COVID-19 contributes to a hypercoagulable state and thrombotic events are fairly common. Due to the frequency of arterial and venous thrombosis as well as micro-vascular thrombosis demonstrated on lung histology, many clinicians all over the world have opted to use therapeutic anticoagulation in patients with severe or critical illness. In addition, there are numerous reports suggesting that the delta variant (B.1.617.2), now the predominant strain circulating widely in India, results in many more thrombotic events and has also contributed to intrauterine deaths. Hence prophylactic anticoagulation is standard practice for all hospitalized patients with COVID-19. Standard prophylactic doses or Intermediate weight-adjusted doses of anticoagulation for thromboprophylaxis in hospital and ICU settings have been found to have similar safety and efficacy in preventing death or thrombosis, with a slightly higher risk of bleeding with Intermediate weight-based dosing anticoagulation. Hence with the present evidence available, we recommend only prophylactic dose anticoagulation in patients with mild, moderate (without hypoxia) or critical illness, though this may need to be individualized in obesity, pregnancy and renal insufficiency.
Overall among those with moderate (with hypoxia), severe and critical COVID-19 studied in the trials considered, therapeutic dose anticoagulation probably prevents clinically defined thrombotic events by 39% (risk ratio (RR) 0.61 (95% confidence interval (CI) 0.45 to 0.82); moderate certainty in the evidence). It may not reduce mortality, and we are very uncertain of its effect on organ support free days. It does, however, probably improve chance of survival without organ support at 28 days by 6% (95% CI 1% to 10%; moderate certainty in the evidence).
When the moderate (with hypoxia) to severe group of patients were considered separately, the initiation of therapeutic anticoagulation also led to a probable decrease in thrombotic events by 37% (95% CI 7% to 57%), probable increase in organ support free days (OSFD) by 5% (95% CI 1% to 10%), along with a probable increased risk of bleeding.
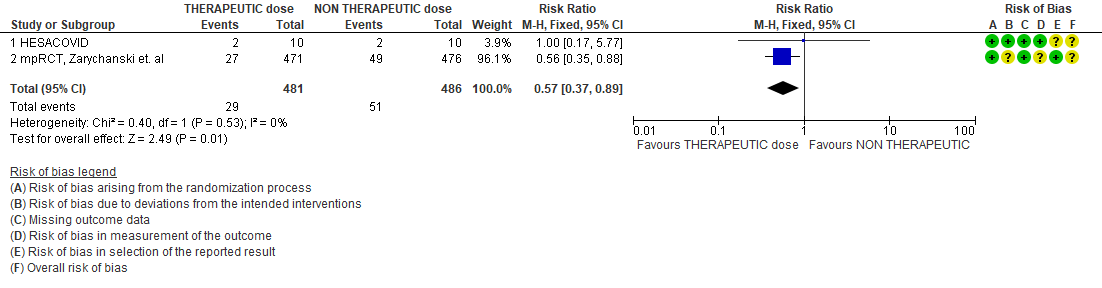
When the critical severity group was evaluated separately, therapeutic anticoagulation probably reduced thrombotic events by 43% (95% CI 11% to 63%) with probably no decrease in all-cause mortality or OSFD. In addition, there was probably no increase in bleeding (RR 1.39; 95% CI 0.71 to 2.71). Due to the increasing reports of thrombosis in all categories of patients in India, clinician discretion is advised for this critical category of patients. The group debated at length the advantages and disadvantages of therapeutic dose anticoagulation in this group. The group felt in the critical setting they are unable to pick up a thrombotic event easily which may impact eventual mortality and morbidity, and it is most often based on a clinical suspicion which are they are often unable to confirm as these patients are not amenable to easy shifting for a confirmatory radiology test. However, the group felt it is easy to pick up major bleeding. In addition, fatal bleeding events, reported only by the mpRCT (non-critical) trial, were only 3 in the therapeutic dose group as compared to 1 in the non-therapeutic dose group, though the actual numbers were not reported in the critical category. Despite the uncertainty reflected in the various guidelines from NIH or NICE (1,2) where due to the lack of trial data they are unable to categorically recommend therapeutic over prophylactic dose, clinicians in India are increasingly recommending an intermediate or therapeutic dose of anticoagulation in severe and critical categories. However, more trials are required to support a conclusion that therapeutic dose anticoagulation is beneficial in the critical category.
Overall therapeutic dose anticoagulation is a low-cost intervention and needs to be weighed against hospitalization and ICU care costs that may result due to a thrombotic event. In addition, clinicians need to make a judgement call regarding the balance between risk of thrombosis and the risk of bleeding in an individual patient.
Date of latest search: 9th June 2021
Date of completion of Summary of findings table and presentation to Expert Working Group: 24th May and 9th June 2021
Date of planned review: 27th December 2021
Evidence synthesis team: Sushil S (SS), Jisha Sara John (JSJ), Richard Kirubakaran, Bhagteshwar Singh & Priscilla Rupali.
We acknowledge gratefully the assistance received from the authors of the multi-platform RCT [mpRCT] (ATTACC, ACTIV-4a, and REMAP-CAP platforms), specifically Ewan Goligher, who provided valuable assistance in evidence clarification and sharing of additional protocol documents.
