 12.74; 95% CI 0.66 to 246.44) revealed that evidence was very uncertain that REGEN-COV 1.2gm, 2.4gm and 8 gm has less infusion related adverse events compared to placebo.
3. Adverse events leading to drug interruption: Very low certainty of evidence in 2670 patients 1.2gm arm (RR 6.68; 95% CI 0.27 to 163.83), and 2855 patients in 8gm arm (RR 5.46; 95% CI 0.22 to 133.93) revealed a very uncertain effect that REGEN-COV 1.2gm and 8 gm had less adverse events that lead to drug interruption as compared to placebo.
g. Time to clinical recovery: The median time to resolution of symptoms in both REGEN-COV dose arms (1.2g and 2.4g) vs placebo was 10 vs 14 days (p<0.0001). - If the viral load was > 6 log copies then Regeneron did reduce symptoms faster by 5 days (p<0.0001)
- If viral load was < 6 log copies it may reduce symptoms but by only 2 days. (p= not significant)
- If Ab negative at baseline, either dose reduced symptoms by 3 to 4 days. (p=<0.0002)
- If Ab positive at baseline 2.4g dose of Regeneron may reduce symptoms by 4 days (p=0.05)
h. Difference in Viral load:Time to negative PCR as specified by the PICO was not available. However, a difference in viral loads was measured. In the modified full analysis set (mFAS) the viral load was 0.75 log less with either 1.2g or 2.4g dose of REGN-COV which is not clinically relevant.
- In those with a baseline viral load >6 log copies, there was a 1 log drop in the viral load when 1.2g (1.01 log copies) or 2.4g (1.04 log copies) of REGN-COV was used vs placebo
- If seronegative at baseline, there was a drop in the intervention arm by 1 log copies, when 1.2g (0.86 log copies) or 2.4g (1.04 copies) dose of REGN-COV was used.
- In those seropositive at baseline with 1.2g (0.16 log copies) or 2.4g (0.43 log copies) the drop in viral load was clinically and statistically insignificant.
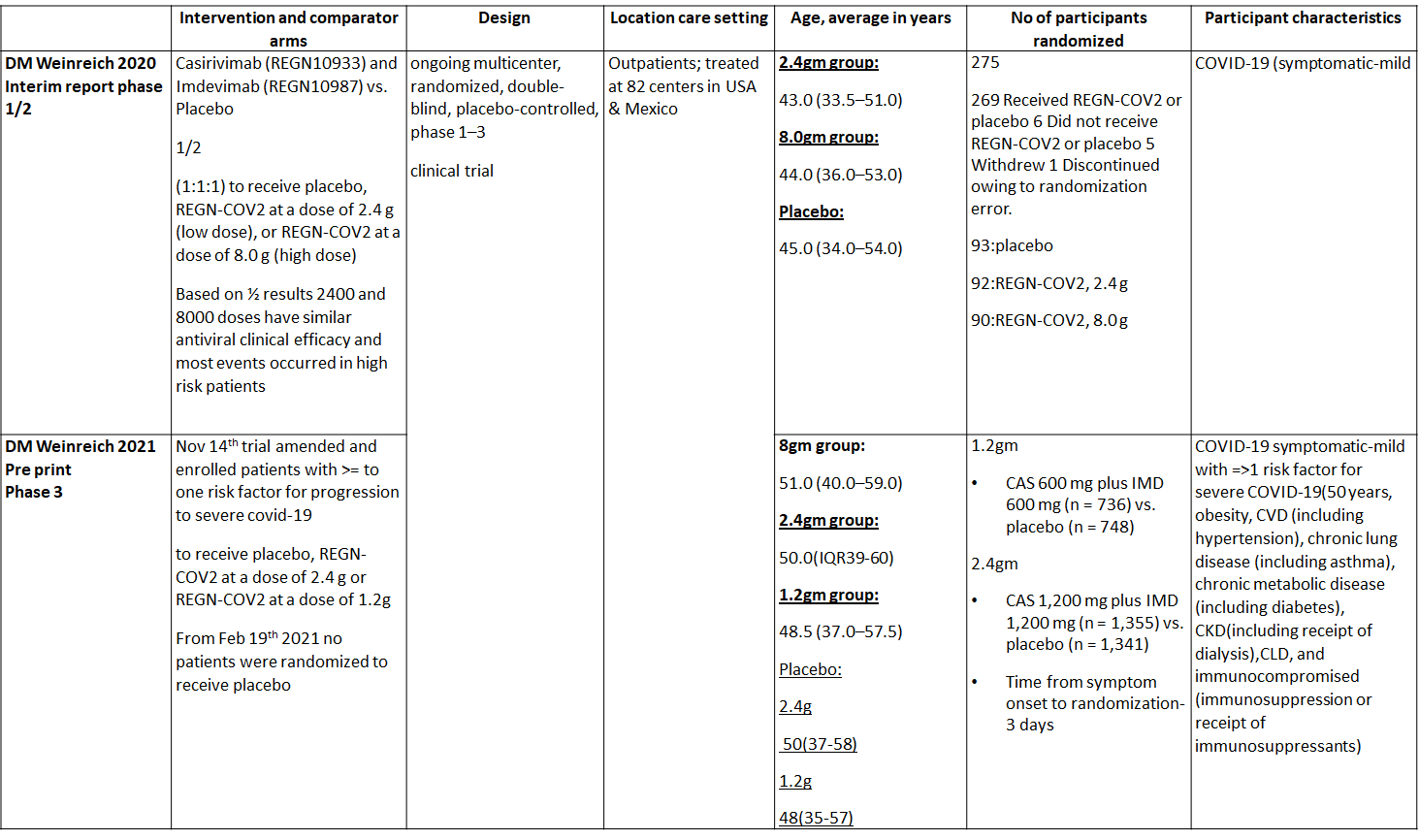
1. Progression to Hospital admission (each dose)
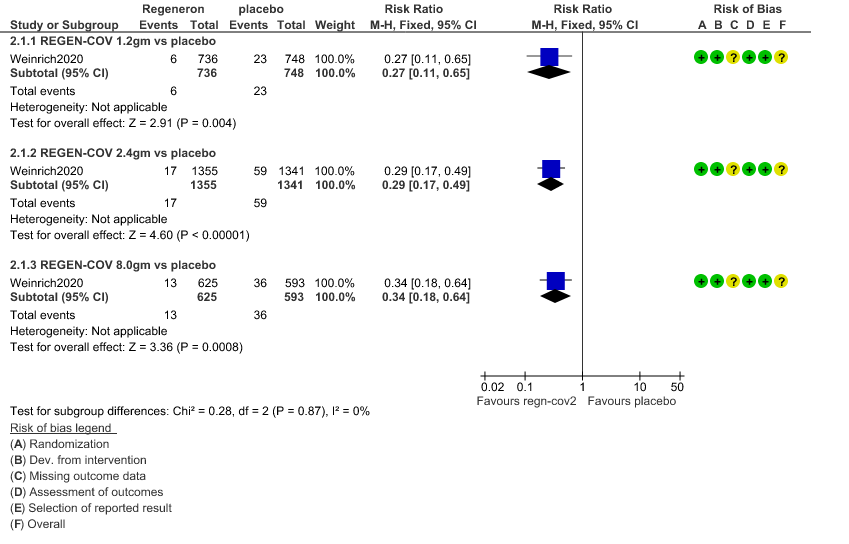
2. Progression to Invasive mechanical ventilation
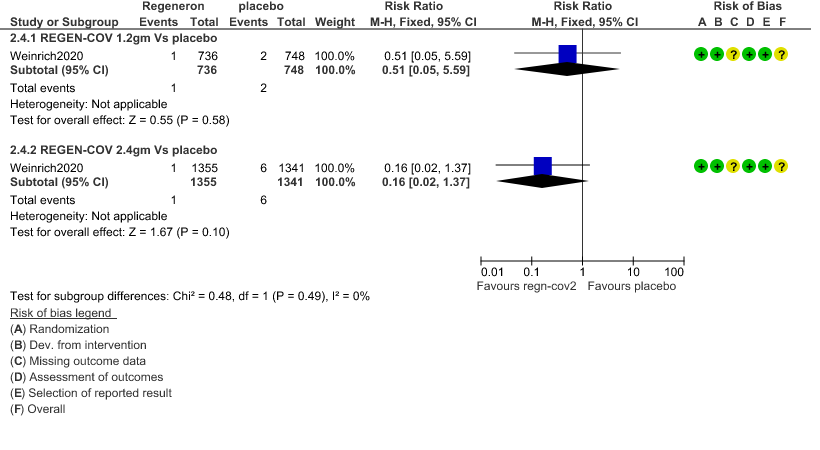
3. Admission to critical care/Intensive care unit
4. Duration of Hospital stay (Length of Stay)
5. Mortality
6. Adverse Events
a. Serious Adverse events
b. Infusion related adverse events
c. Adverse events leading to drug interruption