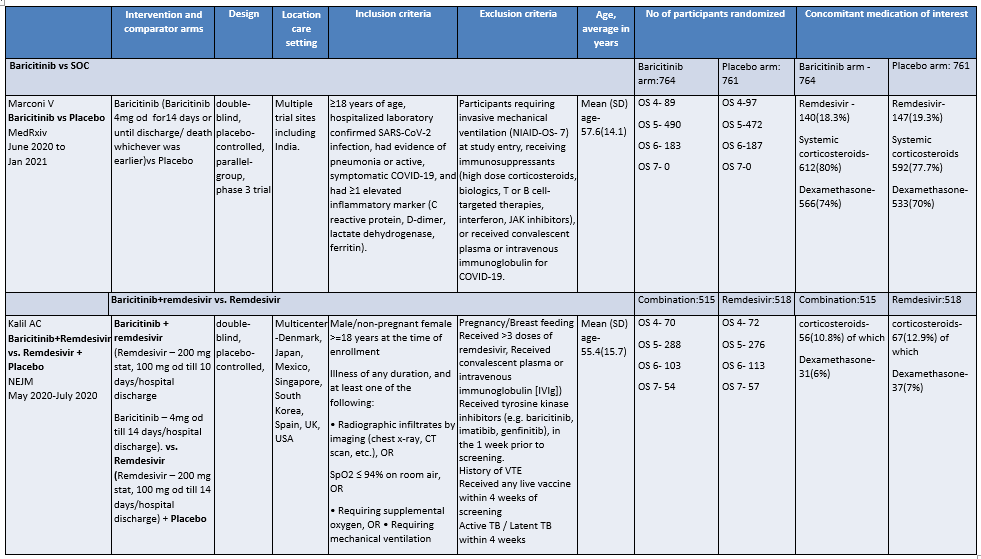
Table 1: Baricitinib vs placebo in hospitalized patients with COVID-19
Explanations
a. Marconi et al (3): ≥18 years of age, hospitalized laboratory confirmed SARS-CoV-2 infection, had evidence of pneumonia or active, symptomatic COVID-19, and had ≥1 elevated inflammatory marker (C reactive protein, D-dimer, lactate dehydrogenase, ferritin); Kalil AC(2) et al: Male/non pregnant female >=18 years at the time of enrollment Illness of any duration, and at least one of the following: Radiographic infiltrates by imaging (chest x-ray, CT scan, etc.), SpO2 ≤ 94% on room air, requiring supplemental oxygen, requiring mechanical ventilation
b. Downgraded by one level for serious imprecision as the lower limit of 3% is clinically not significant.
c. Downgraded by one level for serious imprecision as the confidence interval is wide ranging from 0.63-1.10.
d. Downgraded by one level for serious imprecision as the event rate was low in each arm and OIS criteria not met.
References
- Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021 Mar 4;384(9):795–807.
-
Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial [published online ahead of print, 2021 Aug 31] [published correction appears in Lancet Respir Med. 2021 Sep 8;:]. Lancet Respir Med. 2021;S2213-2600(21)00331-3. doi:10.1016/S2213-2600(21)00331-3
Table 2: Baricitinib compared to placebo for hypoxic COVID-19 patients with moderate illness.
Explanations
a. Data was extracted from two studies [Marconi (3) and Kalil (2)et al] for this category -moderate severity.
b. Downgraded by one level for serious imprecision as the 95% CI for the risk ratio included no effect ranging from 0.96-1.27 and the optimal information size (OIS) criteria were not met.
c. Downgraded by one level for very serious imprecision as the 95% CI is wide, ranging from 0.63-1.10.
References
- Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021 Mar 4;384(9):795–807.
-
Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial [published online ahead of print, 2021 Aug 31] [published correction appears in Lancet Respir Med. 2021 Sep 8;:]. Lancet Respir Med. 2021;S2213-2600(21)00331-3. doi:10.1016/S2213-2600(21)00331-3
Table 3: Baricitinib compared to placebo for COVID-19 patients with severe to critical illness.