1. Outcomes in all categories of patients
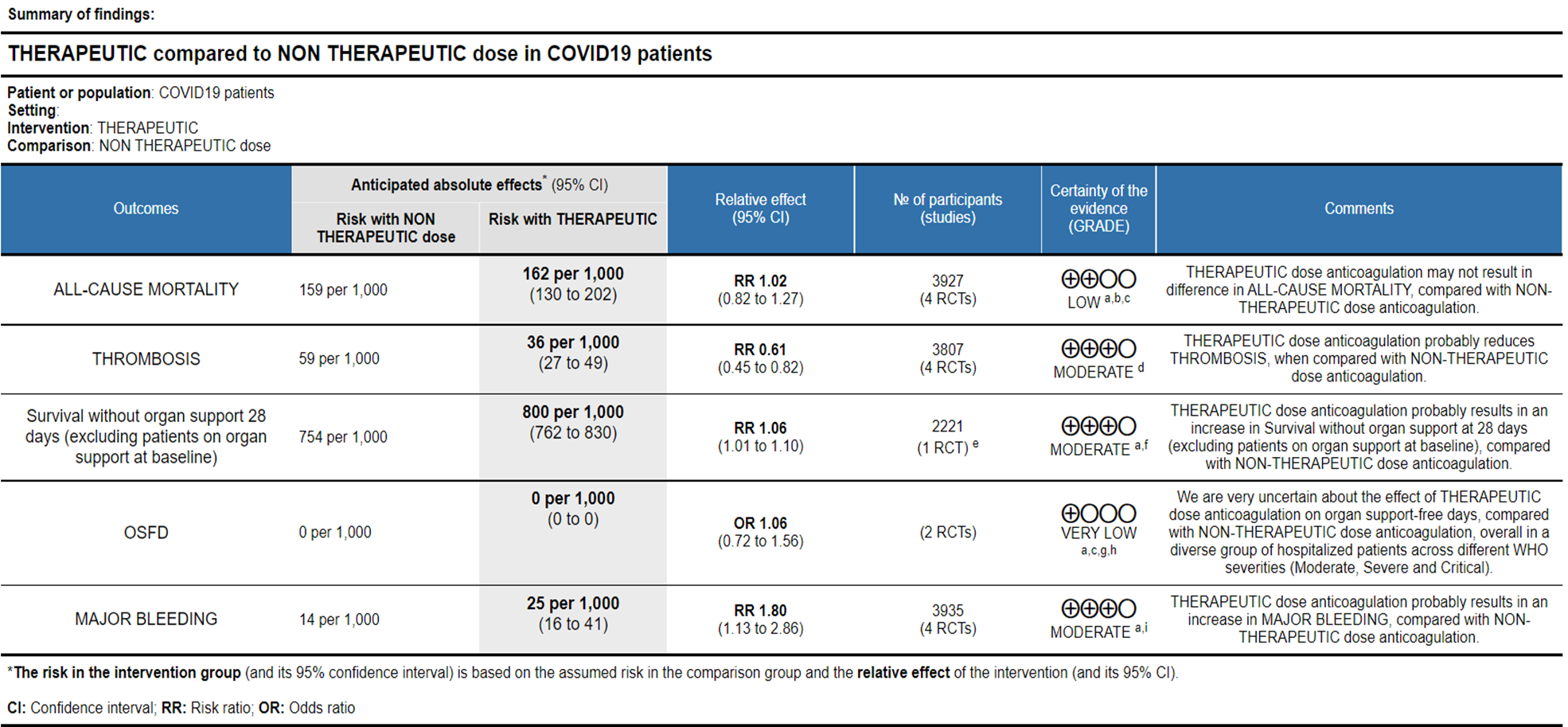
Ref: All Cause Mortality (13-16), Thrombosis (13-16), Survival without organ support 28 days (15), OSFD (14,15), Major Bleeding (13-16)
Explanations:
a. Not downgraded since risk of bias (RoB) assessment with RoB 2.0 tool scored 'some concerns' in only 1 domain of each of the studies, for this outcome. In the mpRCT studies [14;15], Domain 2 was marked down for 'some concerns' in view of significant deviations in intended interventions in trial, which probably did not affect outcomes. In HESACOVID trial [13], Domain 5 was marked down for 'some concerns' in view of not enough information being provided to completely rule out risk of bias in selection of the reported result.
b. Downgraded by 1 level for serious indirectness: the differences in mechanisms of action and drug delivery caused concerns in comparability between the 2 interventions (Rivaroxaban & Heparins) for these outcomes. Aspects like the anti-inflammatory effects of heparins and dosages used in the Rivaroxaban regimen were also discussed in this regard. These putative differences in pharmacological characteristics between Rivaroxaban and Heparin, beyond their direct antithrombotic effects, informed the decision to downgrade for Indirectness in the outcomes not related to thrombosis or bleeding.
c. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from appreciable benefit to harm.
d. Downgraded by 1 level for serious risk of bias; RoB assessment with RoB 2.0 tool scored 'some concerns' in 2 domains of the mpRCT studies [14;15]. In addition to Domain 2 as across all outcomes (see explanation a.), Domain 4 of RoB 2.0 tool was assessed to have some concerns because of the open-labelled nature of the trials which may have impacted aspects of assessment. This would not have a bearing on 'harder' outcomes like mortality, OSFD or major bleeding.
e. Data provided in mpRCT [15] for this outcome was an Adjusted Proportional Odds Ratio (using a bayesian approach) of 1.30 (1.06 - 1.62). We decided to compute the RR from the numbers provided in the supplementary data for this outcome, assuming a baseline risk of 75.4%.
f. Downgraded by 1 level for serious imprecision; 95% CI ranges from a clinically unimportant benefit to appreciable benefit.
g. Downgraded by 1 level for serious inconsistency: the I-squared is 84%, and there is minimal overlap of the CIs of individual ORs.
h. Downgraded by 1 level for serious indirectness: there was a very different baseline odds of higher organ-support free days: in one trial (mpRCT, Zarychanski et al. [14]) all participants were receiving organ support at baseline; in the other (mpRCT, Lawler et al. [15]) very few received organ support at baseline.
i. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from a clinically unimportant harm to appreciable harm.
