Explanations
a. Explanations Downgraded by one-level for risk of bias as study has some concerns
b. Downgraded by two levels for serious imprecision as the 95%CI is wide: 0.08 (0.00-1.40) and event rate is low.
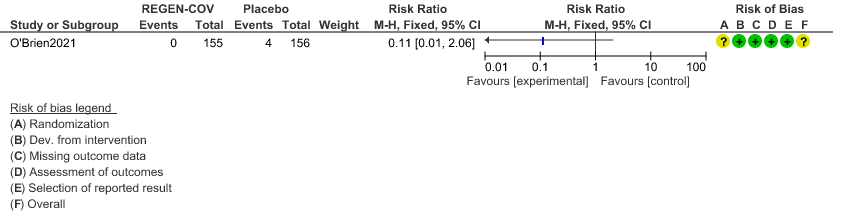
c. Downgraded by two levels for serious imprecision as the 95%CI is wide:0.11 (0.01-2.06) and event rate is low.
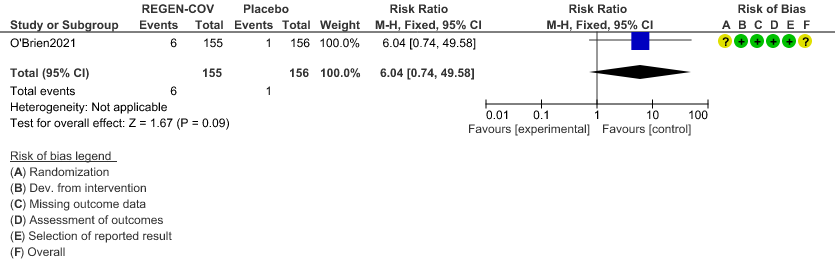
d. Downgraded by two levels for serious imprecision as the 95%CI is wide: 6.04(0.74-49.58) and event rate is low.
REGEN-COVT™ is a cocktail made up of two noncompeting, neutralizing human IgG1 antibodies that target the receptor binding domain of the SARS-CoV-2 spike protein, thereby preventing viral entry into human cells through the angiotensin-converting enzyme 2 (ACE2) receptor(4). The combination is manufactured by Regeneron pharmaceuticals. The drug was approved by the FDA for emergency use on 21 November 2020 in patients ≥12 yrs. of age, weighing at least 40 kilograms with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progressing to severe COVID-19. The authorized dosage is 1,200 mg of Casirivimab and 1,200 mg of Imdevimab administered together as a single intravenous (IV) infusion over 60min as soon as possible after positive viral test for SARS-CoV-2 and within 10 days of symptom onset. In this study however, this is used in a subcutaneous dose. The CDSCO in India, on 5 May 2021, granted an EUA to Roche (Genentech) and Regeneron for use of REGEN-C0VTM in the country. The announcement came in light of the second wave of the COVID-19 pandemic in India(5). This review aims to provide a summary of the available evidence from randomized clinical trials of REGEN-COVTM for treatment of COVID-19 which could guide clinicians and researchers regarding the appropriate use of this drug in the future.
We searched PubMed, Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language. We also reviewed reference lists of systematic reviews and included studies. We performed all searches up to 13th July 2021.
We searched the above databases and found 11 records. After removing duplicates and excluding those that did not match our PICO question, one randomized controlled trial was selected and included for the analysis.
We extracted data for the following outcomes, pre-defined by the Expert Working Group:
- Critical (primary for this review):
- Admission into hospital (all cause)
- Time to clinical recovery
- Progression to severe disease- requiring O2, non-invasive or invasive mechanical ventilation or ICU care
- Important (secondary):
- Time to viral clearance (negative PCR)
- Death
- Progression to symptoms
- Adverse events:
- All
- Serious
- Infusion-related adverse events (if reported, record rate of infusion used)
Two reviewers independently assessed eligibility of search results using the online Rayyan tool. Data extraction was performed by one reviewer, and checked by another, using a piloted data extraction tool on Microsoft Word. Risk of bias (RoB) was assessed using the Cochrane RoB v2.0 tool, with review by a second reviewer and compared with covidnma database. If there was a difference in more than one domain it was assessed by a third independent author. We used risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs) using Review Manager (RevMan) v5.4. We used GRADE methodology to make summary of findings tables on GRADEPro GDT.