Explanations
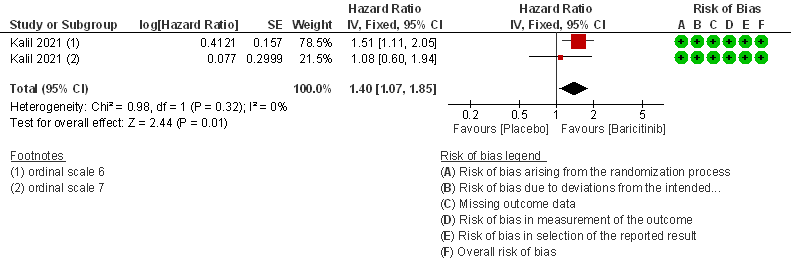
a. Subgroup data from Marconi (3)and Kalil (2)et al, hypoxic hospitalized patients requiring NIV or high-flow oxygen devices (ordinal scale -6) and hypoxic hospitalized patients requiring mechanical ventilation or ECMO (ordinal scale-7) .
b. Downgraded by one level for serious imprecision as the 95% CI is wide, ranging from 0.46-1.07.
c. Downgrade by one level for serious indirectness as the effect was more in patients in ordinal scale 6 (Hospitalized patients requiring NIV or high-flow oxygen devices) than in ordinal scale 7 (hospitalized patients requiring mechanical ventilation or ECMO).
References
- Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021 Mar 4;384(9):795–807.
-
Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial [published online ahead of print, 2021 Aug 31] [published correction appears in Lancet Respir Med. 2021 Sep 8;:]. Lancet Respir Med. 2021;S2213-2600(21)00331-3. doi:10.1016/S2213-2600(21)00331-3
Baricitinib, an orally administered, selective inhibitor of Janus kinase (JAK) 1 and 2, inhibits the intracellular signaling pathway of cytokines known to be elevated in severe Covid-19, including interleukin-2, interleukin-6, interleukin-10, interferon-γ, and granulocyte–macrophage colony stimulating factor; acts against SARS-CoV-2 through the impairment of AP2-associated protein kinase 1 and the prevention of SARS-CoV-2 cellular entry and infectivity and improves lymphocyte counts in patients with Covid-19 (2). Emerging data suggests that disease severity in COVID-19 infection may be due in part to a dysregulated inflammatory response. It is postulated that mitigating the immune response and preventing a hyperinflammatory state may further improve clinical outcomes.
To identify all available systematic reviews pertaining to our PICO question, a systematic search of PubMed, covid-nma.org and the Epistemonikos databases was conducted. The search strategy was designed and validated by the Group’s information specialist. The search results were screened independently by two reviewers against the pre-defined criteria set out above, using Rayyan.
Seven systematic reviews were identified using databases, of which 2 were excluded as they were narrative reviews. For eligibility, 4 were identified and all 4 systematic reviews had the same study. For randomized controlled trials, 25 were identified using databases, of which 6 were removed as they were duplicates. Of the 19 RCTs screened, 2 were press releases and 15 were study protocols or trial registries and hence they were excluded. The remaining 2 RCTs were assessed for eligibility and included for analysis.
For the risk of bias, Cochrane ROB2 tool was used. For all outcomes, one review author performed the assessment, and cross-checked it against the assessment by another review author. If there was a difference in more than one domain, it was assessed by a third independent author.
Data was extracted for the following outcomes as per the pre-defined PICO question by the anti-inflammatory expert working group:
Primary:
- Overall mortality
- Time to clinical recovery
Secondary:
- Length of hospital stay
- Need for NIV/invasive mechanical ventilation
- Duration of invasive ventilation
- Duration of stay in critical care
Adverse events:
- All
- Serious
- Nosocomial/opportunistic infections – may be measured indirectly by need for antibacterial or antifungal agents
- Anemia
- Shingles
- Infusion-related adverse events
Data was entered into Review manager version 5.4 for meta-analysis. The results were entered into Grade ProGDT (online), to create the summary of findings (GRADE) table. We used risk ratios (RR) for dichotomous outcomes with 95% confidence intervals (CIs).
We found 2 randomized controlled trials that met our search criteria.