2. Outcomes in the WHO moderate (on oxygen) and severe category of patients
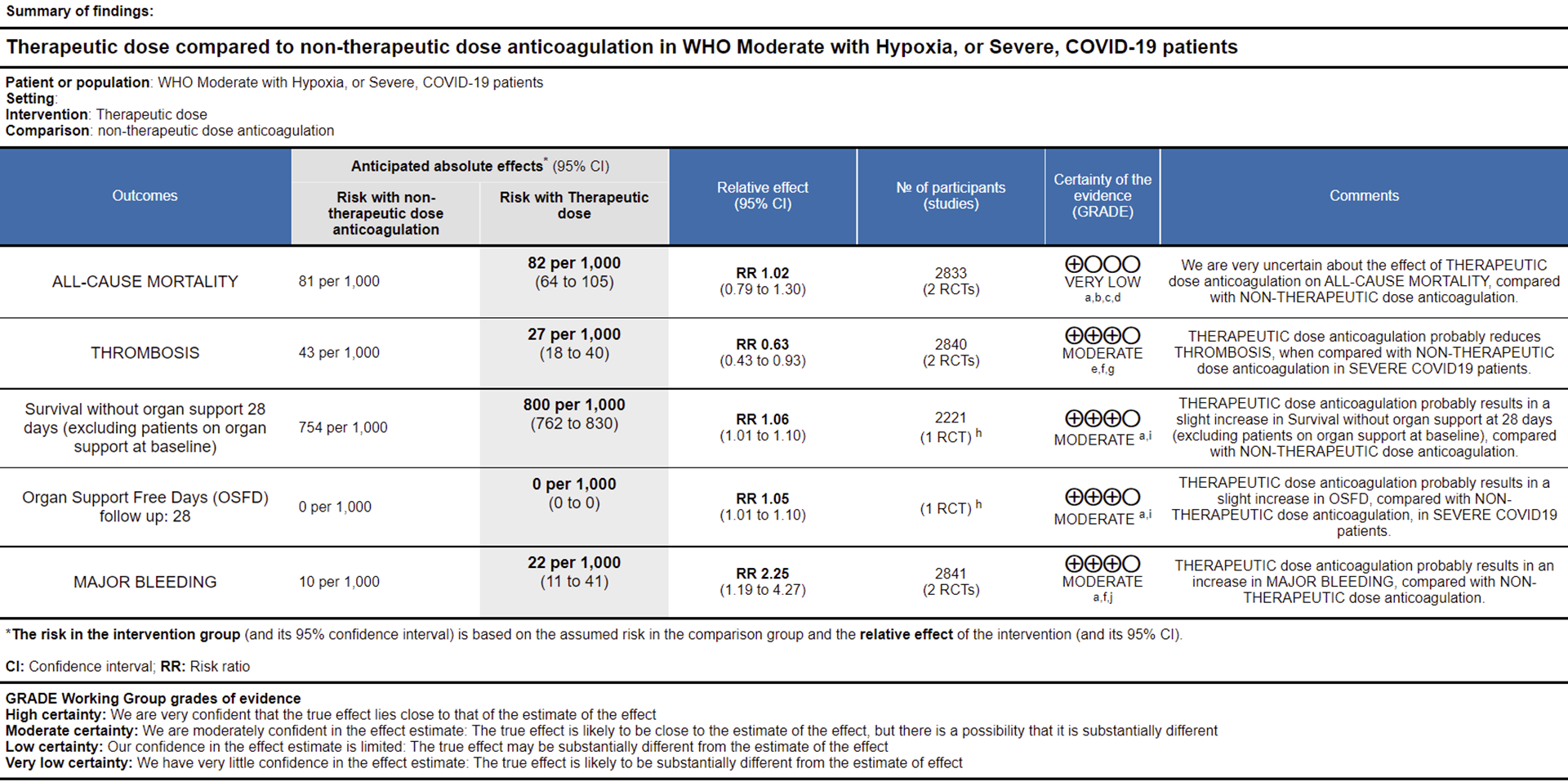
Ref: All Cause Mortality (15,16), Thrombosis (15,16), Survival without organ support 28 days (15), OSFD (15), Major Bleeding (15,16)
Explanations
a. Not downgraded since RoB assessment with RoB 2.0 tool scored 'some concerns' in only 1 domain of each of the studies [15;16], for this outcome. Domain 2 was marked down for 'some concerns' in view of significant deviations in intended interventions in trial; these probably did not affect outcomes.
b. Downgraded by 1 level for serious inconsistency; the I-squared is 67%.
c. Downgraded by 1 level for serious indirectness: the differences in mechanisms of action and drug delivery caused concerns in comparability between the 2 interventions (Rivaroxaban & Heparins) for these outcomes. Aspects like the anti-inflammatory effects of heparins and dosages used in the Rivaroxaban regimen were also discussed in this regard. These putative differences in pharmacological characteristics between Rivaroxaban and Heparin, beyond their direct antithrombotic effects, informed the decision to downgrade for Indirectness in the outcomes not related to thrombosis or bleeding.
d. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from appreciable benefit to harm.
e. Downgraded by 1 level for serious risk of bias; RoB assessment with RoB 2.0 tool scored 'some concerns' in 2 domains for mpRCT (Lawler et al. [15]), and in one domain for the other trial [16], for measurement of this outcome. In the mpRCT (Lawler et al. [15]), In addition to Domain 2 (see explanation a.), Domain 4 of RoB 2.0 tool was assessed to have 'some concerns' because of the open-labelled nature of the trials which may have impacted aspects of assessment of this outcome [15;16]. This would not have a bearing on 'harder' outcomes like mortality, OSFD or major bleeding.
f. Not downgraded for indirectness in these outcomes (Thrombosis/Bleeding), as the direct anti-thrombotic effect of therapeutic anticoagulation form both Rivaroxaban and Heparins could be considered comparable.
g. Not downgraded for imprecision as, even at the upper 95% CI, the benefit was considered clinically significant by the expert working group.
h. Data provided in mpRCT (Lawler et al [15]), was as Adjusted Proportional Odds Ratios (using a Bayesian approach). We decided to compute the RR, assuming a baseline risk of 75.4%, which we have mentioned in effects column.
i. Downgraded by 1 level for serious imprecision; 95% CI ranges from a clinically unimportant benefit to appreciable benefit.
j. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from clinically low harmful effect to appreciable harm.
