Explanations
a. Downgraded by one level for serious imprecision as the confidence interval is very wide
b. Downgraded by 2 levels because of High Risk of Bias
c. Downgraded by 2 levels for very serious imprecision because of wide CI
d. Downgraded by 1 level for serious imprecision as the mean difference of less than a day is not clinically significant
e. Downgraded by 1 level for serious imprecision as optimal information size criteria were not met, very few events documented
f. Downgraded by 1 level because of some concerns and high Risk of Bias in studies.
g. Downgraded by 1 level for serious inconsistency as the I2 was greater than 50%
h. Downgraded by 1 level for serious indirectness as all categories of severity are included
i. downgraded by 1 level as outcomes at 6 and 14 days were included
Subgroup Analysis
Explanations
a. Downgraded by 2 level due to wide 95% CI, and with low number of events
b. Downgraded by 1 level due to some concerns in ROB
c. Downgraded by1 level due to wide 95% CI
d. Downgraded by 2 levels for very serious imprecision, as the CI were wide and event rates were very low; optimal information size (OIS) was inadequate
e. Downgraded by 2 levels for very serious ROB.
f. Downgraded by 1 level for serious imprecision, as the event rate was low and OIS was inadequate.
g. The ECG measurement at a homecare setting was not reliable.
Explanations
a. Downgraded by 1 level, some concerns in two studies
b. Downgraded by 2 levels for very serious imprecision, due to wide 95% CI, OIS not met.
c. Downgraded by 1 level for serious ROB, some concerns in a single study
d. Downgraded by 1 level for serious inconsistency, I2 value is 58%
e. Downgraded by 1 level for serious imprecision, as the effect estimate is 0.70 days is not clinically significant
f. Downgraded by 2 levels for very serious ROB, single study with High ROB
g. Downgraded by 1 level for serious indirectness, as time to clinical improvement is measured as fever resolution.
h. Downgraded by 1 level for serious imprecision, due to wide 95% CI, OIS not met.
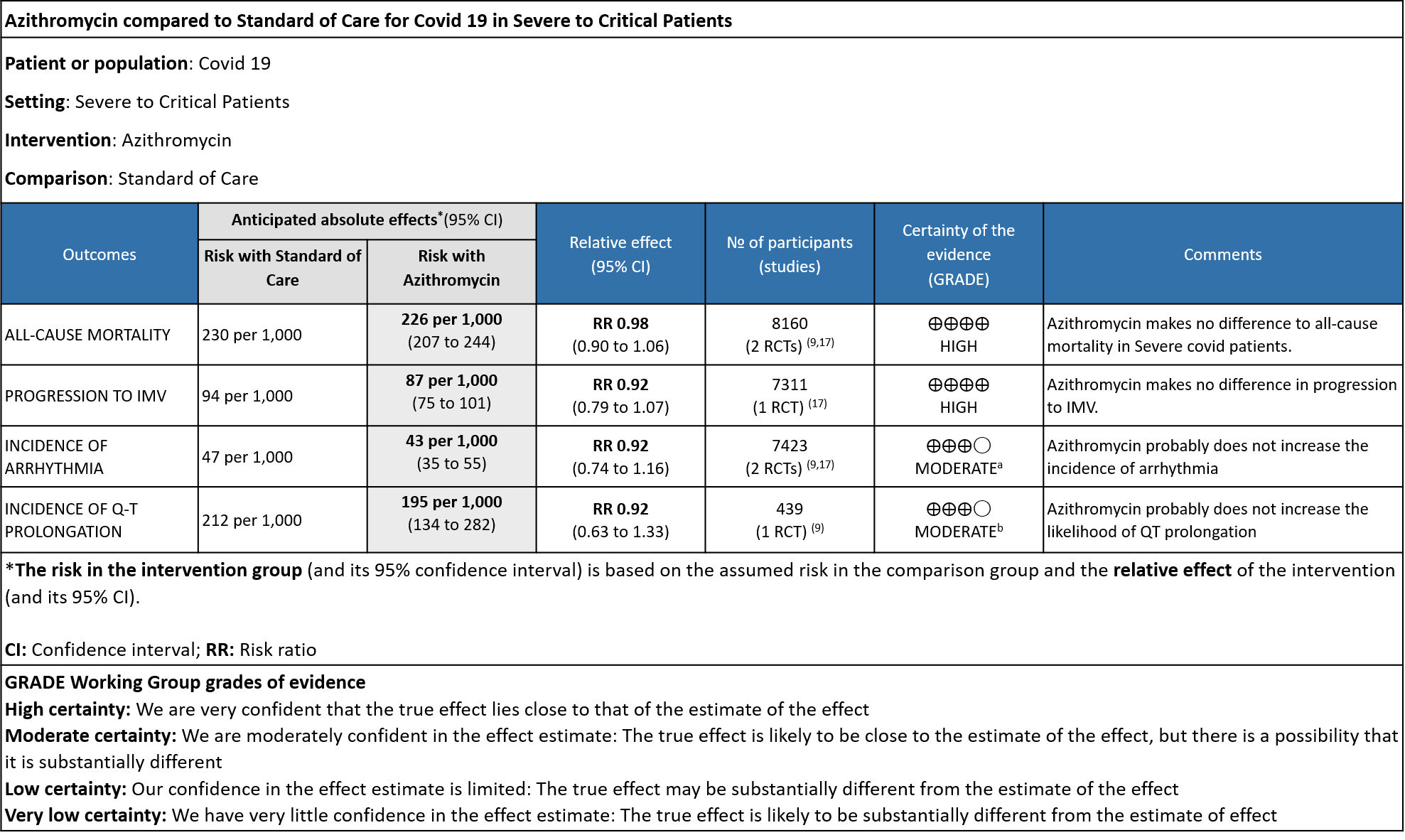
Explanations
a. Downgraded by 1 level, for serious imprecision, as CI was wide (0.74 to 1.16)
b. Downgraded by 1 level, for serious imprecision, as CI was wide (0.63 to 1.33)
Azithromycin is a synthetic macrolide which has been used against bacterial, rickettsial and mycobacterial diseases. It also has antiviral and anti-inflammatory actions and hence has been used previously for treatment of SARS-CoV or MERS-CoV. In vitro studies suggest possible effect against SARS-CoV-2 also (1). Azithromycin’s effect on pH of the Golgi network and recycling endosome is postulated to interfere with intracellular SARS-CoV-2 activity and replication(4). The virus has a furin-like cleavage site in the spike protein and as this drug might reduce levels of furin(enzyme), it could interfere with the cell entry of SARS-CoV-2. Azithromycin’s ability to reduce levels of proinflammatory cytokines such as IL-6 could reduce the intensity of the SARS-CoV-2 triggered cytokine storm, along with associated tissue damage(5). It is an easily available drug and has been utilized for Covid 19 rampantly in India(6). The doses used are 250mg or 500mg of the drug per day for duration of 3 to 10 days. This review aims to provide a summary of the available evidence from randomized clinical trials of Azithromycin for treatment of acute COVID-19, so the Expert Working Group can provide a recommendation to guide clinicians and researchers regarding the appropriate use of this drug.
Towards identifying all relevant studies, we conducted a systematic search of PubMed, covid-nma.org and the Epistemonikos databases in July 2021. We also reviewed reference lists of systematic reviews and included studies.
We included randomized controlled trials (RCTs) testing Azithromycin at any dose for any duration, in people with confirmed COVID‐19, and extracted data for the following pre-defined outcomes:
- Critical (primary for this review):
- Progression to:
- Oxygen therapy
- Ventilation: non-invasive or invasive
- Critical or Intensive care (any reason)
- Duration of hospitalization
- Need for hospitalization (out-patients)
- Progression to:
- Important (secondary):
- Mortality (all-cause) – at 28-30 days, or in-hospital
- Time to clinical improvement
- Time to negative PCR for SARS-CoV-2
- Complications of COVID-19:
- thrombotic events
- pulmonary infections/fibrosis
- Long covid/post-acute sequalae COVID
- Secondary infections
- Adverse events: All and serious adverse events
Two reviewers independently assessed eligibility of search results using Rayyan. We identified a total of 78 articles of which 9 were excluded as they were duplications and 55 as they were not the right study design or were for the wrong drug as intervention. The remaining 14 articles comprised of 12 RCT’s and 2 systematic reviews.