We also performed a subgroup analysis to assess efficacy of Baricitinib among the various levels of severity
1. Baricitinib compared to placebo in COVID-19
2. Baricitinib compared to placebo in moderate COVID-19
3. Baricitinib compared to placebo in severe to critical COVID-19
BARICITINIB COMPARED TO STANDARD OF CARE/PLACEBO FOR COVID-19:
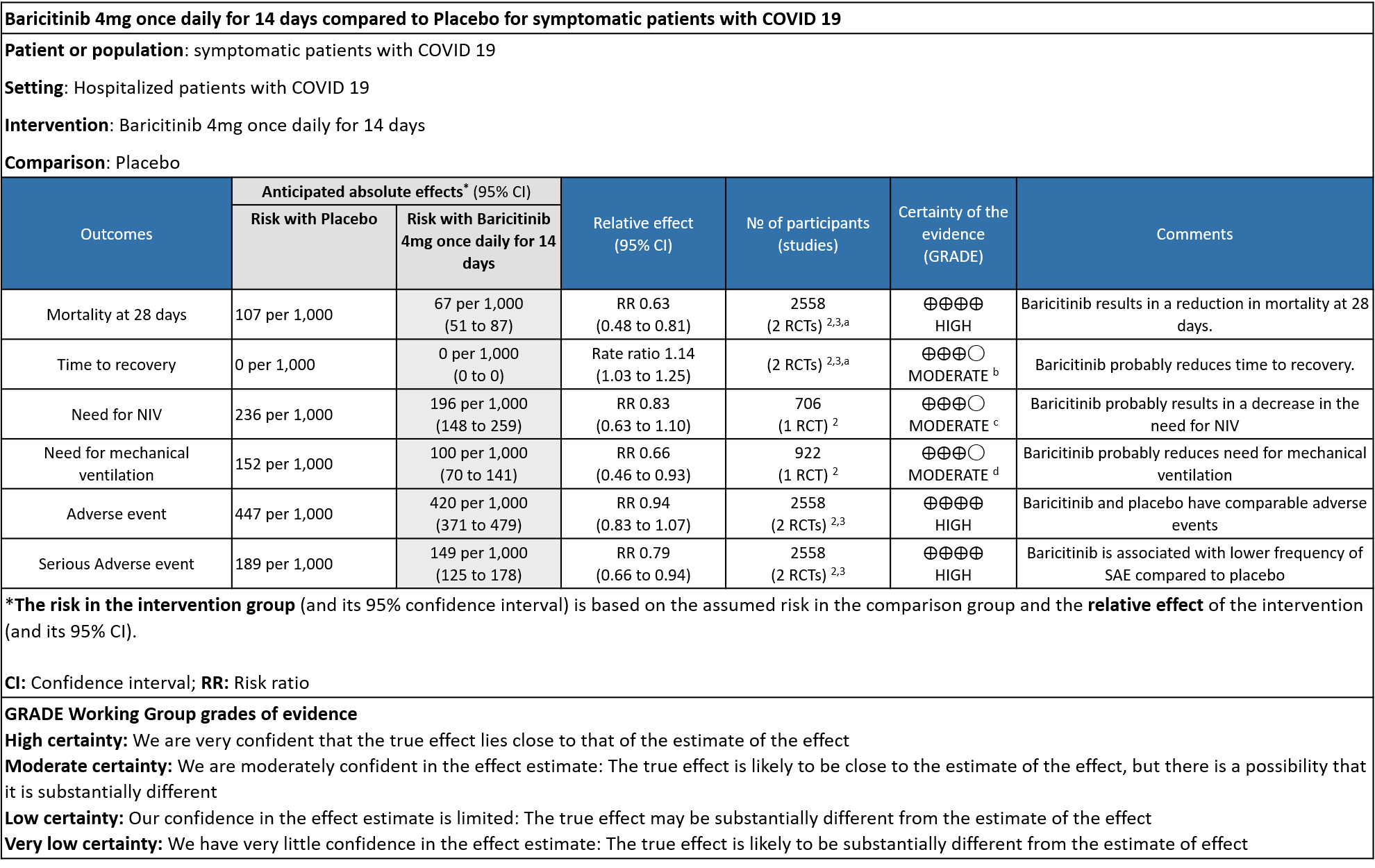
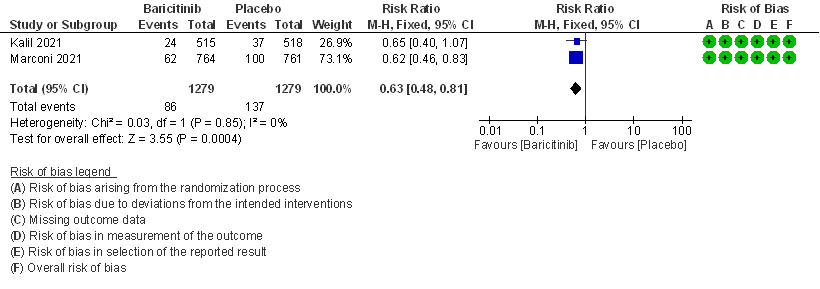
- All-cause mortality at 28 days: High certainty evidence from 2 RCTs (2,3) that included 2588 patients with COVID-19 disease indicated that Baricitinib reduces mortality at 28 days by 37% (RR=0.63; 95% CI 0.48-0.81). The number needed to treat (NNT) to is 25 patients to prevent 1 death with COVID-19.