3. Outcomes in critical category of patients
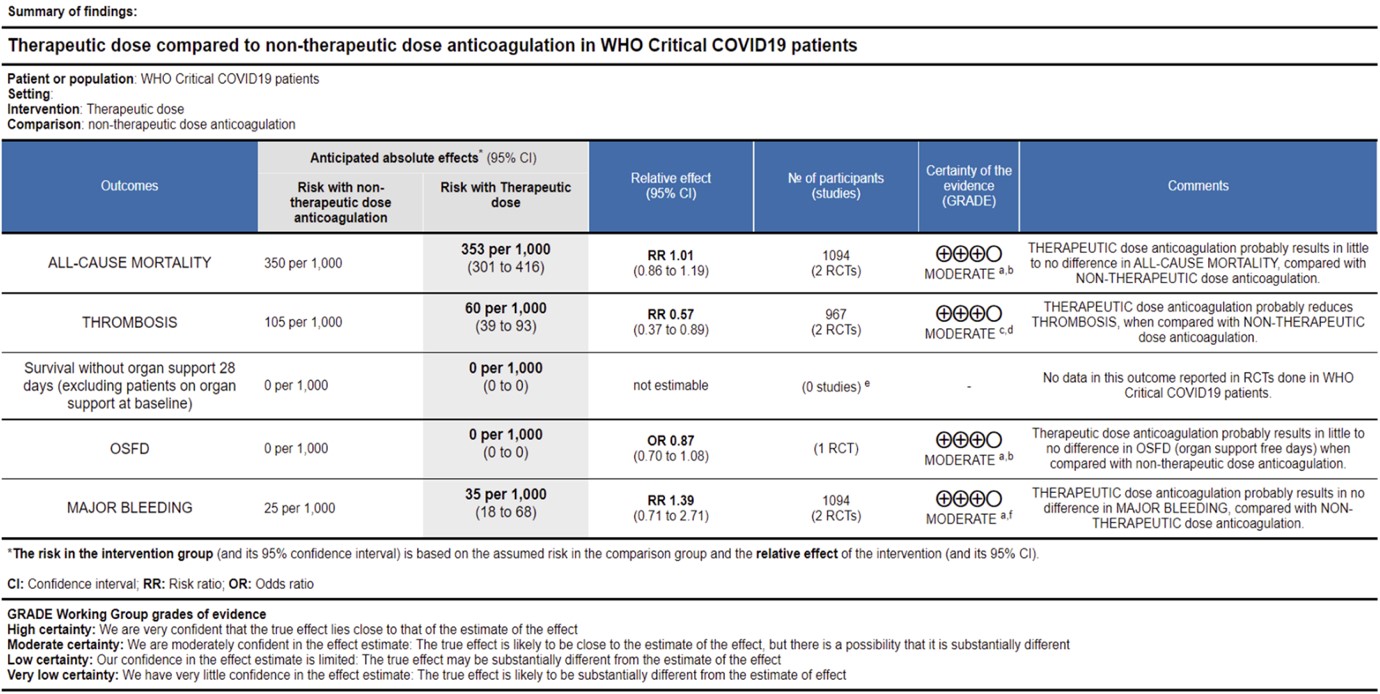
Ref: All Cause Mortality (13,14), Thrombosis (13,14), OSFD (14), Major Bleeding (13,14)
Explanations
a. Not downgraded since RoB assessment with RoB 2.0 tool scored 'some concerns' in only 1 domain of each of the studies, for this outcome. In the mpRCT (Zarychanski et al. [14]), Domain 2 was marked down for 'some concerns' in view of significant deviations in the intended interventions in trial, which probably did not affect In HESACOVID trial [13], Domain 5 was marked down for 'some concerns' in view of not enough information being provided to completely rule out risk of bias in selection of the reported result.
b. Downgraded by 1 level for serious imprecision; 95% CI ranges from appreciable benefit to harm.
c. Downgraded by 1 level for serious risk of bias; RoB assessment with RoB 2.0 tool scored 'some concerns' in 2 domains for mpRCT (Zarychanski et al. [14]), and in one domain for other trials, for measurement of this outcome. In the mpRCT (Zarychanski et al. [14]), Domain 2 was marked down for 'some concerns' - see explanation a. Domain 4 was assessed to have 'some concerns' because of the open-labelled nature of the trials which may have impacted aspects of assessment of this outcome. This would not have a bearing on 'harder' outcomes like mortality, OSFD or major bleeding.
d. Not downgraded for imprecision as, even at the upper 95% CI, the benefit was considered clinically significant by the expert working group.
e. No data contributed towards this outcome from available trials in WHO Critical COVID-19 patients.
f. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from appreciable benefit to appreciable harm.
Since December 2019, the worldwide pandemic of COVID-19, caused by the SARS-CoV-2 virus has adversely impacted humanity in diverse ways. Clinical studies of hospitalized patients with COVID-19 initially showed flu-like symptoms, most commonly cough, sore throat, fever, myalgia, and fatigue at onset of COVID-19 illness, which can then proceed to develop into a viral pneumonia with varying severity [3].
Abnormal coagulation profiles and thrombotic complications, both venous and arterial, are common among the hospitalized severe and critically ill patients [4], with pulmonary embolism the most common site [5].