Two reviewers extracted data from each included study, and assessed risk of bias using the Cochrane Risk of bias (RoB) v2.0 tool. The entire RoB assessment was scrutinized by the whole team for this review, to reach consensus.
The systematic reviews were assessed by the AMSTAR grading tool and were found to be moderate to high quality evidence. We used RevMan 5.4 to perform meta‐analysis using a random‐effects model for outcomes where pooling of effect estimates was appropriate. We used risk ratios (RR) for dichotomous outcomes with 95% confidence intervals (CIs). We used the I2 statistic to measure residual heterogeneity. We used GRADE methodology to assess the certainty in the evidence, and documented this in a ‘Summary of findings’ table using GradeProGDT.
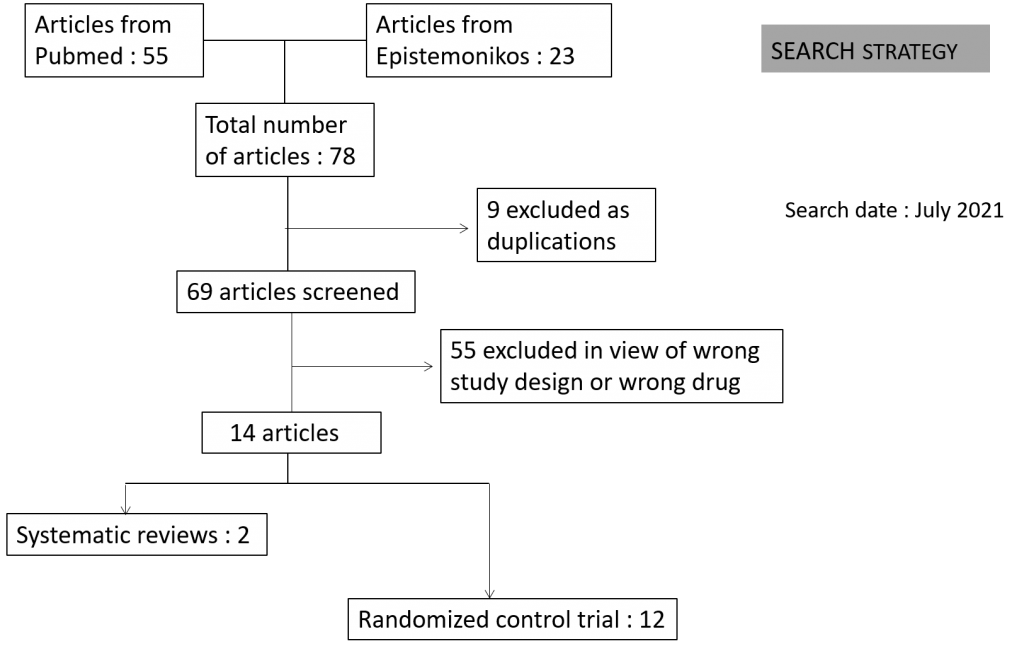
We found 12 RCTs that matched the PICO question as pre-defined by the expert working group. These included 4 studies with ambulatory patients who did not need hospitalization, 6 studies with patients with non-severe COVID 19 but were hospitalized during study period and 2 studies that included patients who had severe to critical COVID 19. Hence recommendation being considered for: Symptomatic confirmed COVID 19 infection
Categories:
- Ambulatory,
- Hospitalised non severe
- Hospitalised severe to critical
Azithromycin in Comparison to Standard of Care in Covid 19
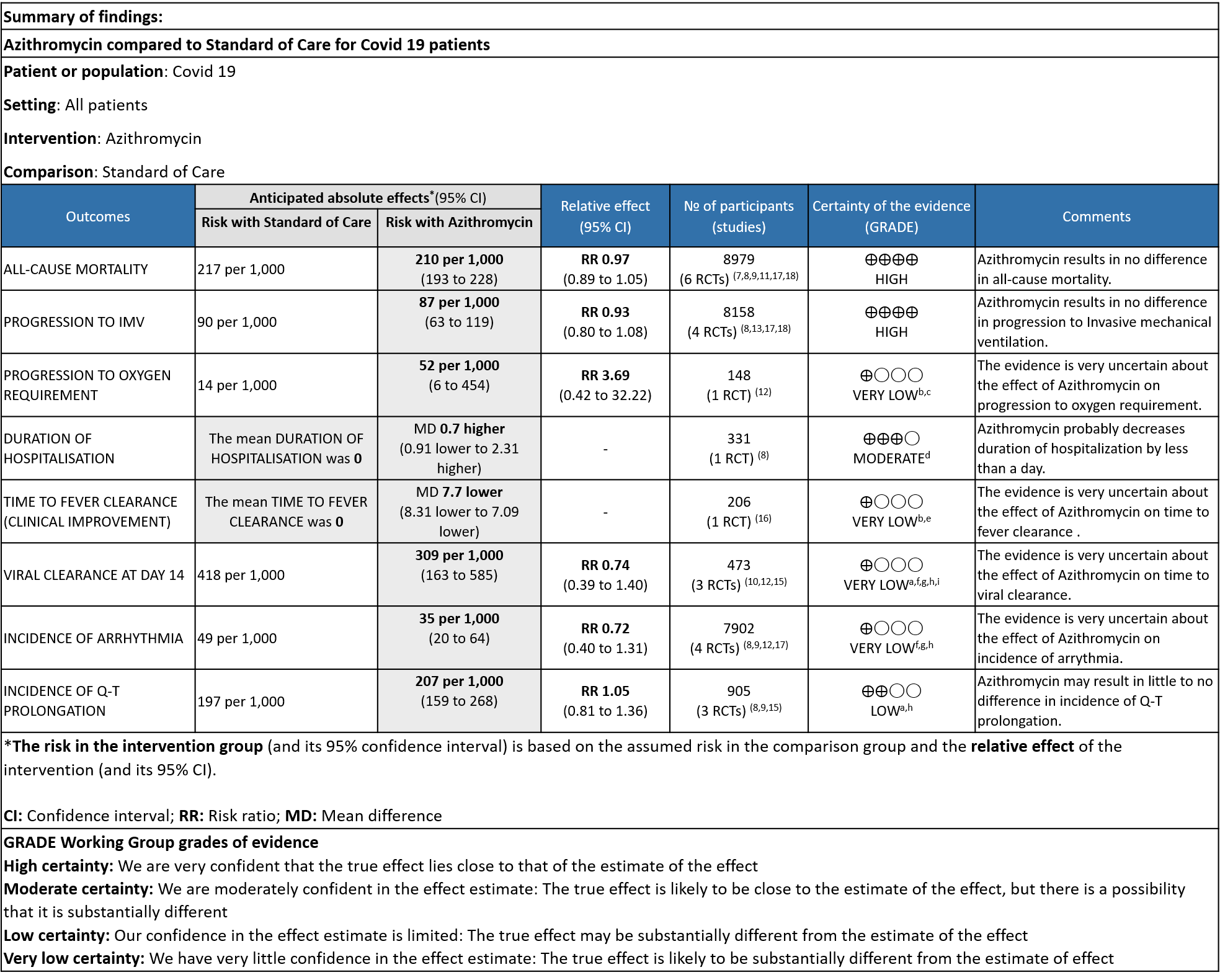
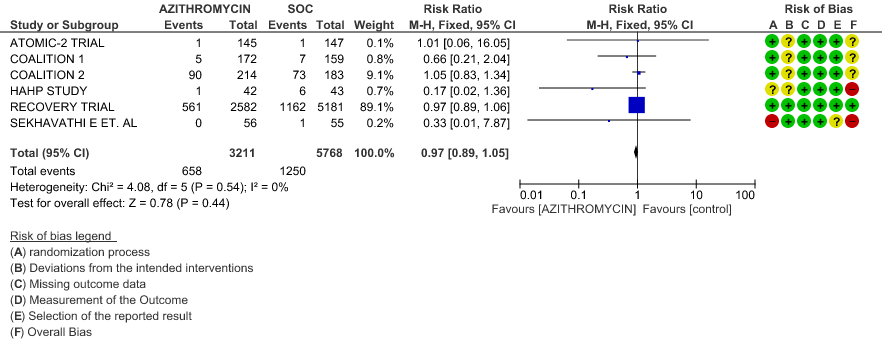
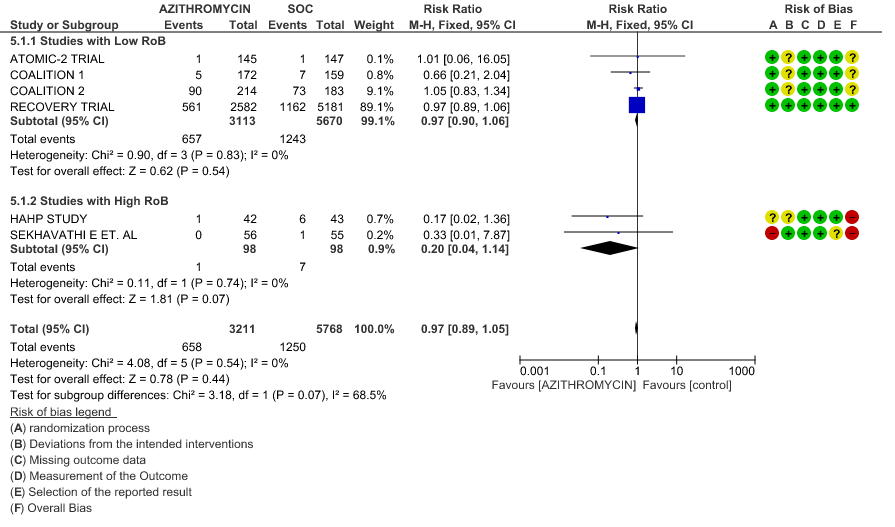
- ALL-CAUSE MORTALITY: High certainty of evidence from 6 RCT’s in a total of 8979 patients reveals that Azithromycin does not reduce all-cause mortality (RR 0.97; 95% CI 0.89 to 1.05).
