Baricitinib is a Janus kinase (JAK) inhibitor which has been used in Rheumatoid arthritis for many years as an anti-inflammatory agent. In COVID-19 infection it has been used as an anti-inflammatory agent and has also been thought to have potential antiviral effects by interfering with viral entry (1).
Baricitinib was found to reduce mortality and progression to invasive mechanical ventilation in hypoxic moderate, severe and critical COVID-19 infection with the number needed to prevent one death being approximately 25 across all the severity groups. We also found that Baricitinib reduced the time to clinical recovery and length of hospital stay, with a slight decrease in the progression to non-invasive ventilation (NIV) as well.
The risk of adverse events and serious adverse events are uncertain, but appeared to be low in the short term. However, it is important to mention that the long-term effects were not assessed in either of the randomized controlled trials (RCTs) available. However, there is considerable clinical experience with use of this drug in other clinical conditions (such as rheumatoid arthritis), where it has a well-defined and acceptable safety profile. At present there appears to be no data for the use of this drug in the non-hospitalized, mild COVID-19 infection. The safety and efficacy of its concomitant use with other immunomodulators like Tocilizumab is also unavailable at the moment. With the present data available it is recommended that Baricitinib should NOT be given along with Tocilizumab as it may lead to severe immunosuppression.
Taking the evidence into account, the group recommends for a conditional use in hypoxic patients with moderate to critical COVID-19 infection, if there is a rapid progression of respiratory requirements despite use of steroids.
Date of latest search: 21st July 2021
Date of completion & presentation to Expert Working Group: 29th July 2021
Date of planned review: 29th January 2022
Evidence synthesis team: Karthik G, Annabelle Kaunds, Sujith Chandy, Bhagteshwar Singh, Richard Kirubakaran and Priscilla Rupali.
Table 1: Baricitinib vs placebo in hospitalized patients with COVID-19
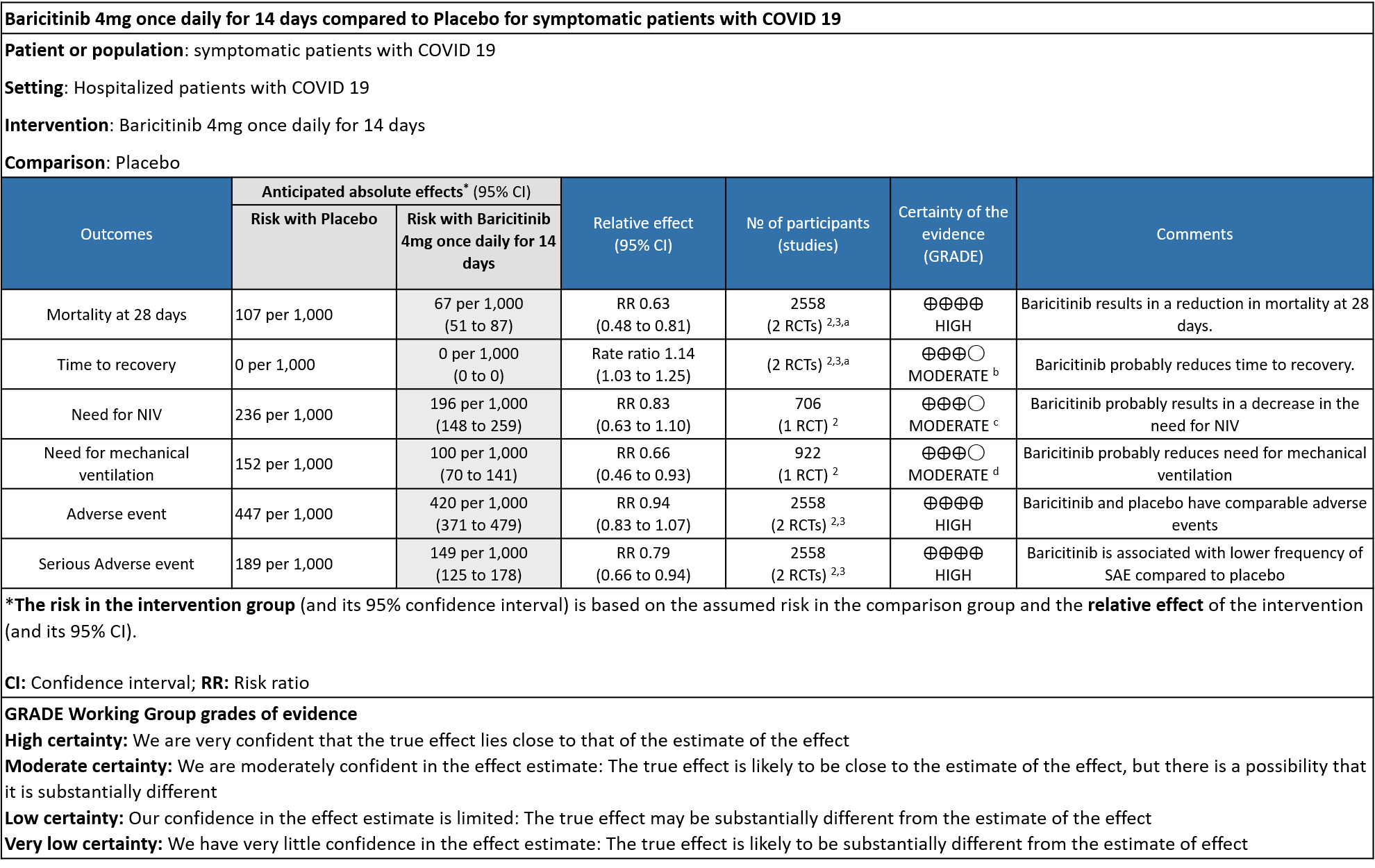
Explanations
a. Marconi et al (3): ≥18 years of age, hospitalized laboratory confirmed SARS-CoV-2 infection, had evidence of pneumonia or active, symptomatic COVID-19, and had ≥1 elevated inflammatory marker (C reactive protein, D-dimer, lactate dehydrogenase, ferritin); Kalil AC(2) et al: Male/non pregnant female >=18 years at the time of enrollment Illness of any duration, and at least one of the following: Radiographic infiltrates by imaging (chest x-ray, CT scan, etc.), SpO2 ≤ 94% on room air, requiring supplemental oxygen, requiring mechanical ventilation
b. Downgraded by one level for serious imprecision as the lower limit of 3% is clinically not significant.
c. Downgraded by one level for serious imprecision as the confidence interval is wide ranging from 0.63-1.10.
d. Downgraded by one level for serious imprecision as the event rate was low in each arm and OIS criteria not met.