In addition, multiple autopsy reports show unprecedented pulmonary microvascular thrombosis and endothelial damage [6] which could be related to the direct viral cytopathic effect on the endothelial cells due to shared receptors with the alveolar cells [7]. Other etiopathogenetic mechanisms include immune/cytokine mediated dysregulation of pro-coagulant & anti-fibrinolytic pathways.
Though hypercoagulability in COVID-19 is now well-recognized, uncertainty still exists as to how best to manage clotting risk in these patients. In addition, an increased risk of hypercoagulability leading to increased thrombotic events has been reported and recognized extensively in the media and among peers. There is a prevailing assumption that the delta variant in India may be contributing to an increased number of thrombotic events, however this needs to be systematically studied and documented.
Over the past year, several guidance documents have recommended the use of anticoagulation in hospitalized patients with COVID-19 [8]. Most of these guidelines recommend the use of unfractionated heparin (UFH) or low molecular weight heparin (LMWH), though the evidence is scarce with regard to which dose of anticoagulation i.e., prophylactic, intermediate, or therapeutic (“full”) dose should be employed in each severity group of COVID-19.
At present there is now fairly broad-based consensus from national and international guidelines that the standard of care is prophylactic dose anticoagulation to all in-patients with COVID-19 pneumonia. However, it remains unclear if specific severity sub-groups of patients will benefit from intermediate or therapeutic dose anticoagulation in the absence of a confirmed thrombotic event.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Pubmed), Epistemonikos (COVID Living Overview of Evidence [L*OVE] platform), and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language published from March 2020 up to 15th April 2021. We also reviewed reference lists of systematic reviews and included studies.
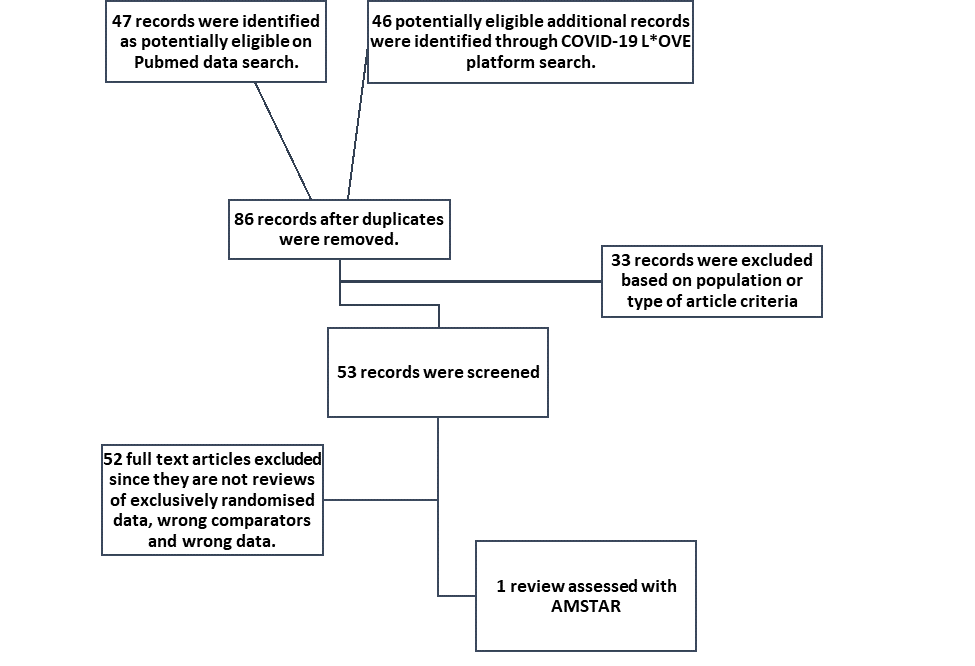
We searched the Pubmed database and found 47 systematic reviews, and from COVID Living Overview of Evidence(L*OVE) platform found 46 potentially eligible records. After removing duplicates, and excluding reviews that did not include exclusively randomized control trial (RCT) data or did not include intended interventions, we found only one systematic review looking specifically at the outcome of mortality [9]. When analysed with the AMSTAR 2 tool [10] it was found to be of low quality, and also did not include most outcomes of interest as defined by the working group’s PICO.
So, we decided to proceed with a rapid review of available RCTs that compare outcomes between anticoagulation doses in COVID-19 patients.
Two reviewers (SS & JSJ) independently assessed eligibility of search results. One reviewer extracted data from each included study, and both assessed risk of bias using the Cochrane Risk of bias (RoB) v2.0 tool [11].
We extracted data for the following outcomes, pre-defined by the Expert Working Group:
- Critical (primary for this review):
- Mortality (all-cause) – at 21-30 days, or in-hospital
- Thrombotic events
- Important (secondary):
- Time to clinical improvement
- Organ support free days (OSFD): - ventilator, inotropic requirements.
- Survival without organ support at day 28
- Duration of hospitalization
- Bleeding events
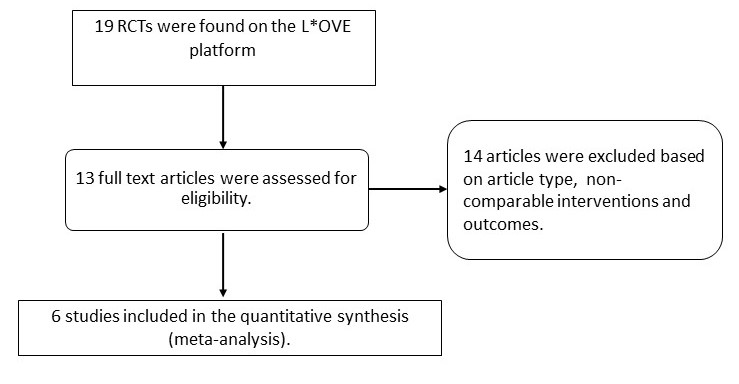
On searching Pubmed & COVID L*OVE platform and when restricting to RCTs, we found 19 records. After excluding extraneous records and trials that did not report any outcomes that could provide usable data for the review, we assessed 6 RCTs which compared differing doses of anticoagulation in COVID-19 patients.