- PROGRESSION TO INVASIVE MECHANICAL VENTILATION (IMV): High certainty evidence from 4 RCT’s in 8158 patients revealed that Azithromycin does not reduce progression to invasive mechanical ventilation (RR 0.93; 95% CI of 0.80 to 1.08).
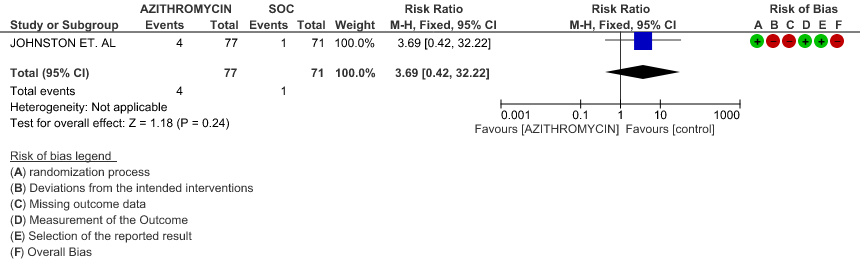
- PROGRESSION TO OXYGEN REQUIREMENT: Low certainty evidence from 1 RCT in 148 patients revealed a very uncertain effect of Azithromycin on progression to oxygen requirement (RR 3.69 with a 95% CI of 0.42 to 32.22).
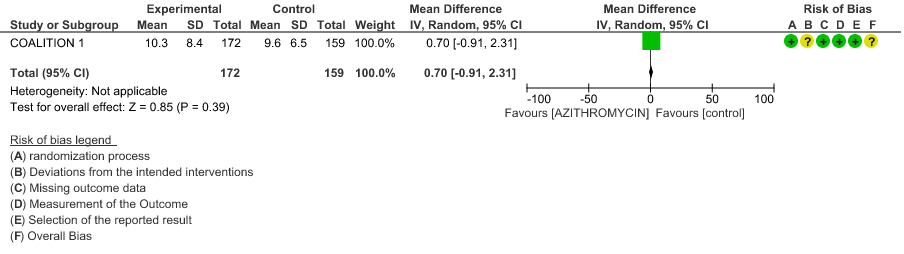
- DURATION OF HOSPITALISATION: Moderate certainty evidence from 1 RCT in 331 patients revealed that Azithromycin probably decreases duration of hospitalization by less than a day (MD 0.7 higher; 95% CI 0.91 lower to 2.31 higher).
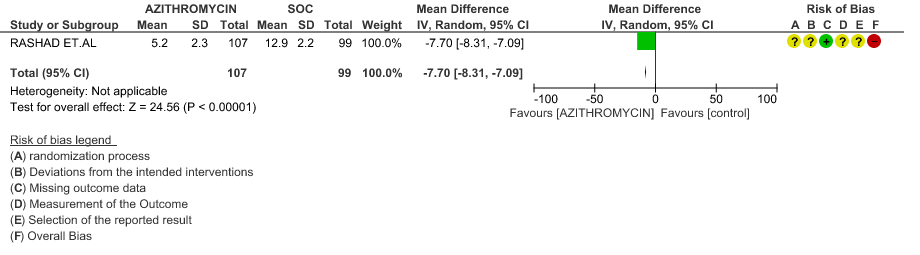
- TIME TO CLINICAL IMPROVEMENT (time to fever clearance): Very low certainty evidence from single study with high risk of bias is from 1 RCT in 206 patients revealed a very uncertain effect of Azithromycin on time to fever clearance (MD 7.7 lower; 95% CI 8.31 lower to 7.09 lower).
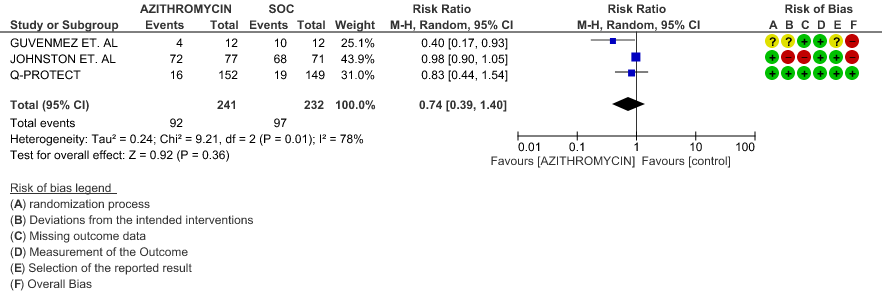
- VIRAL CLEARANCE ON DAY 14: Very low certainty evidence from 3 RCT’s in 473 patients revealed a very uncertain effect of Azithromycin on time to viral clearance (RR 0.74; 95 % CI 0.39 to 1.40).
- INCIDENCE OF ARRHYTHMIA: Very low certainty evidence from 4 RCT’s in 7902 patients revealed a very uncertain effect of Azithromycin on incidence of arrhythmia (RR 0.72; 95% CI 0.40 to 1.31).
- INCIDENCE OF QT-PROLONGATION: Low certainty evidence from 4 RCT’s in 905 patients revealed that Azithromycin may result in no difference in incidence of Q-T prolongation (RR 1.05; 95% CI 0.81 to 1.3).