References
- Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021 Mar 4;384(9):795–807.
-
Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial [published online ahead of print, 2021 Aug 31] [published correction appears in Lancet Respir Med. 2021 Sep 8;:]. Lancet Respir Med. 2021;S2213-2600(21)00331-3. doi:10.1016/S2213-2600(21)00331-3
Table 2: Baricitinib compared to placebo for hypoxic COVID-19 patients with moderate illness.
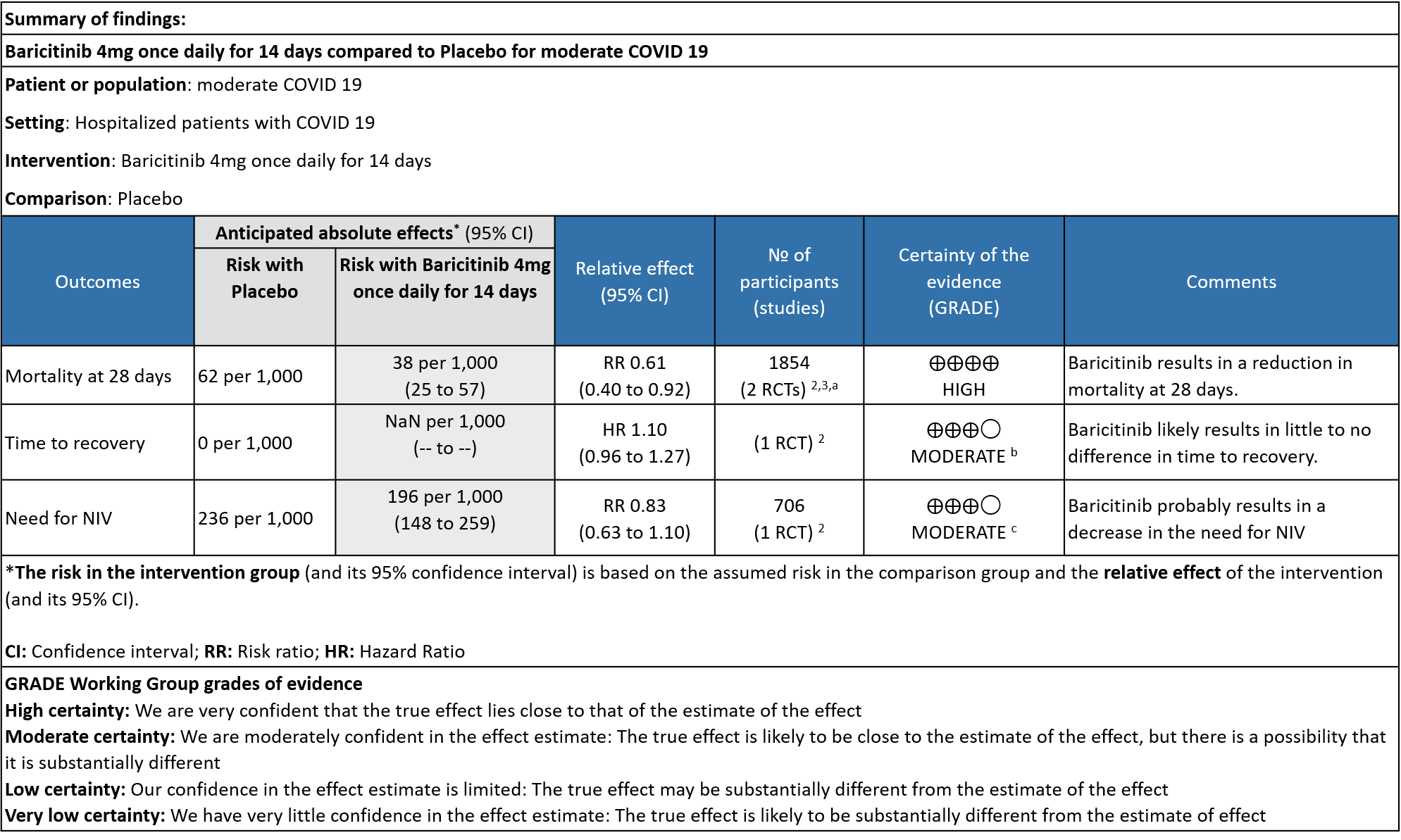
Explanations
a. Data was extracted from two studies [Marconi (3) and Kalil (2)et al] for this category -moderate severity.
b. Downgraded by one level for serious imprecision as the 95% CI for the risk ratio included no effect ranging from 0.96-1.27 and the optimal information size (OIS) criteria were not met.
c. Downgraded by one level for very serious imprecision as the 95% CI is wide, ranging from 0.63-1.10.
References
- Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021 Mar 4;384(9):795–807.
-
Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial [published online ahead of print, 2021 Aug 31] [published correction appears in Lancet Respir Med. 2021 Sep 8;:]. Lancet Respir Med. 2021;S2213-2600(21)00331-3. doi:10.1016/S2213-2600(21)00331-3
Table 3: Baricitinib compared to placebo for COVID-19 patients with severe to critical illness.