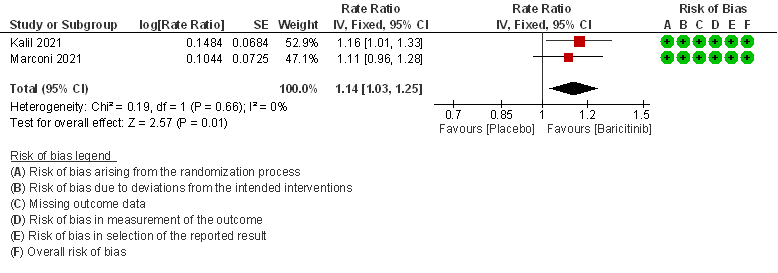
- Rate ratio for individuals recovered: Moderate certainty evidence from 2 RCTs (2,3) that included 2588 patients with COVID-19 disease indicates that Baricitinib probably reduces rate of recovery by 14% (95% CI 3% to 25%) as compared to placebo.
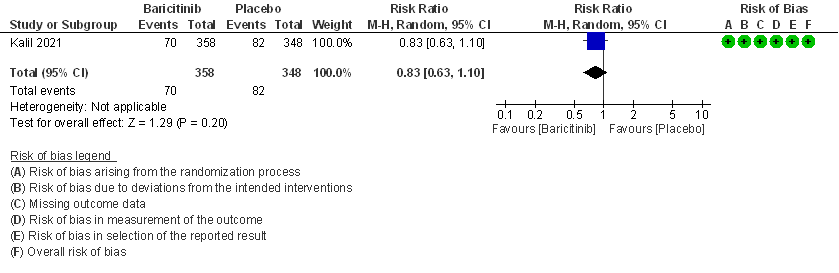
- Need for non-invasive ventilation: Moderate certainty evidence from 1 RCT (2) that included 706 patients with COVID-19 disease indicates that Baricitinib probably results in a reduction in need for NIV by 17% (RR =0.83; 95% CI 0.63 to 1.10) or increases it by 10% compared to placebo. Based on the data, we anticipate that for 1000 patients with COVID-19 infection given Baricitinib, there may be 40 fewer (from 88 fewer to 23 more) requiring non- invasive ventilation as compared to placebo.
- Need for mechanical ventilation: Moderate certainty evidence from 1 RCT (2) that included 922 patients with COVID-19 disease indicates that Baricitinib probably results in a reduction in need for mechanical ventilation by 34% (RR= 0.66 95% CI 0.46 -0.93) compared to placebo. Based on the data, we anticipate that for 1000 patients with COVID-19 disease treated with Baricitinib there may be 52 fewer (from 11 to 82 fewer) requiring mechanical ventilation per 1000 people. The number needed to treat (NNT) to prevent 1 additional patient requiring mechanical ventilation is 20.
- Time to clinical recovery(days): This was lower in the Baricitinib group by about a day in both the studies – 10(9-11) in Baricitinib group vs 11(10-12) in placebo group [Ref Marconi et al(3)] and 7(6-8) in Baricitinib group vs 8(7-9) in placebo group [Ref Khalil et al(2)].
- Length of hospital stay(days): This was almost equal in both the groups– 12.9(0.4)days in Intervention group vs 13.7(0.40)days in control group [Ref Marconi et al (3)] and 8 (5-15)days in the intervention group vs 8(5-20)days in the control group.
- Duration of invasive mechanical ventilation (IMV) or ECMO: In those already on IMV or ECMO at baseline, the duration of IMV was 20 (9-28) days in Baricitinib vs 25(11-28) days in the control group. In those in whom IMV and ECMO was not present at baseline and was newly required during the course of the hospitalization it was 16 (8-28) days in the Baricitinib group vs 27(12-28) days in the control group.
- All adverse events: High certainty evidence from 2 RCTs that included 2558 patients with COVID-19 disease revealed that Baricitinib does not increase adverse events as compared to placebo RR=0.94 (95% CI 0.83-1.07).