We used RevMan v5.4 (12) to perform meta‐analysis using fixed-effect & random‐effects models for outcomes where pooling of effect estimates was appropriate. We used risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). Since the guidelines were going to be specific to each severity category we grouped studies as per their inclusion criteria into their severity categories and combined them as such to provide pooled estimates. We used the I2 statistic to measure residual heterogeneity. We used GRADE methodology to assess the certainty in the evidence, and documented this in a ‘Summary of findings’ table using GradeProGDT.
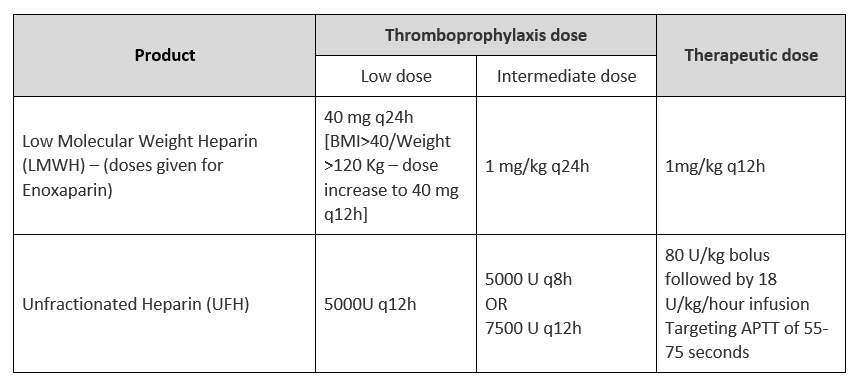
There are myriad dosing strategies for anticoagulation based on indication, organ dysfunction, BMI and adverse drug reactions, based on available literature and package insert recommendations. For purposes of our guidelines in the expert working group meeting it was decided to broadly group prophylactic & intermediate doses as non-therapeutic anticoagulation (up to and including 1mg/kg of enoxaparin or equivalent subcutaneously once daily) AND higher doses would be therapeutic dose anticoagulation (usually 1mg/kg enoxaparin or equivalent subcutaneously twice daily).
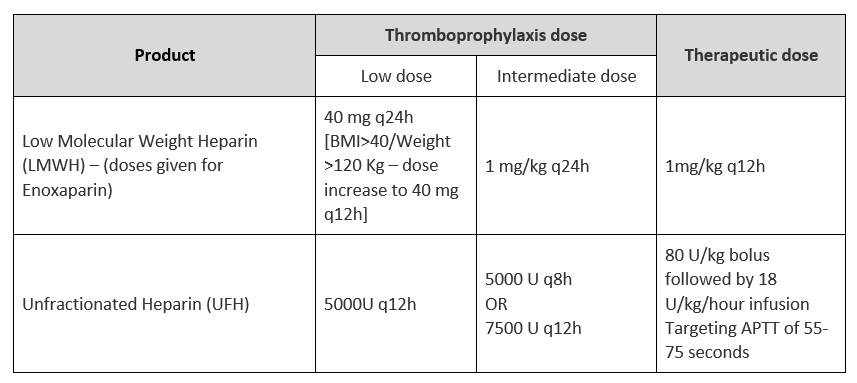
Though we are using the term anticoagulation, the intent of use of anticoagulation in all these trials was prophylaxis of thrombotic events, but two different doses are being compared in each of these trials: therapeutic vs. non-therapeutic, the latter of which may be prophylactic or intermediate dose.
Of the 6 RCTs found, four attempted to compare therapeutic dose anticoagulation with non-therapeutic doses:
- HESACOVID [13]: compared prophylactic vs. therapeutic dose of anticoagulation (20 participants).
- mpRCT (Critically ill; Zarychanski et al.) pre-print [14]: a collaboration of 3 RCT platforms, ACTIV-4a, REMAP-CAP and ATTACC, looking specifically at critically ill patients (total of 1074 participants) – comparing therapeutic vs non-therapeutic dose of anticoagulation.
- mpRCT (Non-critically ill; Lawler et al.) pre-print [15]: is a collaboration of above mentioned 3 RCT platforms, looking specifically at non-critically ill patients (total of 2291 participants) – comparing therapeutic vs non-therapeutic dose of anticoagulation.
- COALITION-ACTION [16]: Looked specifically at the WHO moderate to severe categories of participants.
Two randomized trials studied the effect of intermediate vs. prophylactic dose anticoagulation:
- INSPIRATION [17]: compared prophylactic vs. intermediate dose of anticoagulation (562 participants).
- Usha Perepu et al, preprint [18]: compared prophylactic vs. intermediate dose of anticoagulation (176 participants).
Since we were providing category specific recommendations, HESACOVID [13] and mpRCT critical [14] were analysed together to enable evidence synthesis and a recommendation in the critical category of patients. In addition, mpRCT non-critically ill [15] and COALITION ACTION [16] were analysed together to provide a moderate-severe category specific recommendation as well. The results of INSPIRATION and Usha Perepu et al were kept separate from these other trials, in view of different comparator arms which were prophylactic and intermediate dose of anticoagulation. They are not presented in the results of this review.
The critical outcomes that were available and extracted for analysis from these studies included:
- All-cause mortality (21-30 days),
- Thrombosis,
- Organ support free days (i.e., number of days without need for ICU-level organ support, including invasive and non-invasive mechanical ventilation)
- Survival without organ support at 28 days
- Major bleeding events.