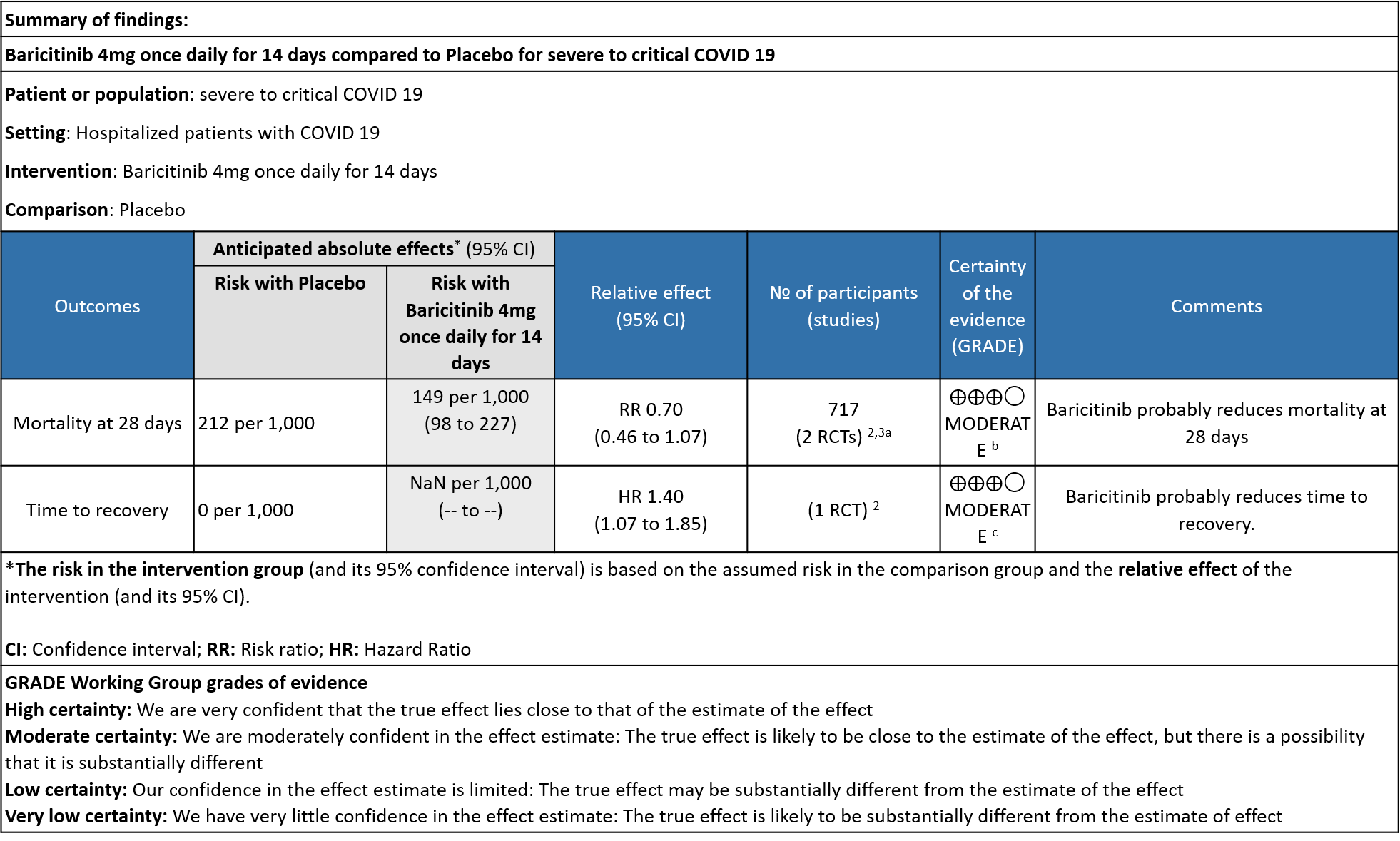
Explanations
a. Subgroup data from Marconi (3)and Kalil (2)et al, hypoxic hospitalized patients requiring NIV or high-flow oxygen devices (ordinal scale -6) and hypoxic hospitalized patients requiring mechanical ventilation or ECMO (ordinal scale-7) .
b. Downgraded by one level for serious imprecision as the 95% CI is wide, ranging from 0.46-1.07.
c. Downgrade by one level for serious indirectness as the effect was more in patients in ordinal scale 6 (Hospitalized patients requiring NIV or high-flow oxygen devices) than in ordinal scale 7 (hospitalized patients requiring mechanical ventilation or ECMO).
References
- Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021 Mar 4;384(9):795–807.
-
Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial [published online ahead of print, 2021 Aug 31] [published correction appears in Lancet Respir Med. 2021 Sep 8;:]. Lancet Respir Med. 2021;S2213-2600(21)00331-3. doi:10.1016/S2213-2600(21)00331-3
Baricitinib, an orally administered, selective inhibitor of Janus kinase (JAK) 1 and 2, inhibits the intracellular signaling pathway of cytokines known to be elevated in severe Covid-19, including interleukin-2, interleukin-6, interleukin-10, interferon-γ, and granulocyte–macrophage colony stimulating factor; acts against SARS-CoV-2 through the impairment of AP2-associated protein kinase 1 and the prevention of SARS-CoV-2 cellular entry and infectivity and improves lymphocyte counts in patients with Covid-19 (2). Emerging data suggests that disease severity in COVID-19 infection may be due in part to a dysregulated inflammatory response. It is postulated that mitigating the immune response and preventing a hyperinflammatory state may further improve clinical outcomes.
To identify all available systematic reviews pertaining to our PICO question, a systematic search of PubMed, covid-nma.org and the Epistemonikos databases was conducted. The search strategy was designed and validated by the Group’s information specialist. The search results were screened independently by two reviewers against the pre-defined criteria set out above, using Rayyan.
Seven systematic reviews were identified using databases, of which 2 were excluded as they were narrative reviews. For eligibility, 4 were identified and all 4 systematic reviews had the same study. For randomized controlled trials, 25 were identified using databases, of which 6 were removed as they were duplicates. Of the 19 RCTs screened, 2 were press releases and 15 were study protocols or trial registries and hence they were excluded. The remaining 2 RCTs were assessed for eligibility and included for analysis.
For the risk of bias, Cochrane ROB2 tool was used. For all outcomes, one review author performed the assessment, and cross-checked it against the assessment by another review author. If there was a difference in more than one domain, it was assessed by a third independent author.
Data was extracted for the following outcomes as per the pre-defined PICO question by the anti-inflammatory expert working group:
Primary:
- Overall mortality
- Time to clinical recovery
Secondary:
- Length of hospital stay
- Need for NIV/invasive mechanical ventilation
- Duration of invasive ventilation
- Duration of stay in critical care
Adverse events:
- All
- Serious
- Nosocomial/opportunistic infections – may be measured indirectly by need for antibacterial or antifungal agents
- Anemia
- Shingles
- Infusion-related adverse events
Data was entered into Review manager version 5.4 for meta-analysis. The results were entered into Grade ProGDT (online), to create the summary of findings (GRADE) table. We used risk ratios (RR) for dichotomous outcomes with 95% confidence intervals (CIs).
We found 2 randomized controlled trials that met our search criteria.