SUBGROUP ANALYSIS
Azithromycin in Comparison to Standard of Care in Ambulatory Patients with Covid 19
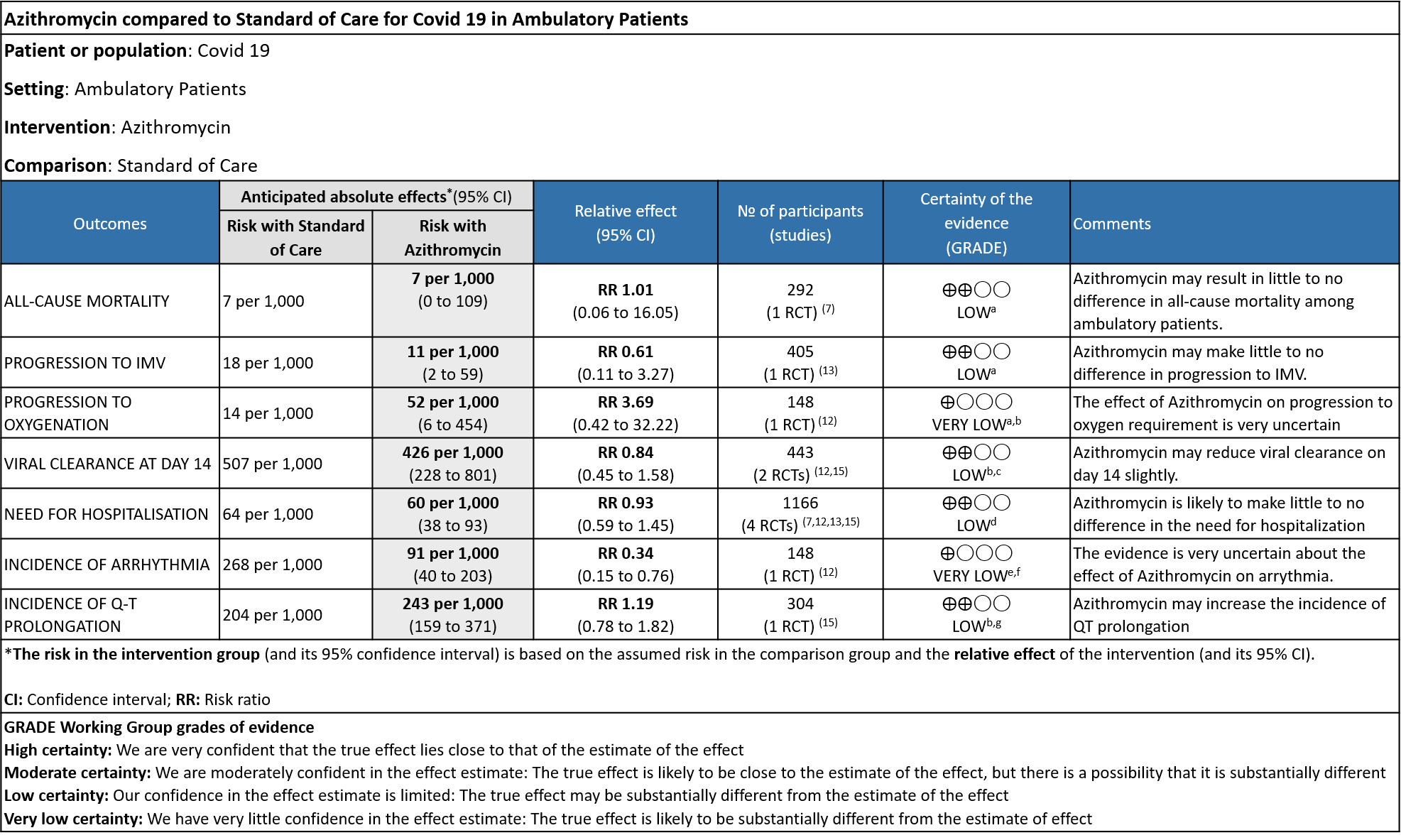
- ALL-CAUSE MORTALITY: Low certainty evidence from 1 RCT in 292 patients revealed that Azithromycin may result in no difference to all-cause mortality in ambulatory patients (RR 1.01 with a 95% CI of 0.06 to 16.05).
- PROGRESSION TO IMV: Low certainty evidence from 1 RCT in 405 patients revealed that Azithromycin may reduce progression to IMV slightly (RR 0.61 with 95% CI of 0.11 to 3.27).
- PROGRESSION TO OXYGEN REQUIREMENT: Low certainty evidence from 1 RCT in 148 patients, revealed a very uncertain effect of Azithromycin on progression to oxygen requirement (RR 3.69 with 95% CI of 0.42 to 32.22).
- DURATION OF HOSPITALISATION: Low certainty evidence from 4 RCT’s in 1066 patients revealed that Azithromycin may make no difference in the need for hospitalization (RR 0.93 with 95% CI of 0.59 to 1.45).
- VIRAL CLEARANCE ON DAY 14: Low certainty evidence from 2 RCT in 443 patients revealed that Azithromycin may reduce viral clearance on day 14 slightly (RR 0.84 with 95% CI of 0.45 to 1.58).
- INCIDENCE OF ARRHYTHMIA: Very low certainty evidence from 1 RCT in 148 patients revealed a very uncertain effect of Azithromycin on arrhythmia (RR 0.34 with 95% CI of 0.15 to 0.76).
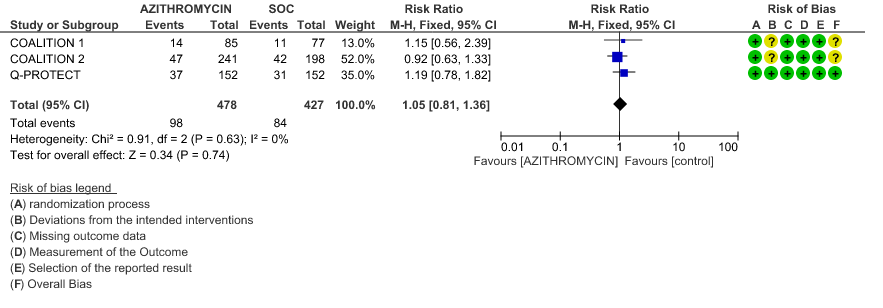
- INCIDENCE OF QT-PROLONGATION: Low certainty evidence from 1 RCT in 304 patients revealed that Azithromycin may increase the incidence of QT prolongation (RR 1.19 with 95% CI of 0.78 to 1.82)
Azithromycin in Comparison to Standard of Care in Hospitalized Non-Severe Patients with Covid 19
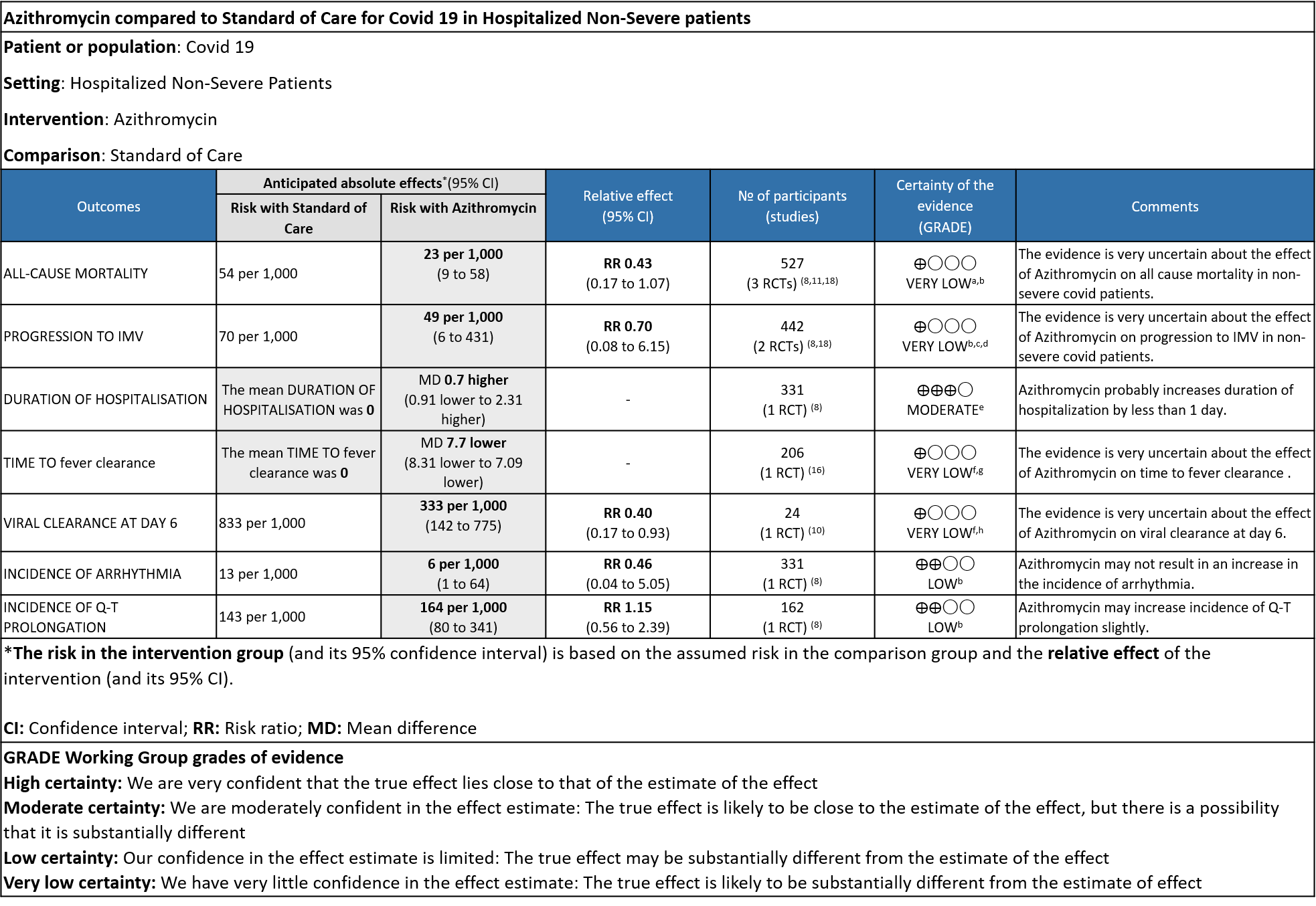
- ALL-CAUSE MORTALITY: Very low certainty evidence from 3 RCT’s in 527 patients, revealed a very uncertain effect of Azithromycin on all-cause mortality (RR 0.43 with 95% CI of 0.17 to 1.07).