We included 4 trials with 3,927 patients of which 1 trial did not contribute much data (13). The two trials which did not include a therapeutic dose of anticoagulation as a comparator arm were excluded from this analysis (17,18). The patients were compared across different pre-specified COVID19 severity groups, for the different outcomes as mentioned above (See summary of characteristics tables below). The duration of administration of anticoagulation varied from a minimum of 96 hours to 14 days or till the patient got better.
We tailored the severity strata in the study according to WHO COVID-19 Clinical management: living guidance severity classification (Interim document originally published under the title "Clinical management of COVID-19: interim guidance, 27 May 2020"(19).
| | WHO severity criteria correlate |
|---|---|
| mpRCT (Critically ill) - Zarychanski et al. | WHO Critical |
| mpRCT (non-Critically ill) - Lawler et al. | WHO Severe & Moderate |
The group of patients studied in the mpRCT(Critically ill) study (14), correlated directly with WHO critical severity criteria. The group of patients studied in the mpRCT(non-Critically ill) study(15), correlated with WHO severe category also overlapping with a few in the WHO moderate category.
Another aspect to be factored into the analysis was that, in COALITION-ACTION trial (16), the overwhelming majority of patients in the therapeutic dose arm(90.3%) received Rivaroxaban. This may have led to indirectness. Aspects like the anti-inflammatory effects of heparins, absorption of oral drugs and dosages used in the Rivaroxaban regimen were also discussed in this regard. These putative differences in pharmacological characteristics between Rivaroxaban and Heparin, beyond their direct antithrombotic effects, informed the decision to downgrade for indirectness in the outcomes not related to thrombosis or bleeding. (See further detailed explanations in SoF tables)
Overall analysis: Using GRADE methodology certainty of evidence is shown in Summary of Findings tables.
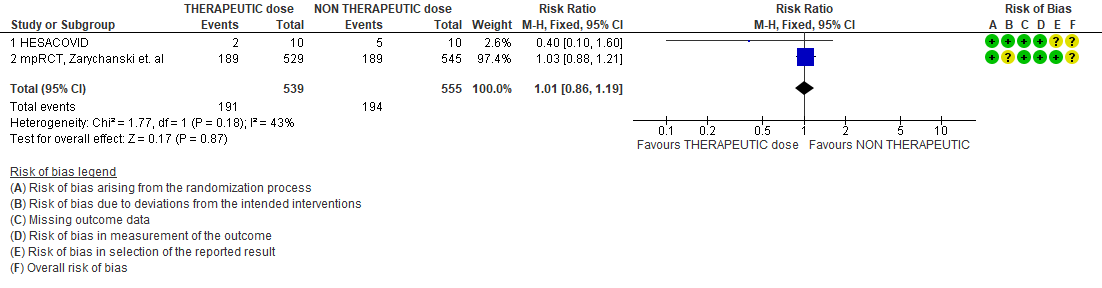
- All-cause Mortality- Low certainty of evidence in 4 trials with 3927 patients, revealed that therapeutic dose anticoagulation may result in little to no difference in all-cause mortality, compared with non-therapeutic dose anticoagulation (RR 1.02, 95 % CI=0.82 to 1.27).
- Thrombosis- Moderate certainty of evidence in 4 trials with 3807 patients, revealed that therapeutic dose anticoagulation probably reduces thrombosis, when compared with non-therapeutic dose anticoagulation (RR= 61, 95 % CI = 0.45 to 0.82).
- Major bleeding- Moderate certainty of evidence in 3 trials with 3321 patients, revealed that therapeutic dose anticoagulation probably results in an increase in major bleeding, compared with non-therapeutic dose anticoagulation (RR 1.80, 95% CI = 1.13 to 2.86). There were 3 cases of fatal bleeds in mpRCT (non-critically ill) when given therapeutic dose anticoagulation vs 1 in the prophylactic dose anticoagulation.
- OSFD- Very low certainty of evidence in 2 trials with 3301 patients, revealed a very uncertain effect of therapeutic dose anticoagulation on organ support-free days in hospitalized patients, compared with non-therapeutic dose anticoagulation when patients from WHO moderate, severe & critical COVID19 strata were combined from available trials (RR=1.06,95%CI 0.72-1.56).
Since guidelines are to provide recommendations for each severity strata, disaggregated data was also assessed to provide analysis to help formulate recommendations specific to each severity strata.
Analysis according to severity strata
WHO Critical group: Very low certainty evidence from 2 trials (13,14) showed that using therapeutic dose anticoagulation did not significantly reduce mortality in critical COVID19 illness (RR-1.01, 95 % CI = 0.86 to 1.19). However, moderate certainty evidence did show decreased thrombosis in patients (RR-0.57, 95 % CI = 0.37 to 0.89) but with no increase in bleeding (RR-1.39, 95 % CI = 0.71 to 2.71). In addition, moderate certainty evidence from 1 trial (28), showed that therapeutic anticoagulation did not improve days free of organ support compared to prophylactic dose anticoagulation [Odds Ratio (OR) 0.87, 95% CI 0.70 to 1.08]. However, the median OSFD in the therapeutic dose anticoagulation group was 3 days vs 5 days in the prophylactic dose anticoagulation group. It was also noted that 41% received low dose prophylactic dose anticoagulation and 51% received an intermediate dose prophylactic dose anticoagulation.
Bayesian analysis from Zarychanski et al (14). showed a posterior probability of futility of 99.8% and posterior probability of inferiority of 89.4% for impact of therapeutic anticoagulation on OSFD. DSMB stopped recruitment since pre-specified futility boundary for therapeutic anticoagulation was achieved. However, this was for OSFD not for mortality or thrombotic events.