- Serious adverse events: High certainty evidence from 2 RCTs that included 2558 patients with COVID-19 disease revealed that Baricitinib reduced serious adverse events by 21% (95% CI 6% to 34%) compared to placebo.
BARICITINIB COMPARED TO PLACEBO IN MODERATE COVID-19 WITH HYPOXIA
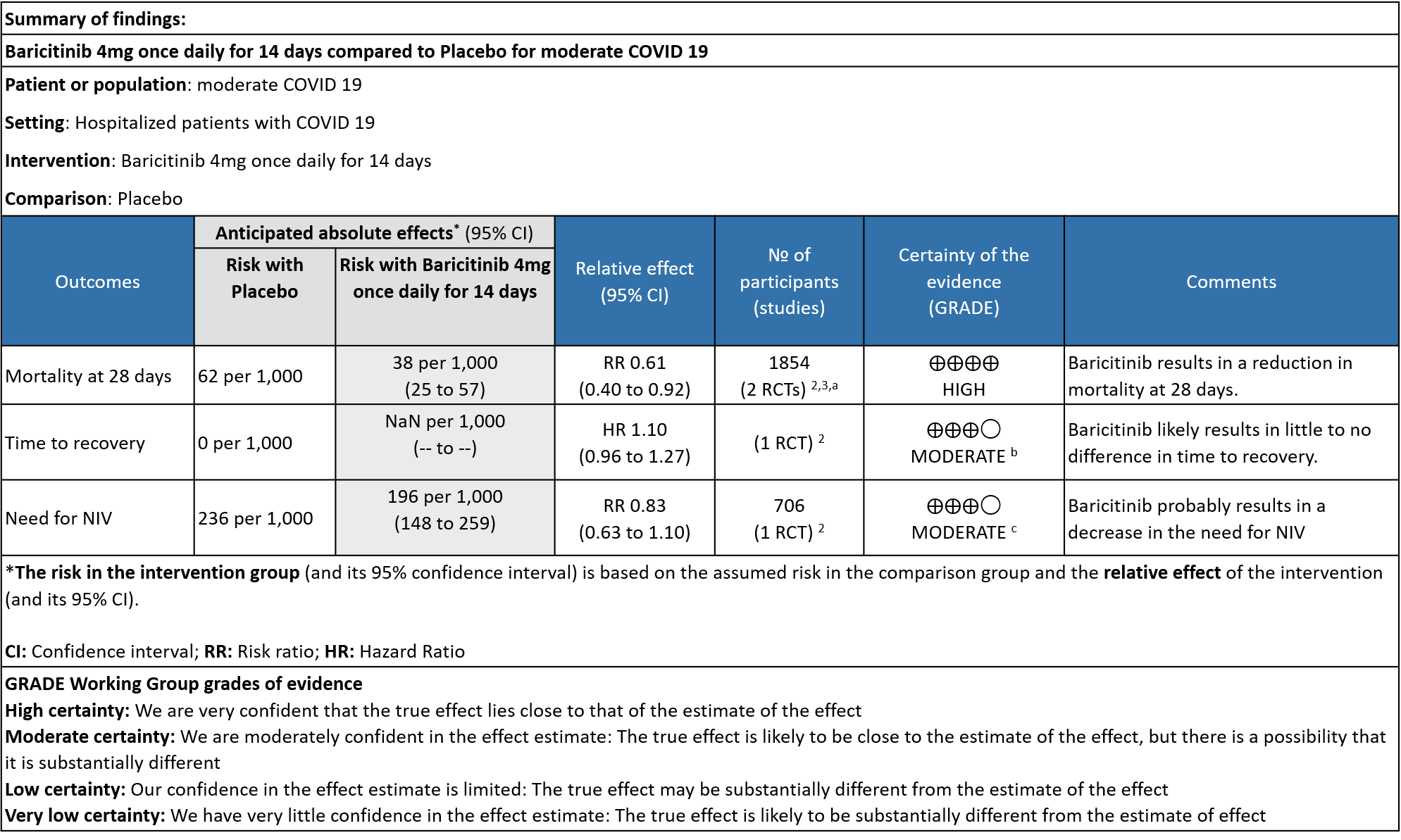
- All-cause mortality: High certainty evidence in 2 RCTs that included 1854 patients with moderate COVID-19 disease indicated that Baricitinib reduces mortality at 28 days by 39% (RR=0.61; 95% CI 0.40-0.92). The number needed to treat (NNT) to prevent one death is 40 patients with moderate COVID-19.