We also performed a subgroup analysis to assess efficacy of Baricitinib among the various levels of severity
1. Baricitinib compared to placebo in COVID-19
2. Baricitinib compared to placebo in moderate COVID-19
3. Baricitinib compared to placebo in severe to critical COVID-19
BARICITINIB COMPARED TO STANDARD OF CARE/PLACEBO FOR COVID-19:
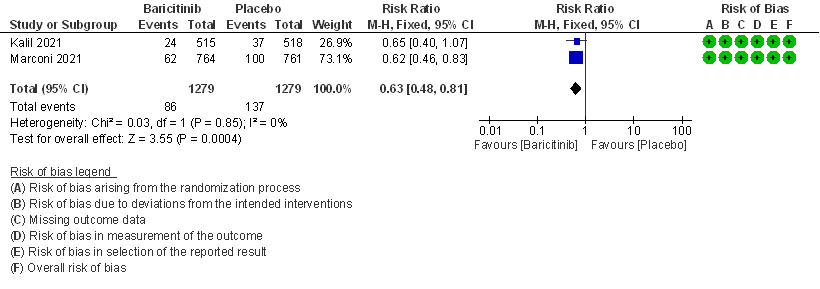
- All-cause mortality at 28 days: High certainty evidence from 2 RCTs (2,3) that included 2588 patients with COVID-19 disease indicated that Baricitinib reduces mortality at 28 days by 37% (RR=0.63; 95% CI 0.48-0.81). The number needed to treat (NNT) to is 25 patients to prevent 1 death with COVID-19.
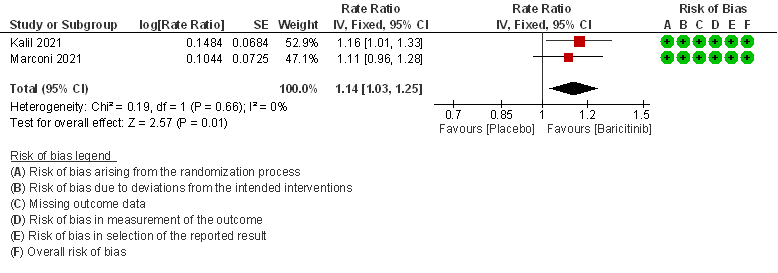
- Rate ratio for individuals recovered: Moderate certainty evidence from 2 RCTs (2,3) that included 2588 patients with COVID-19 disease indicates that Baricitinib probably reduces rate of recovery by 14% (95% CI 3% to 25%) as compared to placebo.
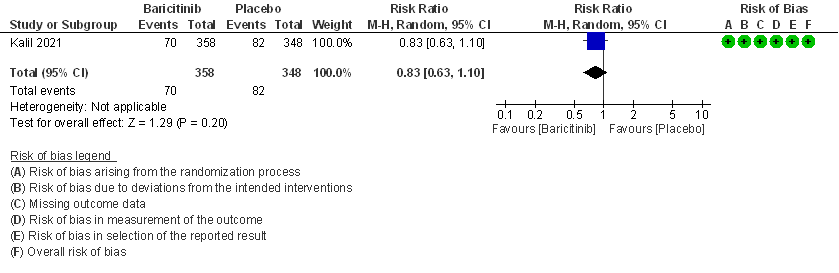
- Need for non-invasive ventilation: Moderate certainty evidence from 1 RCT (2) that included 706 patients with COVID-19 disease indicates that Baricitinib probably results in a reduction in need for NIV by 17% (RR =0.83; 95% CI 0.63 to 1.10) or increases it by 10% compared to placebo. Based on the data, we anticipate that for 1000 patients with COVID-19 infection given Baricitinib, there may be 40 fewer (from 88 fewer to 23 more) requiring non- invasive ventilation as compared to placebo.
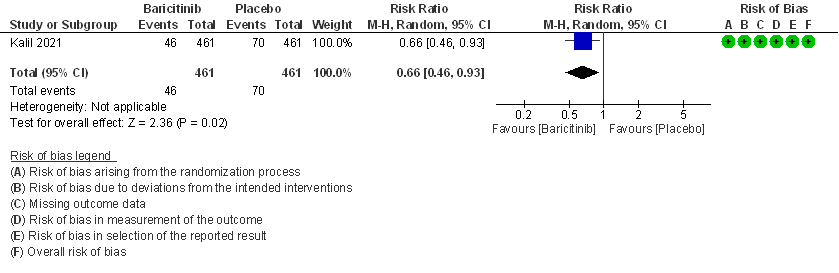
- Need for mechanical ventilation: Moderate certainty evidence from 1 RCT (2) that included 922 patients with COVID-19 disease indicates that Baricitinib probably results in a reduction in need for mechanical ventilation by 34% (RR= 0.66 95% CI 0.46 -0.93) compared to placebo. Based on the data, we anticipate that for 1000 patients with COVID-19 disease treated with Baricitinib there may be 52 fewer (from 11 to 82 fewer) requiring mechanical ventilation per 1000 people. The number needed to treat (NNT) to prevent 1 additional patient requiring mechanical ventilation is 20.
- Time to clinical recovery(days): This was lower in the Baricitinib group by about a day in both the studies – 10(9-11) in Baricitinib group vs 11(10-12) in placebo group [Ref Marconi et al(3)] and 7(6-8) in Baricitinib group vs 8(7-9) in placebo group [Ref Khalil et al(2)].
- Length of hospital stay(days): This was almost equal in both the groups– 12.9(0.4)days in Intervention group vs 13.7(0.40)days in control group [Ref Marconi et al (3)] and 8 (5-15)days in the intervention group vs 8(5-20)days in the control group.
- Duration of invasive mechanical ventilation (IMV) or ECMO: In those already on IMV or ECMO at baseline, the duration of IMV was 20 (9-28) days in Baricitinib vs 25(11-28) days in the control group. In those in whom IMV and ECMO was not present at baseline and was newly required during the course of the hospitalization it was 16 (8-28) days in the Baricitinib group vs 27(12-28) days in the control group.
- All adverse events: High certainty evidence from 2 RCTs that included 2558 patients with COVID-19 disease revealed that Baricitinib does not increase adverse events as compared to placebo RR=0.94 (95% CI 0.83-1.07).