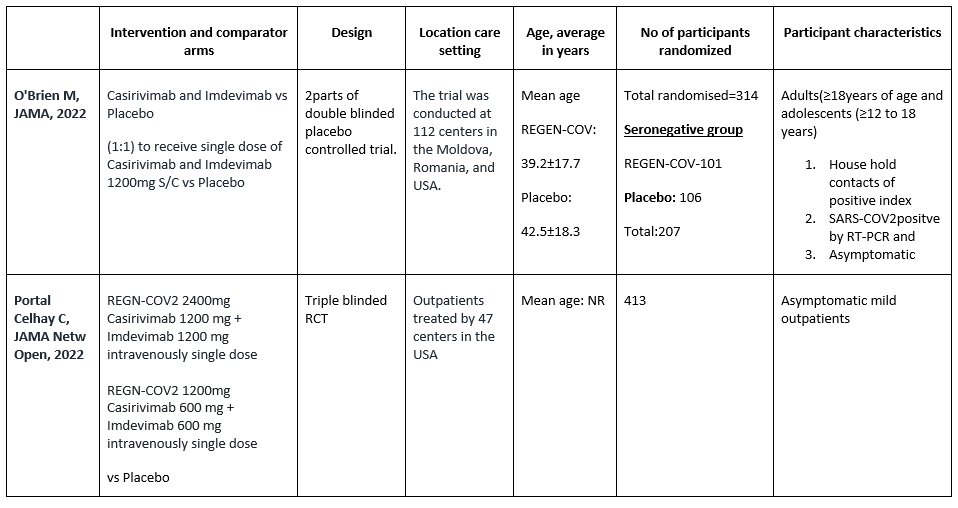
We found one RCT that matched the PICO question as pre-defined by the expert working group. This randomized trial included a total of 314 participants, all of whom were adults and adolescents ≥ 12 years of age and asymptomatic house hold contacts of index cases with SARS-COV2 virus infection. Study was performed at 112 sites in the United States, Romania, and Moldova.
The results are described below; characteristics of the trials are summarized in the Summary of characteristics of included trials table.
The following comparisons were investigated in the trial (we compared outcomes for arms randomized to Casirivimab and Imdevimab combination (REGN-COVTM) vs Placebo as pre specified in our PICO which was formulated by the expert working group.
Outcomes
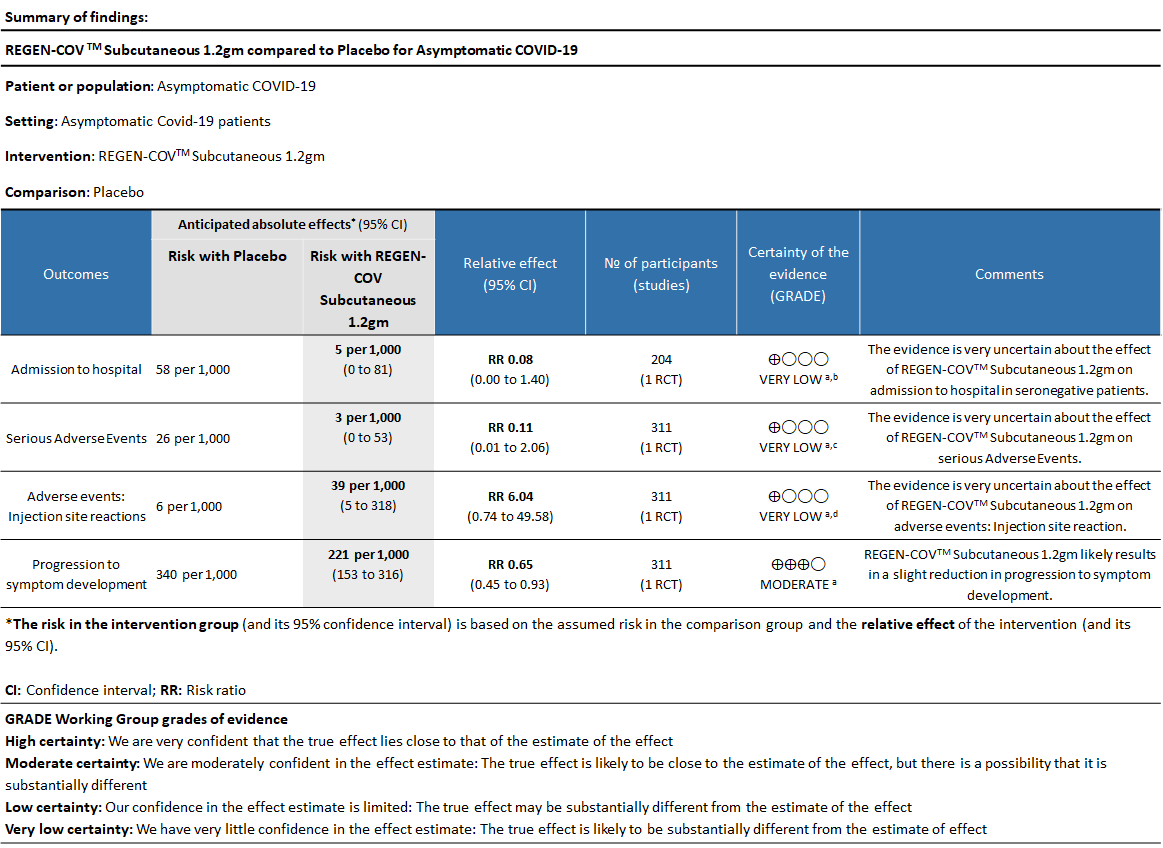
As presented in the ‘Summary of findings’ tables, the evidence is of very low certainty about the effect of Casirivimab and Imdevimab combination (REGEN-COVTM) 1.2 gm as a single dose subcutaneous injection on admission to hospital in seronegative patients and also on serious adverse events and injection site reactions.