- Time to clinical Recovery: Moderate certainty evidence from 1 RCT that indicates that Baricitinib likely results in little to no difference in time to recovery as compared to placebo in moderate COVID- 19 (HR=1.10; 95% CI 0.96-1.27).
- Need for non-invasive ventilation: Moderate certainty evidence from 1 RCT that included 706 patients with moderate COVID-19 disease indicates that Baricitinib probably results in a reduction in need for NIV by 17% (RR=0.83; 95% CI 0.63-1.10) compared to placebo.
BARICITINIB COMPARED TO PLACEBO IN SEVERE TO CRITICAL COVID-19
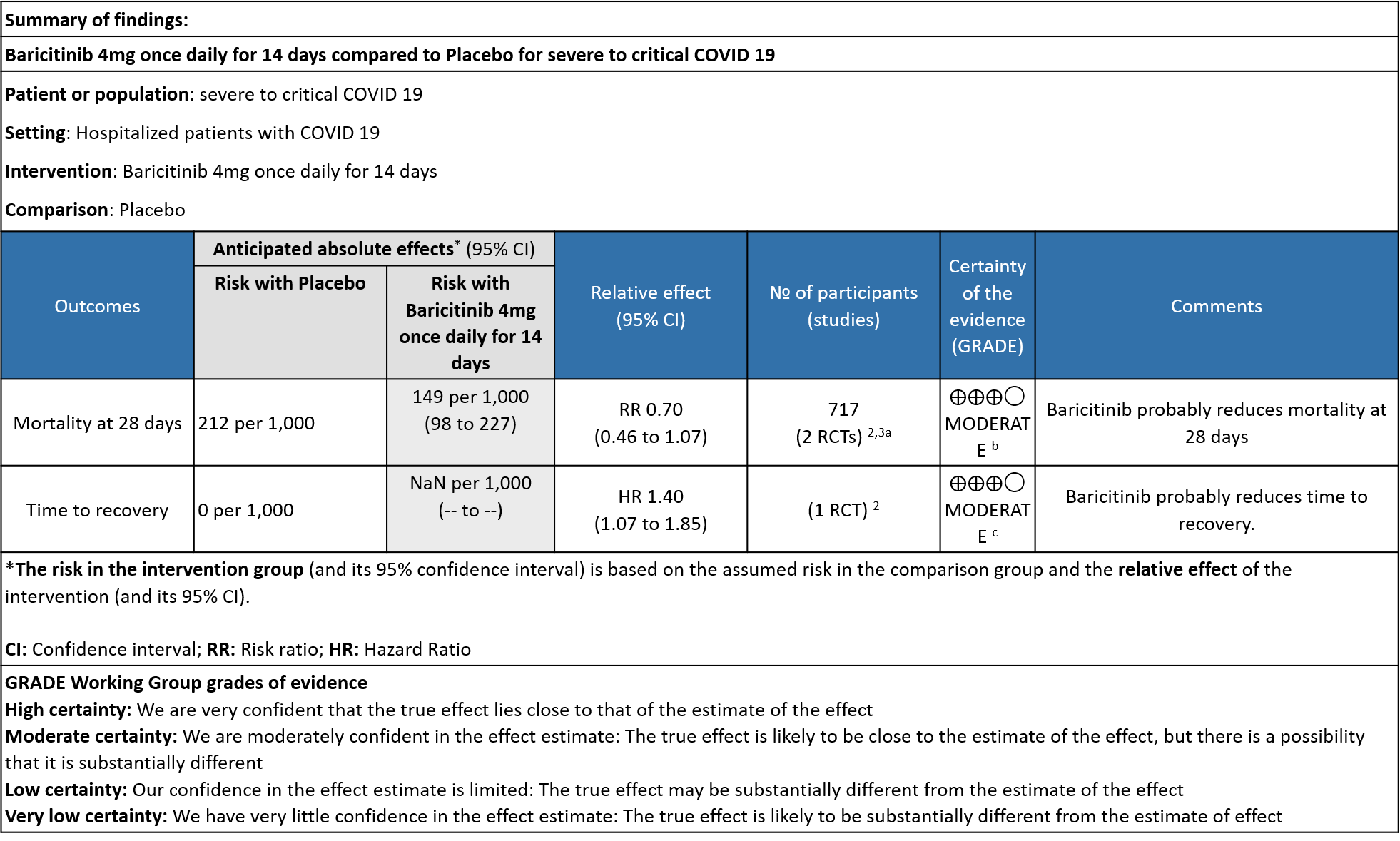
- All-cause mortality: Moderate certainty evidence from 2 RCTs that included 717 patients with severe to critical COVID-19 disease indicated that Baricitinib probably results in a reduction in mortality at 28 days by 30% (RR= 0.70; 95% CI 0.46-1.07). Based on the data, we anticipate that for 1000 patients with severe to critical COVID-19 disease in the Baricitinib arm 63 fewer (from 114 fewer to 15 more) are likely to die. The number needed to treat (NNT) with Baricitinib to prevent 1 death is 17 patients with severe to critical COVID-19.