- PROGRESSION TO IMV: Very low certainty evidence from 2 RCT’s in 442 patients revealed a very uncertain effect of Azithromycin on progression to IMV (RR 0.70 with a 95% CI of 0.08 to 6.15).
- DURATION OF HOSPITALISATION: Moderate certainty evidence from 1 RCT in 331 patients found that Azithromycin probably increases duration of hospitalization by a 1 day (MD 0.7 higher; 95% CI 0.91 lower to 2.31 higher).
- TIME TO CLINICAL IMPROVEMENT (time to fever clearance): Very low certainty evidence (single study with high risk of bias) from 1 RCT in 206 patients revealed a very uncertain effect of Azithromycin on time to fever clearance (MD 7.7 lower; 95% CI 8.31 lower to 7.09 lower).
- VIRAL CLEARANCE ON DAY 6: Very low certainty evidence from 1 RCT (high risk of bias) in 24 patients revealed a very uncertain effect of Azithromycin on viral clearance at day 6 (RR 0.40 with a 95% CI of 0.17 to 0.93).
- INCIDENCE OF ARRHYTHMIA: Low certainty evidence from 1 RCT in 331 patients revealed that Azithromycin may not cause an increase in the incidence of arrhythmia (RR 0.46 with a 95% CI of 0.04 to 5.05).