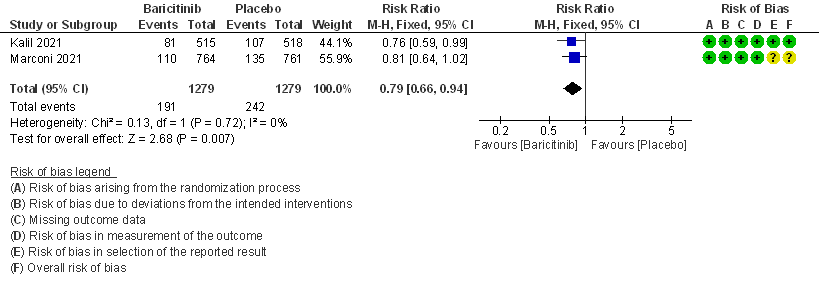
- Serious adverse events: High certainty evidence from 2 RCTs that included 2558 patients with COVID-19 disease revealed that Baricitinib reduced serious adverse events by 21% (95% CI 6% to 34%) compared to placebo.
BARICITINIB COMPARED TO PLACEBO IN MODERATE COVID-19 WITH HYPOXIA
- All-cause mortality: High certainty evidence in 2 RCTs that included 1854 patients with moderate COVID-19 disease indicated that Baricitinib reduces mortality at 28 days by 39% (RR=0.61; 95% CI 0.40-0.92). The number needed to treat (NNT) to prevent one death is 40 patients with moderate COVID-19.
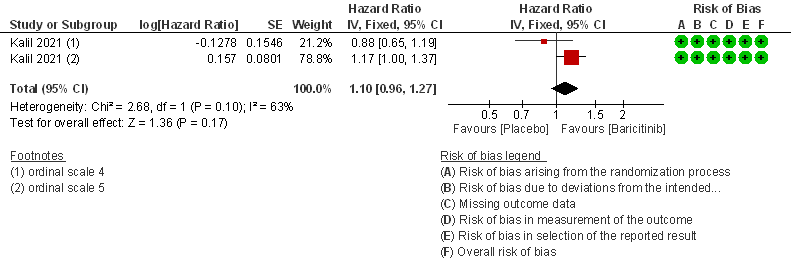
- Time to clinical Recovery: Moderate certainty evidence from 1 RCT that indicates that Baricitinib likely results in little to no difference in time to recovery as compared to placebo in moderate COVID- 19 (HR=1.10; 95% CI 0.96-1.27).
- Need for non-invasive ventilation: Moderate certainty evidence from 1 RCT that included 706 patients with moderate COVID-19 disease indicates that Baricitinib probably results in a reduction in need for NIV by 17% (RR=0.83; 95% CI 0.63-1.10) compared to placebo.
BARICITINIB COMPARED TO PLACEBO IN SEVERE TO CRITICAL COVID-19
- All-cause mortality: Moderate certainty evidence from 2 RCTs that included 717 patients with severe to critical COVID-19 disease indicated that Baricitinib probably results in a reduction in mortality at 28 days by 30% (RR= 0.70; 95% CI 0.46-1.07). Based on the data, we anticipate that for 1000 patients with severe to critical COVID-19 disease in the Baricitinib arm 63 fewer (from 114 fewer to 15 more) are likely to die. The number needed to treat (NNT) with Baricitinib to prevent 1 death is 17 patients with severe to critical COVID-19.
- Time to clinical recovery: Moderate certainty evidence from 1 RCT in 1033 patients indicated that Baricitinib likely results in little to no difference in time to recovery as compared to placebo in severe to critical COVID- 19; HR=1.40(1.07-1.85). The difference seemed to be more marked in those in ordinal scale 6 which is high flow nasal oxygen and non-invasive ventilation as compared to invasive mechanical ventilation which was ordinal scale 7.
The committee had a priori requested analyses of other important subgroups of patients including children, age > 50 years and those with co-morbidities, but unfortunately there was no data to address outcomes in these subgroups specifically. There was no data in either arm with regard to nosocomial or opportunistic infections, anemia, shingles. In addition, this was an oral drug rather than an injection and hence infusion related adverse effects were not considered.
Baricitinib compared to Placebo for COVID-19:
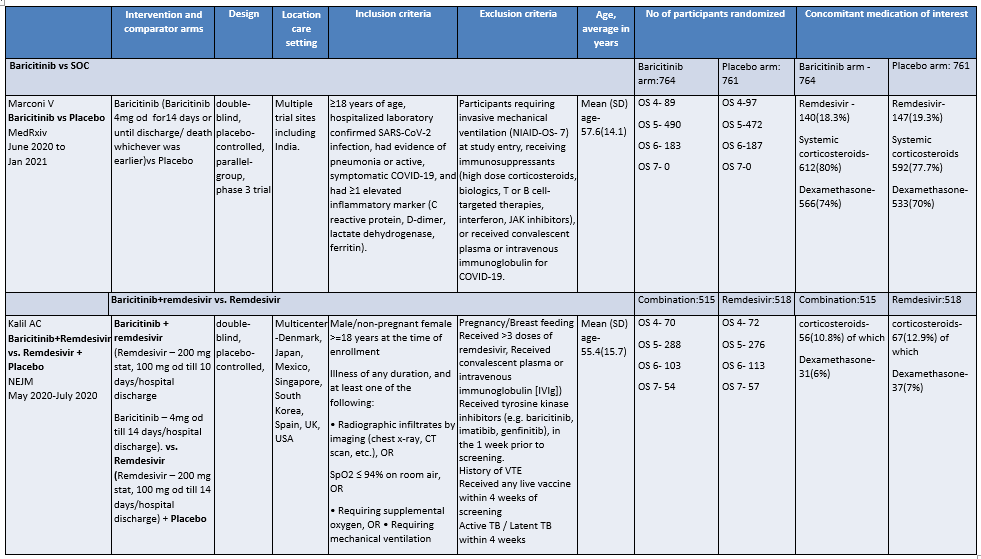
OS - ordinal scale, OS 4: Hospitalized, not requiring supplemental oxygen – requiring ongoing medical care; OS 5: Hospitalized, requiring supplemental oxygen; OS 6 : Hospitalized, on non-invasive ventilation or high flow oxygen devices; OS 7: Hospitalized, on mechanical ventilation or ECMO
I. BARICITINIB COMPARED TO PLACEBO IN PATIENTS WITH COVID 19.
1. All-cause Mortality at 28 days
 2. Rate ratio for individuals recovered with baricitinib as compared to placebo in patients with COVID 19.
2. Rate ratio for individuals recovered with baricitinib as compared to placebo in patients with COVID 19.
3. Need for Non-invasive ventilation in patients treated with baricitinib as compared to placebo in COVID 19 patients.
4. Need for invasive mechanical ventilation in patients treated with baricitinib as compared to placebo in COVID 19 patients.