- Time to clinical recovery: Moderate certainty evidence from 1 RCT in 1033 patients indicated that Baricitinib likely results in little to no difference in time to recovery as compared to placebo in severe to critical COVID- 19; HR=1.40(1.07-1.85). The difference seemed to be more marked in those in ordinal scale 6 which is high flow nasal oxygen and non-invasive ventilation as compared to invasive mechanical ventilation which was ordinal scale 7.
The committee had a priori requested analyses of other important subgroups of patients including children, age > 50 years and those with co-morbidities, but unfortunately there was no data to address outcomes in these subgroups specifically. There was no data in either arm with regard to nosocomial or opportunistic infections, anemia, shingles. In addition, this was an oral drug rather than an injection and hence infusion related adverse effects were not considered.
Baricitinib compared to Placebo for COVID-19:
OS - ordinal scale, OS 4: Hospitalized, not requiring supplemental oxygen – requiring ongoing medical care; OS 5: Hospitalized, requiring supplemental oxygen; OS 6 : Hospitalized, on non-invasive ventilation or high flow oxygen devices; OS 7: Hospitalized, on mechanical ventilation or ECMO
I. BARICITINIB COMPARED TO PLACEBO IN PATIENTS WITH COVID 19.
1. All-cause Mortality at 28 days
 2. Rate ratio for individuals recovered with baricitinib as compared to placebo in patients with COVID 19.
2. Rate ratio for individuals recovered with baricitinib as compared to placebo in patients with COVID 19.
3. Need for Non-invasive ventilation in patients treated with baricitinib as compared to placebo in COVID 19 patients.
4. Need for invasive mechanical ventilation in patients treated with baricitinib as compared to placebo in COVID 19 patients.