- INCIDENCE OF QT-PROLONGATION: Low certainty evidence from 1 RCT in 162 patients revealed that Azithromycin may increase incidence of Q-T prolongation slightly (RR 1.15 with a 95% CI of 0.56 to 2.39).
Azithromycin in Comparison to Standard of Care in Hospitalized Severe to Critical Patients with Covid 19
- ALL-CAUSE MORTALITY: High certainty evidence from 2 large RCT’s with a total number of 8160 patients revealed that Azithromycin did not reduce all-cause mortality in patients with severe to critical COVID 19 (RR 0.98; 95% CI 0.90-1.06).
- PROGRESSION TO IMV: High certainty evidence from 1 large RCT in 7311 patients revealed that Azithromycin did not reduce progression to IMV (RR 0.92 with 95% CI of 0.79 to 1.07).
- INCIDENCE OF ARRHYTHMIA: Moderate certainty evidence from 2 RCT’s in 7423 patients revealed that Azithromycin probably does not increase the incidence of arrhythmia in patients with severe to critical COVID 19 (RR 0.92 with 95% CI of 0.74 to 1.16).
- INCIDENCE OF QT-PROLONGATION: Moderate certainty evidence from 1 RCT in 439 patients revealed that Azithromycin probably does not increase the likelihood of QT prolongation (RR 0.92 with a 95% CI of 0.63 to 1.33).
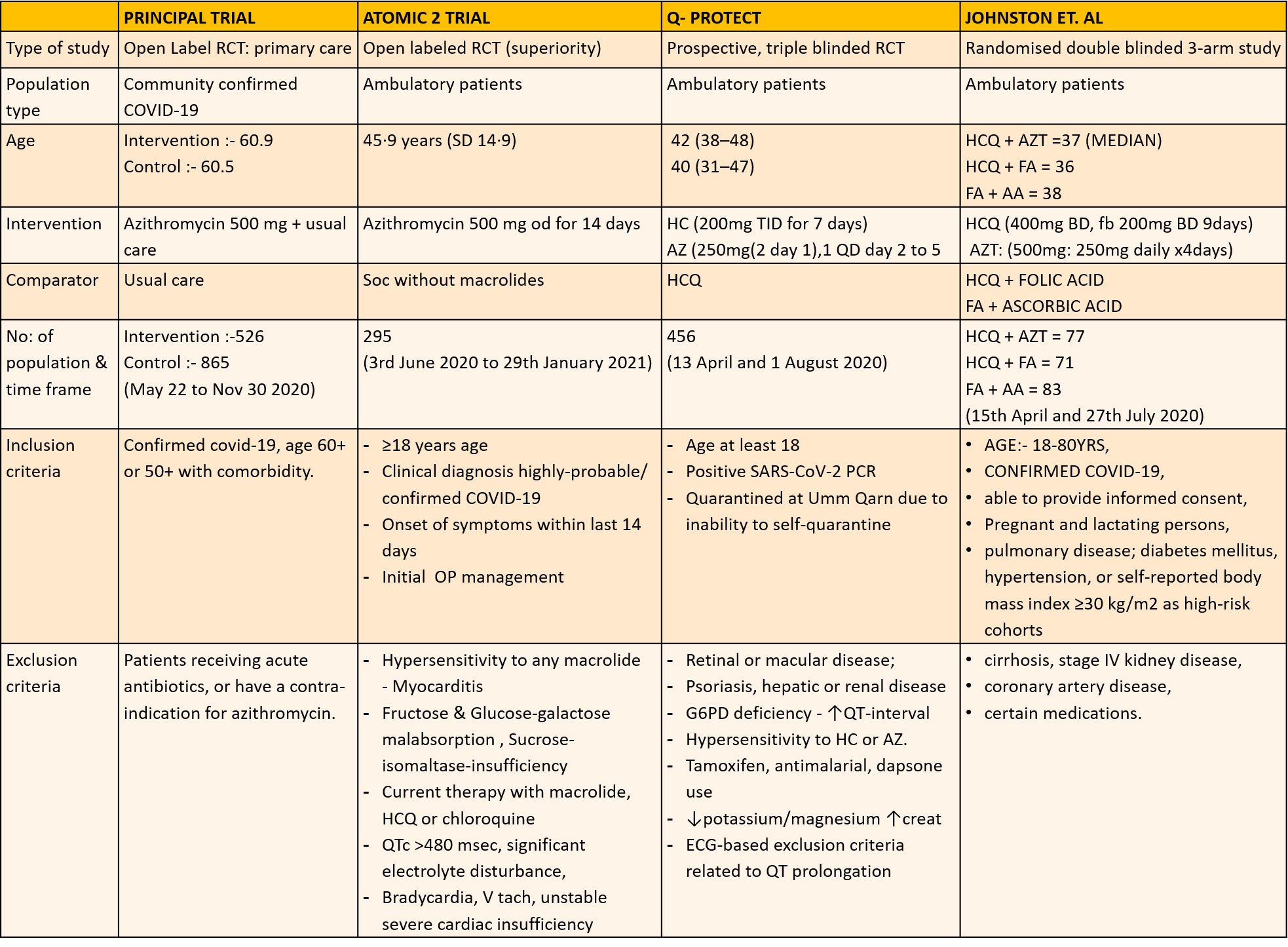
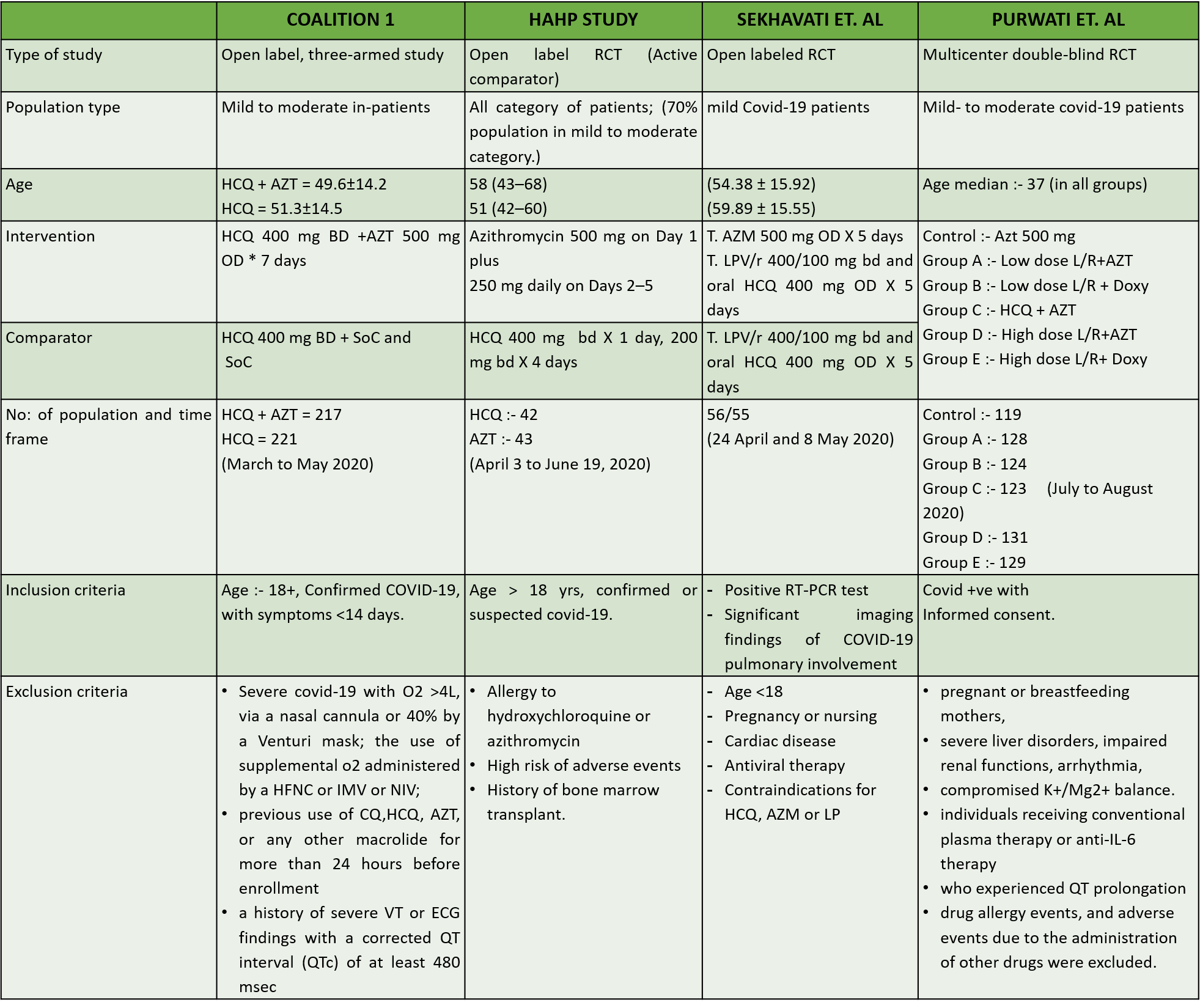
1. Azithromycin compared to standard of care in Covid 19
1a. All-Cause Mortality
1b. All-Cause Mortality – Stratified with RoB
1c. Progression to IMV
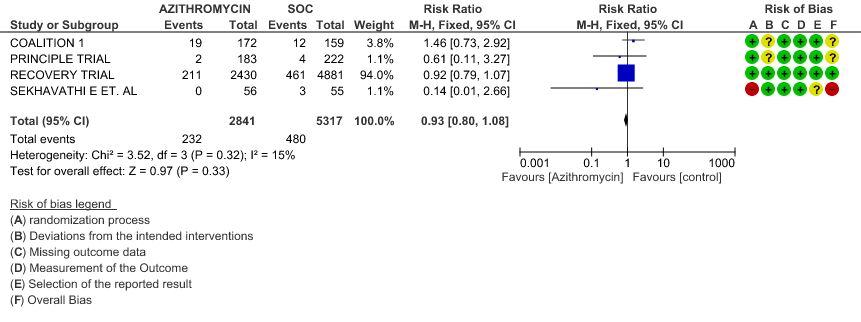
1d. Progression to IMV – Stratified with RoB
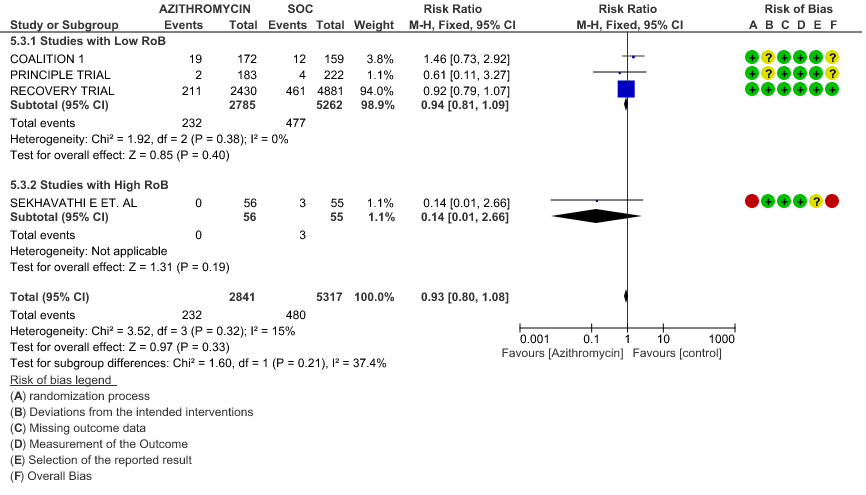
1e. Progression to oxygen requirement
1f. Duration of Hospitalization
1g. Time to clinical improvement
1h. Viral clearance on day 14
1i. Incidence of Arrhythmias
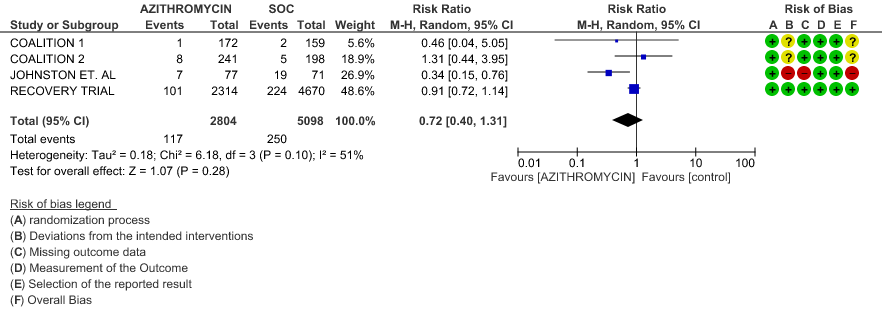
1j. Incidence of QT prolongation
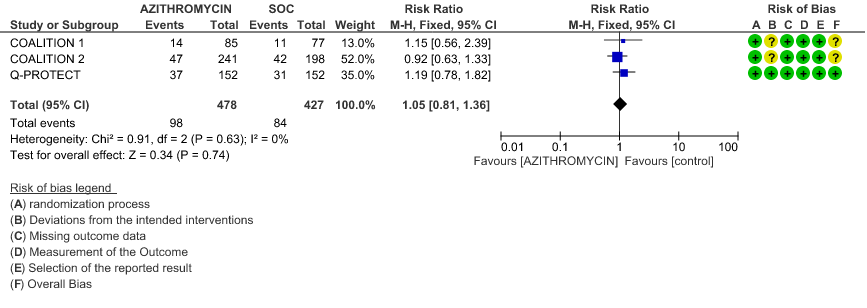
Sub Group Analysis
2. Ambulatory patients
2a. All-Cause Mortality
2b. Progression to IMV
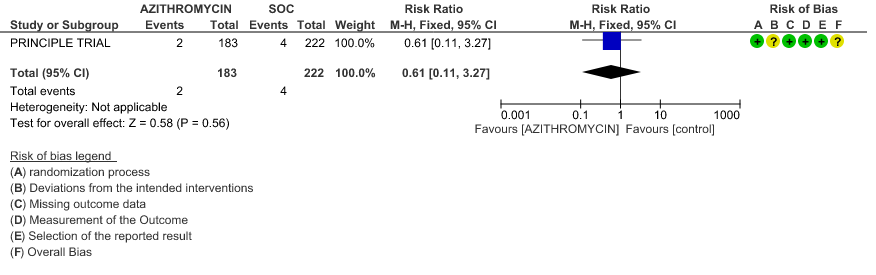
2c. Progression to oxygen requirement
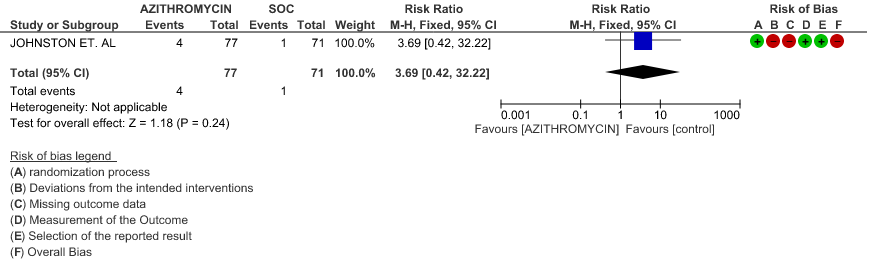
2d. Viral clearance on day 14