5. Adverse events
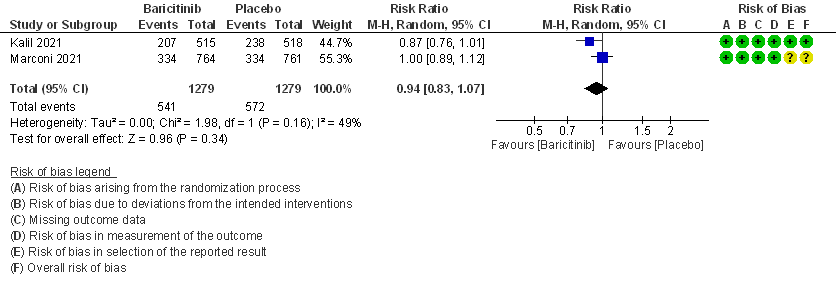
6. Serious Adverse events
II. BARICITINIB COMPARED TO PLACEBO IN COVID 19 PATIENTS WITH MODERATE ILLNESS.
1. All-cause mortality at 28 days.
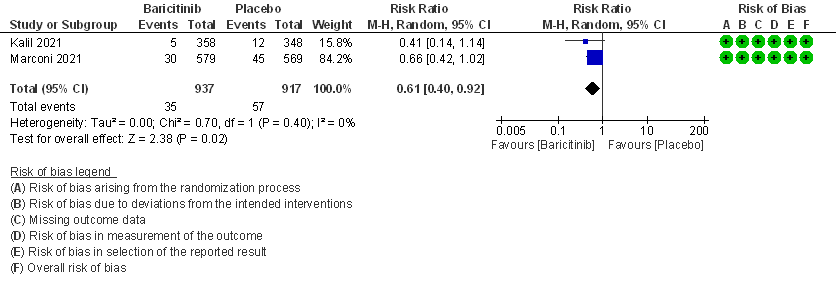
2. Rate ratio for individuals recovered with Baricitinib as compared to placebo in patients with moderate COVID 19.
3. Need for Non-invasive ventilation in patients treated with Baricitinib as compared to placebo in moderate COVID 19 patients.
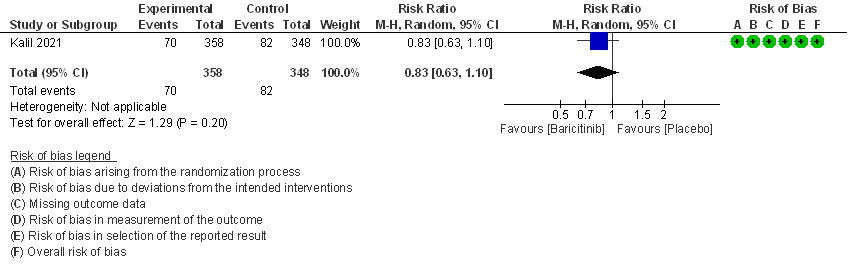
III. BARICITINIB COMPARED TO PLACEBO IN COVID 19 PATIENTS WITH SEVERE ILLNESS.
1. All-cause mortality at 28 days.
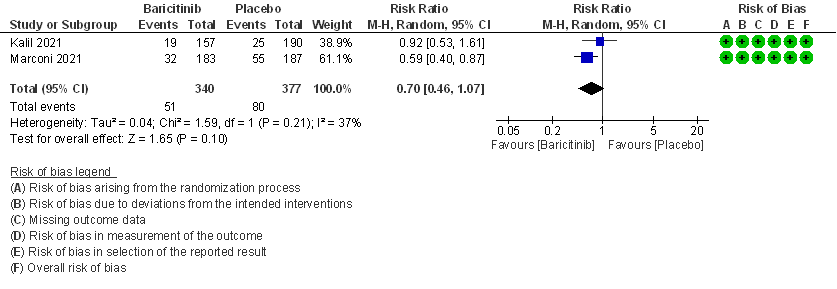
2. Rate ratio for individuals recovered with baricitinib as compared to placebo in COVID 19 patients with severe illness.
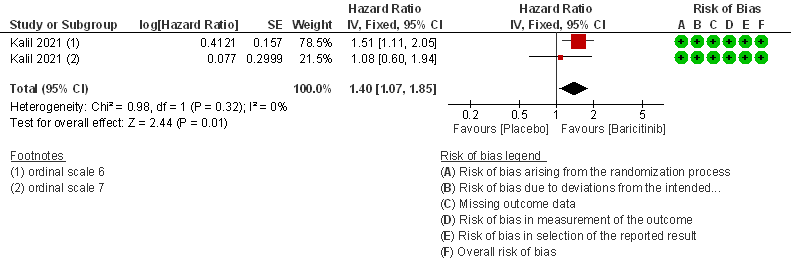
The Anti-inflammatory Expert Working Group met on 29th July 2021 to consider Baricitinib as a treatment for COVID-19. Conflict of interest declarations were reviewed by the Steering Committee; none were found to be relevant to Baricitinib.
A summary and then more detailed explanations of the Expert Working Group's judgements follow.