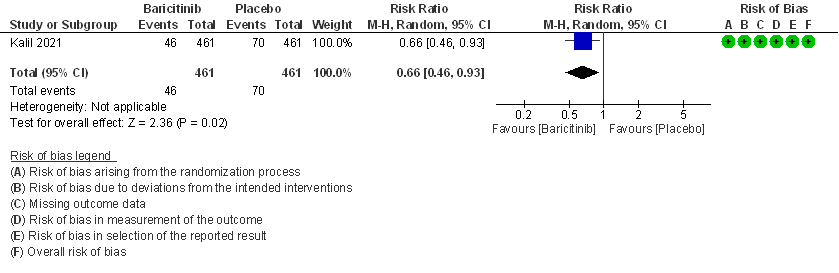
5. Adverse events
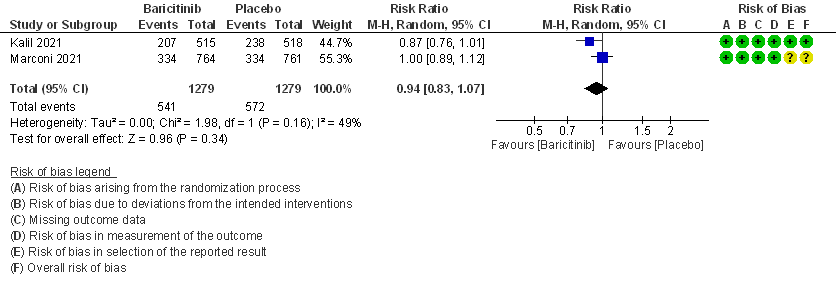
6. Serious Adverse events
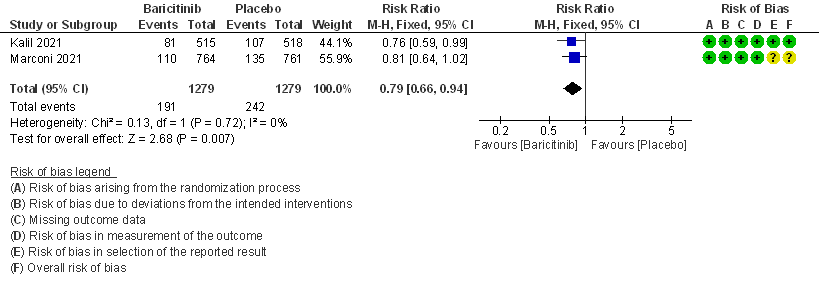
II. BARICITINIB COMPARED TO PLACEBO IN COVID 19 PATIENTS WITH MODERATE ILLNESS.
1. All-cause mortality at 28 days.
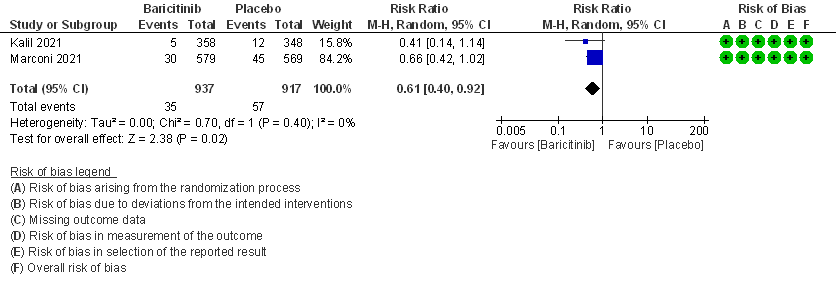
2. Rate ratio for individuals recovered with Baricitinib as compared to placebo in patients with moderate COVID 19.
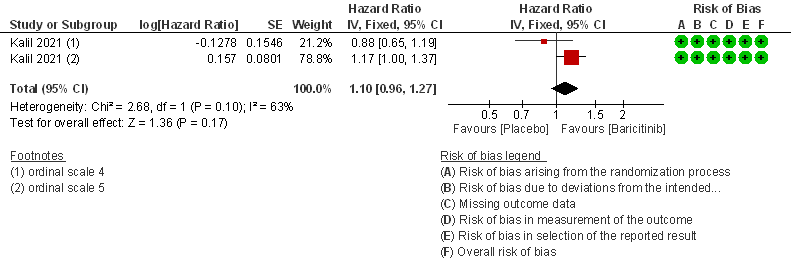
3. Need for Non-invasive ventilation in patients treated with Baricitinib as compared to placebo in moderate COVID 19 patients.
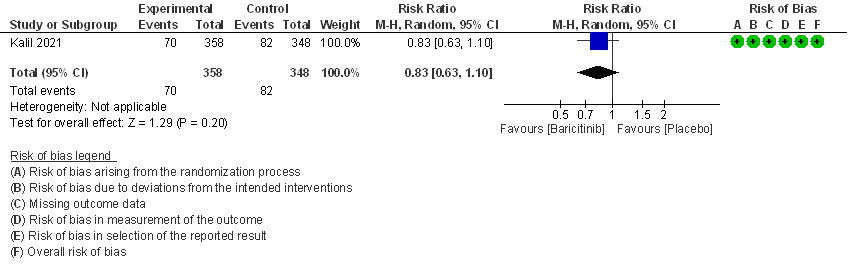
III. BARICITINIB COMPARED TO PLACEBO IN COVID 19 PATIENTS WITH SEVERE ILLNESS.
1. All-cause mortality at 28 days.
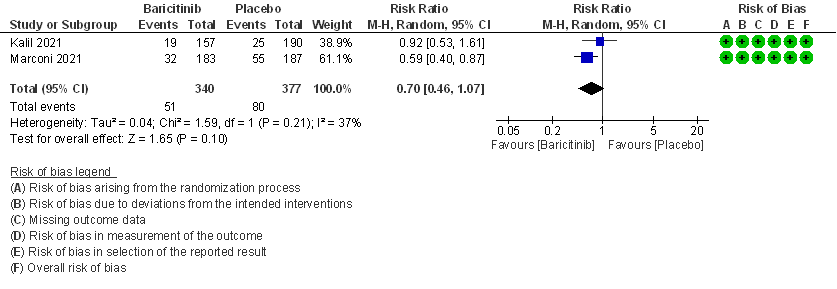
2. Rate ratio for individuals recovered with baricitinib as compared to placebo in COVID 19 patients with severe illness.