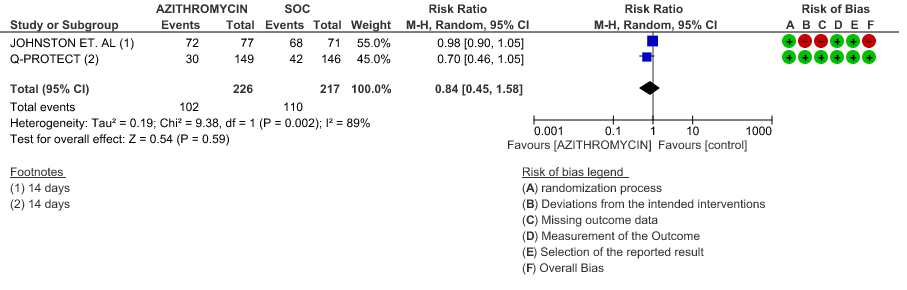
2e. Need for Hospitalisation
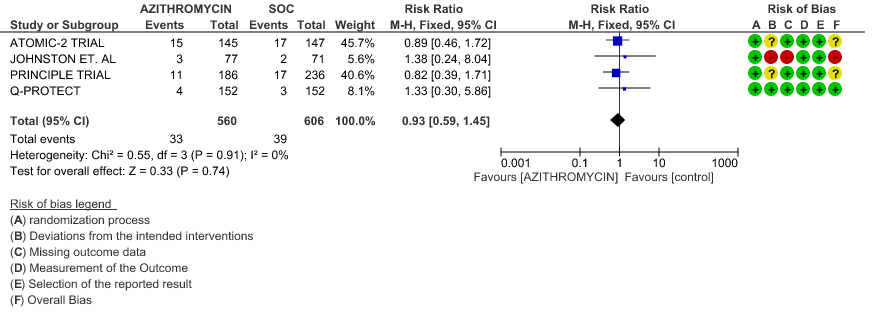
2f. Incidence of Arrhythmias
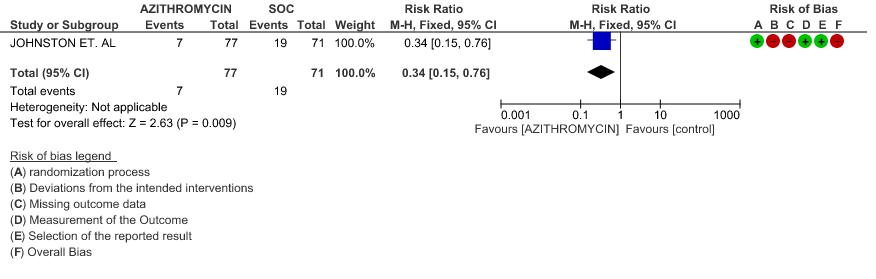
2g. Incidence of QT Prolongation
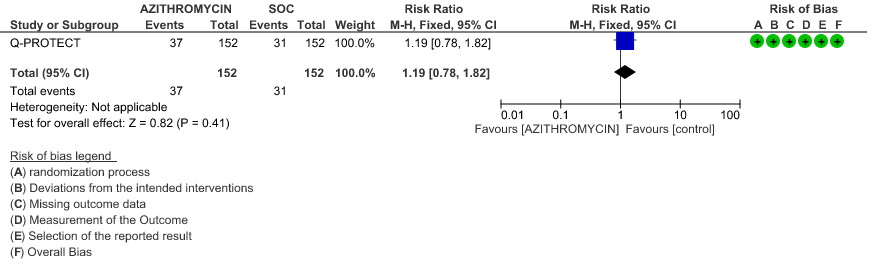
3. Hospitalised Non-severe patients
3a. All-cause mortality
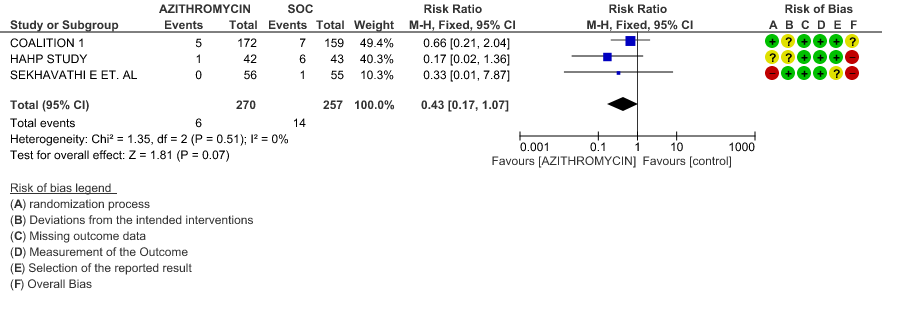
3b. Progression to IMV
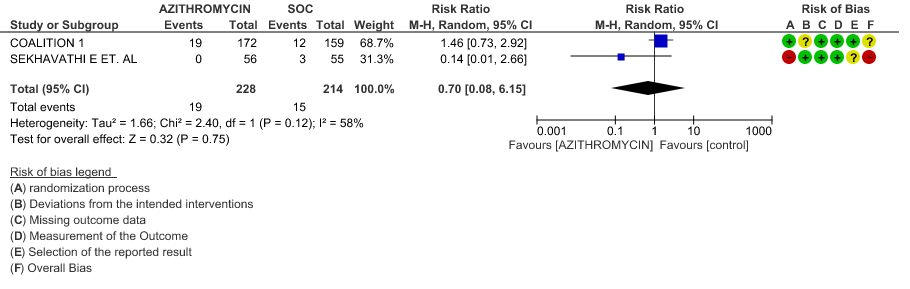
3c. Duration of Hospitalization
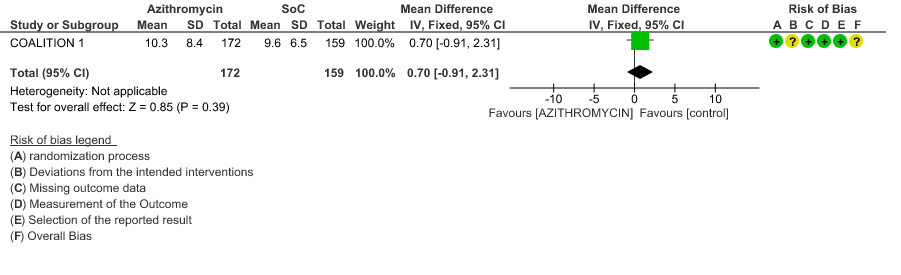
3d. Time to Clinical Improvement
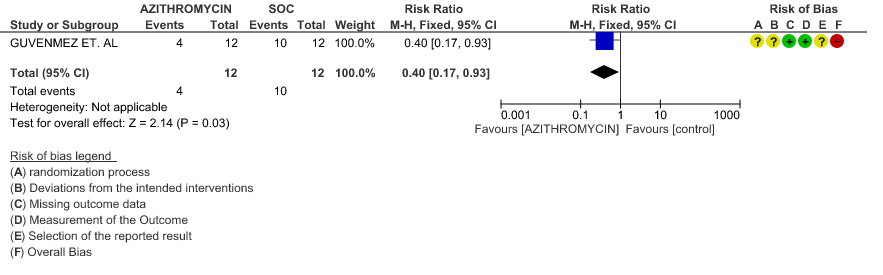
3e. Viral clearance at day 6
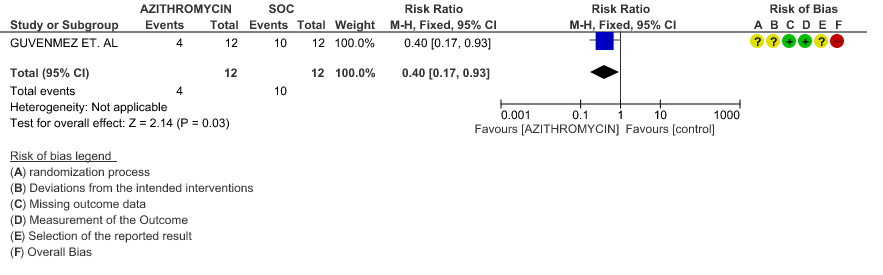
3f. Incidence of Arrhythmias
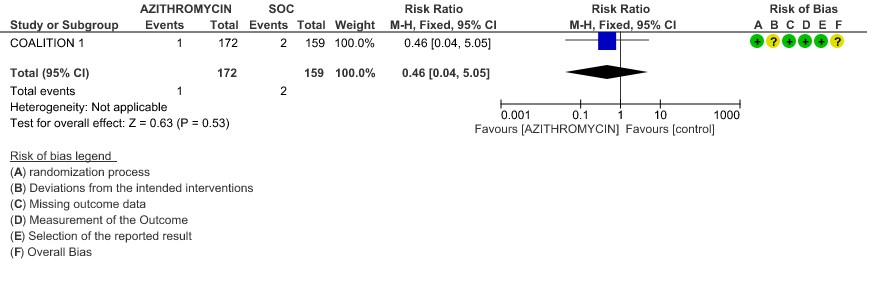
3g. Incidence of QT prolongation
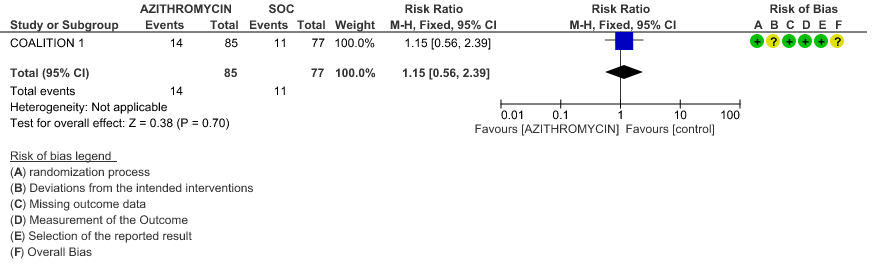
4. Hospitalised severe to critical patients
4a. All-cause mortality
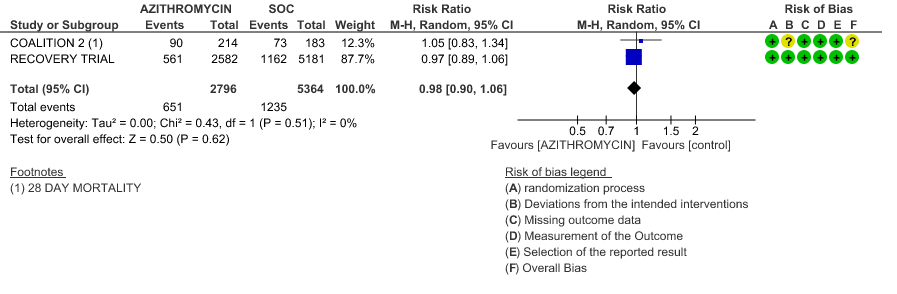
4b. Progression to IMV
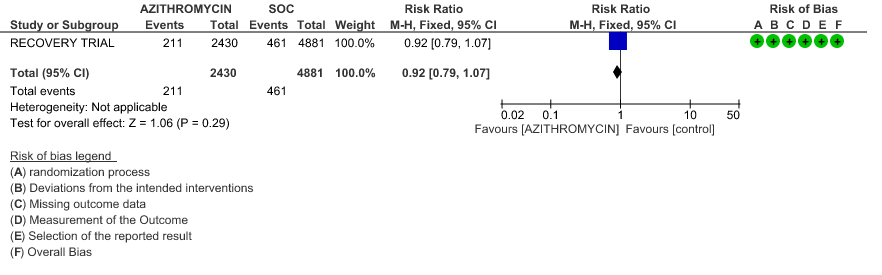
4c. Incidence of Arrhythmias
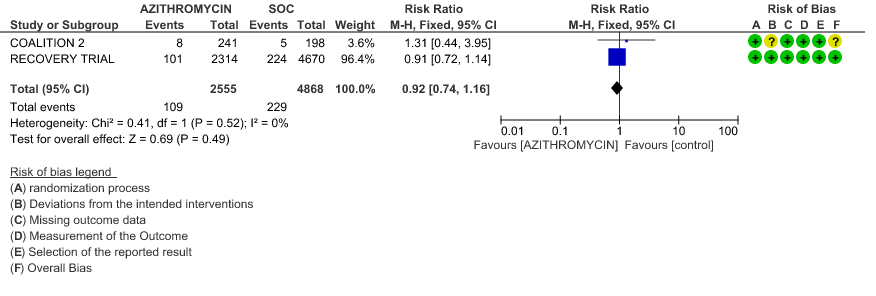
4d. Incidence of QT prolongation