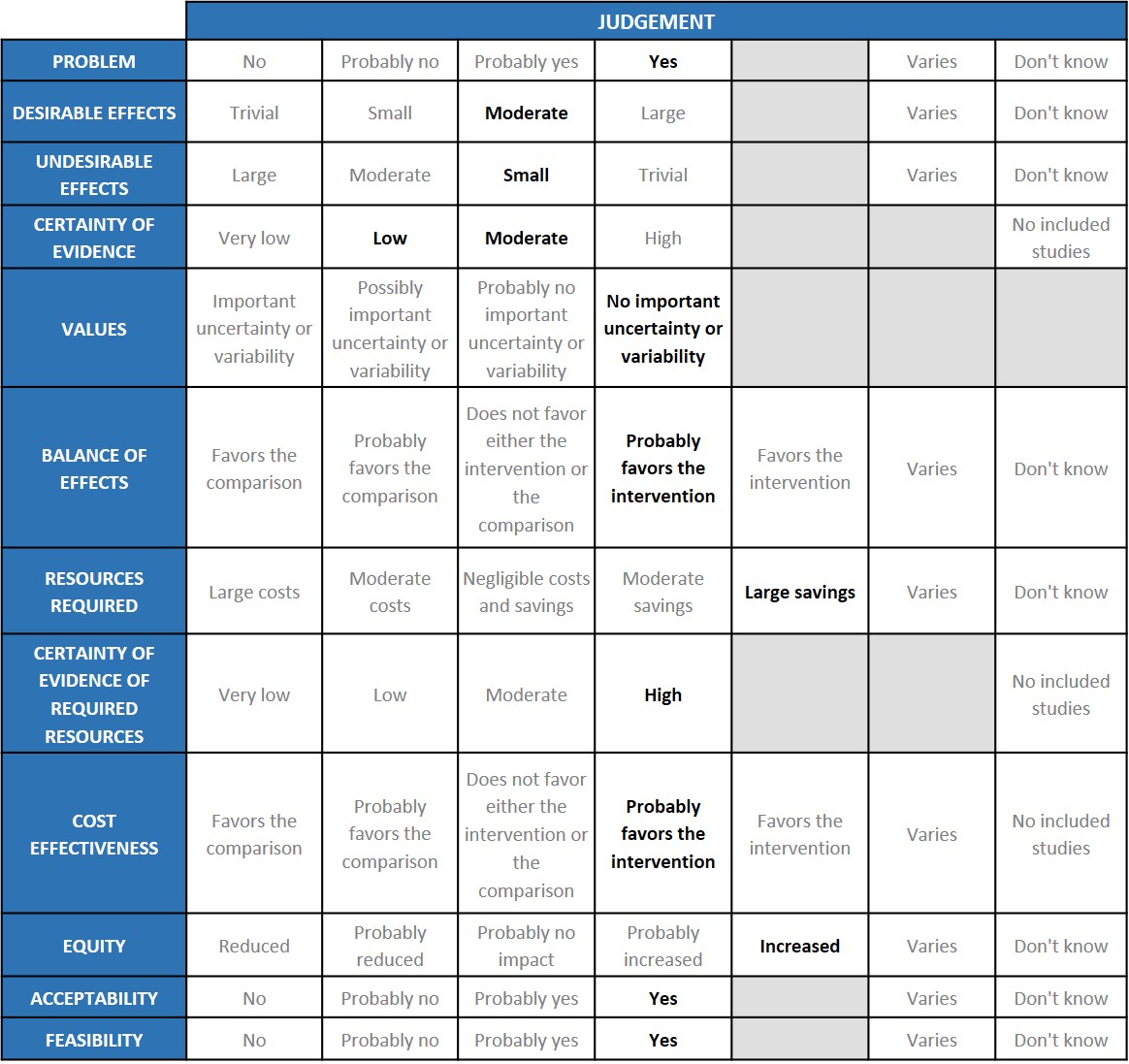
Problem
The COVID-19 pandemic in India with more than 30 million cases and over 0.39 million deaths has significantly impacted and stressed the health structure of the country. With a shortage of intensive care unit beds, oxygen and trained personnel the country is facing a major health crisis. Any intervention which is cheap, easily available and reduces mortality, progression to invasive ventilation and increases clinical recovery will be a major boon.
Desirable effects
The group agreed that the evidence suggests that Baricitinib has a clinically significant effect on mortality and progression to mechanical ventilation overall and across all severity sub groups especially the moderate and severe categories and also reduced time to clinical recovery and length of hospital stay. The group also noted that though the confidence intervals of the point estimates crossed the line of no difference the signal was towards a beneficial effect in the Baricitinib group.
Undesirable effects
The group noted that the pooled data suggested that patients did not experience major adverse events, and in fact serious adverse effects which may be related to either drug or disease were less in the Baricitinib group. Since it is an immunosuppressive drug, nosocomial infections may be higher in our population and not necessarily reflected in the trials which have been done in high income countries. Increased cases of Mucormycosis were noted in the second wave of the epidemic and immune-suppressants especially steroids were noted to be an important risk factor. Considering this fact caution should be exercised when prescribing Baricitinib. JAK inhibitors especially Baricitinib have been used in Rheumatoid arthritis for years. Though Herpes zoster has often been reported, other secondary infections are uncommon. There has been extensive experience in Rheumatoid arthritis where it has been used for years.
Certainty of evidence
Using GRADE methodology, the evidence synthesis team rated Certainty of evidence was found to be moderate for desirable effects like mortality, need for NIV and mechanical ventilation, low for time to recovery and serious adverse events, very low for adverse events in non-severe patients. Similarly, certainty of evidence was moderate for severe to critical COVID patients across the critical outcome of mortality and important outcome of clinical recovery. The group agreed with these judgements and rated the overall certainty of evidence as moderate.
Values
The EWG felt that all the outcomes including those of admission to hospital, mortality and progression to mechanical ventilation were expressed variably in the studies. However, there is no important uncertainty or variability on how people would value the main outcomes.
Balance of effects
The expert working group felt that the balance of effects probably favored the intervention for across all severity subgroups. The trials that were evaluated both showed Baricitinib was beneficial. Though the study -Khalil et al was older it did reveal that when compared to steroids it fared as well as steroids if not better. However, it did not reflect the practice today where all hypoxic patients are automatically given steroids as standard of care. The subsequent study Marconi et al showed that Baricitinib was given in 70% of cases suggesting that Baricitinib was given in addition to steroids. However, it is a slower acting drug and possibly needs to be given earlier in the disease when rapid worsening is expected.
Resources required
The group felt that this is a very cheap drug (generic versions are available) and costs only Rs 420 for 14 days. The group discussed and concluded that this was an efficacious drug proven beneficial and as compared to other monoclonal antibodies available in the market with anti-inflammatory activity, it was much cheaper and would result is large savings. The group includes clinicians in different types of Indian hospitals who have a good idea of drug and hospitalization costs.