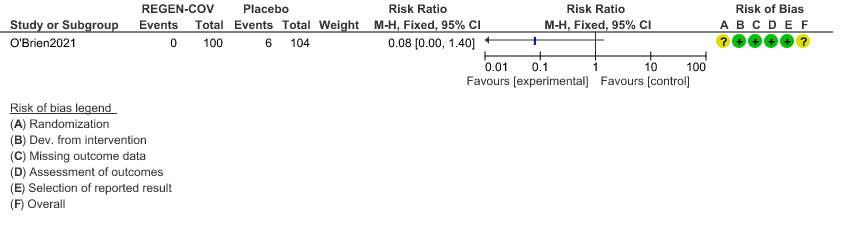
a. Admission to Hospital in Seronegative patients:
Very low certainty of evidence in 206 patients from 1 trial (3) found that the evidence is very uncertain about the effect of REGEN-COVTM subcutaneous 1.2gm injection on reduction of hospital admission in seronegative patients. (RR 1.08; 95 % CI 0.00-1.4)
b. Serious Adverse events:
Very low certainty evidence in 311 patients from 1 trial (3) revealed that the evidence is very uncertain about the effect of REGEN-COVTM Subcutaneous 1.2gm on serious Adverse Events (RR 0.11;95% CI 0.01-2.06)
c. Injection site reactions:
Very low certainty evidence in 311 patients from 1 trial revealed a very uncertain effect of REGEN-COVTM Subcutaneous 1.2gm on Injection site reactions (RR 6.04 ;95% CI 0.74 - 49.58)
d. Progression to symptoms
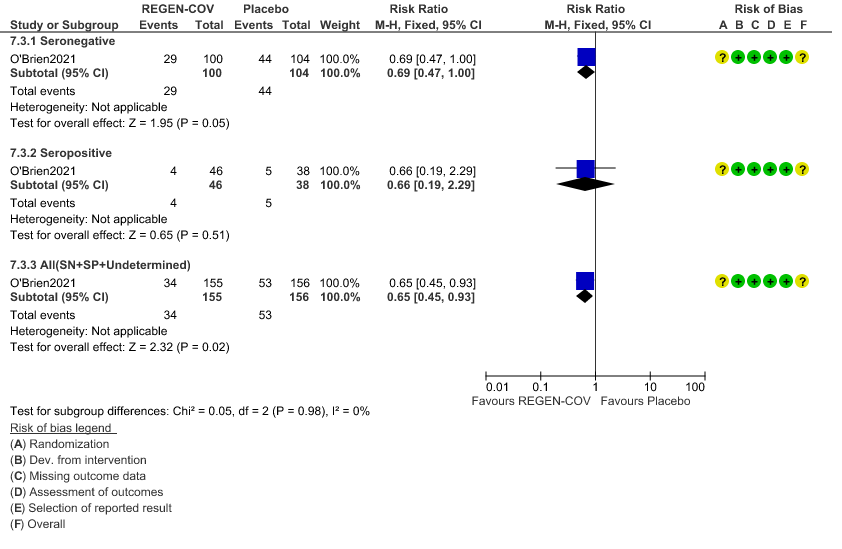
Moderate certainty evidence from 1 trial in 206 patients found that REGEN-COVTM probably reduces progression to symptoms RR 0.65; 95% CI 0.45-0.93).
Seronegative: Progression to symptom development was slightly reduced by REGEN-COV™ with a RR=0.69 (95% CI 0.47-1.00).
Seropositive: Progression to symptom development was not reduced by REGEN-COV™ with a RR=0.66 (95% CI 0.19-2.29).
Subgroup analysis
Disaggregated data could not be obtained for co-morbidities, different age groups, pregnancy, lactation, renal failure, liver failure were excluded from this trial. Also, no data were available separately for immunocompromised individuals or patients with 1 or more risk factors which had a high risk of progression to severe disease or death.
a. Admission to Hospital in Seronegative patients:
b. Serious Adverse events:
c. Injection site reactions:
d. Progression to symptom development