Certainty of evidence of required resources
The group felt that the cost of the drug varied, with generic options varying cost from Rs 420 – Rs 22,000 for 14 days. No studies reporting this were reviewed by the group but the clinicians in the group were aware of the cost and hence felt that there was high certainty of evidence for required resources to implement this intervention.
Cost effectiveness
The group discussed that even though there is mortality reduction according to the evidence in moderate and sever subgroups, there is no evidence of benefit in critical category of patients because Marconi excluded ventilated patients. The group acknowledged the benefit and felt it should be used in hypoxic patients where steroids are used and there is evidence of systemic inflammation. So, the group favored the intervention for moderate and severe categories and was a cost-effective alternative.
Equity
At this point in time this intervention would probably increase equity as the costs are low and there is reduction in mortality and progression to invasive mechanical ventilation.
Acceptability
The group felt that this intervention is likely to have wide acceptance by all the relevant stakeholders (policy-makers, patients and clinicians) as it is an oral drug that is probably quite safe. Taking evidence and cost into account a well informed clinician would be very likely to use it.
Feasibility
This is a feasible intervention if found efficacious as it is easy to deliver and available easily over the counter in the country.
The recommendations are based on 2 large multi-center placebo controlled randomized trials done across several countries (including centers in India). It has been shown to reduce mortality and reduce time to recovery in patients with moderate to severe COVID (ordinal scores of 5 and 6), especially patients requiring high flow oxygen or non-invasive ventilation. The result was not so marked in those who were mechanically ventilated (ordinal scale 7). Given the availability of generic Baricitinib in India and the low cost of the generic drug, it seems to be a very feasible and cost-effective intervention in India (and also in other low to middle income countries). The overall evidence favors its use when combined with Remdesivir or steroids at 4mg once daily dose for 14 days or till discharge/death for moderate to severe COVID. There seems to be an additive effect when the drug is added to either of these agents. Baricitinib has been in use for rheumatoid arthritis for several years and therefore, many clinicians are familiar with the drug. The side effects of Baricitinib are easily manageable and the 2 large randomized trials in COVID have not raised any new safety concerns. Also, administration of the drug is only till hospital discharge or till 14 days (whichever is earlier) and hence the duration of therapy is short. Major increase in infections does not seem to have been a problem in the trials. However, in the Indian setting where infections are inherently more common one would have to wait and see for a delayed increase/reactivation of problems like TB and fungal diseases. Given the potential of the drug to have a steroid - sparing effect in the treatment of moderate to severe COVID, the drug may be of particular importance in diabetics and in patients with pre-existing or co-existing fungal or bacterial infections. When needed, steroids may be added to patients receiving Baricitinib. Currently, (when needed) we would consider giving steroids in the same dose as in the RECOVERY trial. However, it remains to be studied whether a lower dose of steroids would be sufficient in such situations.
Its action is indirect by modifying cytokine release and therefore effects are likely to take a few days. In more severe disease when rapid response is necessary it is probably prudent to consider a parenteral preparation like Tocilizumab.
Since the drug acts at the level of JAKs, it seems likely that the drug will remain effective against the delta and the delta-plus variants. However, this is something which has not been formally evaluated and we will need to wait further data to make any conclusive statements on the efficacy of Baricitinib against the variants of concern.
The group carefully considered a potential subgroup effect across patients with different levels of oxygen and ventilation requirement (i.e. measures of severity), early administration and utility of Baricitinib.
Non-severe patients: Studies included patients with no oxygen requirements which could be extrapolated to a non-severe group; however, there was no mortality benefit noted and the overall certainty of evidence was found to be very low. Hence at this point Baricitinib is not recommended for this group of patients.
Severe patients: Baricitinib benefits hypoxic patients with moderate and severe illness. Moderate certainty of evidence was found in critical outcomes of reduction of mortality and progression to invasive mechanical ventilation. One study excluded those who were invasively ventilated and hence evidence is less certain in this group. However, there is a clear signal towards benefit and hence it is likely that it will work in this group of patients. The NNT was 16 in severe to critical group and 40 in the moderate group.
Baricitinib appears to be an attractive intervention owing to the incremental benefit it provides over steroids in COVID-19 patients with hypoxia with no increase in side effects. Hence it is likely to be used upfront along with steroids in these patients. While the available trials do not signal serious adverse effects and some of the patients were from India, there is a need to monitor safety with regards to secondary infections especially when patients in our setting may have received steroids prior to hospitalization or may get higher doses than recommended. Previous literature from longer term use has reported Varicella Zoster as a secondary infection, however with this dose and duration, it seems unlikely that the incidence would remain the same. The risk of mucormycosis needs to be particularly monitored. The risk of infections in patients who are later transitioned from Baricitinib to tocilizumab needs to be additionally evaluated. Another side effect that warrants monitoring is the risk of thromboembolism (FDA alert). The group will also review new efficacy data about the drug from RCTs worldwide as well as observational data in the Indian setting to update its recommendation.
The use of Baricitinib based on currently available evidence is limited to hypoxic patients who are moderate or severely ill. Areas of equipoise include
1. Correct timing of initiation of Baricitinib (based on oxygen requirement of inflammatory markers) after initiation of steroids
2. Long term adverse effects, when given with steroids in the Indian setting.
3. Further studies should also focus on its indication relative to Tocilizumab
4. Could Baricitinib replace steroids in patients in whom they are relatively contraindicated e.g., uncontrolled diabetics or diabetic ketoacidosis?
5. How could we utilize biomarkers to optimize Baricitinib use?
- Arthur Y Kim, Rajesh T Gandhi. Baricitinib. COVID-19 management in hospitalized adults. In: UpToDate, Martin S Hirsch (Ed), UpToDate, Waltham, MA. (Accessed on 26 August, 2021.)
- Kalil AC, Patterson TF, Mehta AK et al for ACTT-2 Study Group Members. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021;384(9):795. Epub 2020 Dec 11.
-
Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial [published online ahead of print, 2021 Aug 31] [published correction appears in Lancet Respir Med. 2021 Sep 8;:]. Lancet Respir Med. 2021;S2213-2600(21)00331-3. doi:10.1016/S2213-2600(21)00331-3
Covid Management Guidelines India Group – Anti-inflammatory and Antibody working Group – Baricitinib. Covid Guidelines India; Published online on September 17th, 2021; URL: https://indiacovidguidelines.org/baricitinib/ (accessed ).