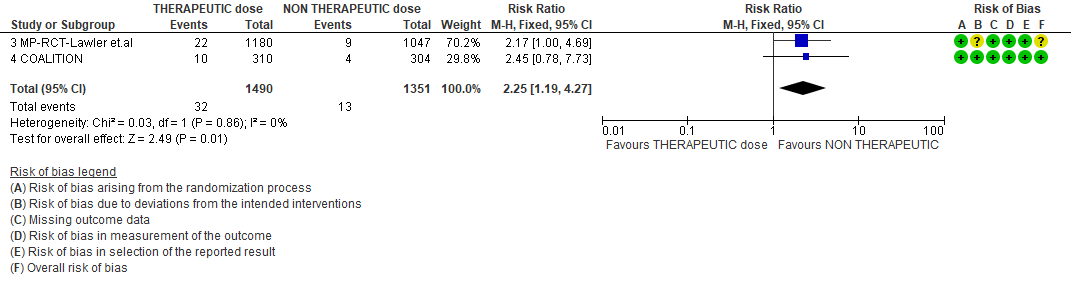
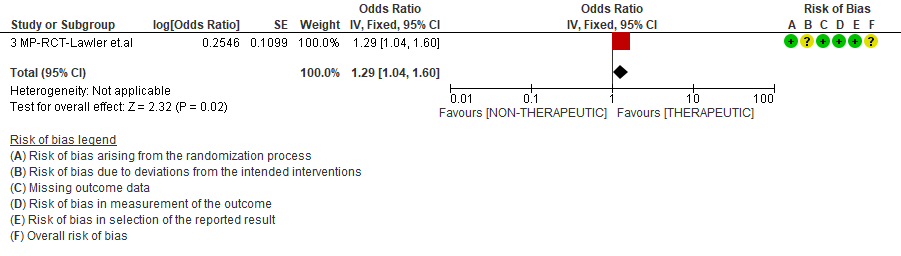
WHO Moderate/Severe group: Very low certainty evidence from 2 trials (15,16) suggested that therapeutic anticoagulation provided no mortality benefit RR 1.02 (95% CI 0.79 to 1.30). However there was moderate certainty evidence that therapeutic dose anticoagulation did appear to prevent thrombosis by 37% (RR 0.63, 95 % CI 0.43 to 0.93) with a clinically appreciable risk of major bleeding of greater than 19%; RR 2.25, 95 % CI 1.19 to 4.27).In addition there was moderate certainty evidence from 1 trial (29) showing that therapeutic dose anticoagulation increased the probability of OSFD with OR 1.05, 95 % CI 1.01 to 1.10 and similarly survival without organ support RR 1.06 (95% CI 1.01 to 1.10). However fatal bleeds in mpRCT non- critically ill [15] revealed 3 bleeds in therapeutic dose anticoagulation vs 1 in the prophylactic dose anticoagulation group.
Bayesian analysis from mpRCT (non-critical) (15) showed a posterior probability of superiority of 99% for therapeutic anticoagulation improving OSFD. In this trial, deep venous thrombosis (DVT) was included as significant thrombosis which seems to have been excluded from mpRCT critical group (14).
Analysis of studies using non-therapeutic doses
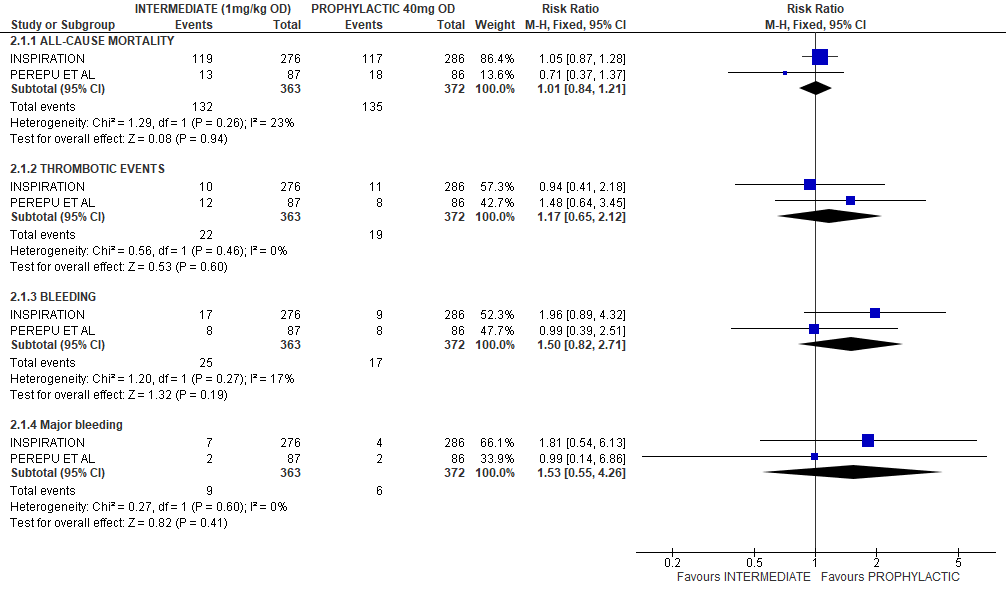
In a brief analysis of RCTs INSPIRATION & Perepu et al.[17,18] which compared prophylactic vs intermediate dose anticoagulation (see table above), no statistically significant differences were noted in the outcomes of all-cause mortality [RR1.01,95% CI 0.84-1.21];thrombotic events [RR 1.17;95% CI 0.65-2.12];bleeding [RR 1.5;95%CI 0.82-2.71]; major bleeding [RR1.53;95%CI 0.55-4.26] assessed. (See relevant forest plot)
Table 1: The four trials analysed for various outcomes
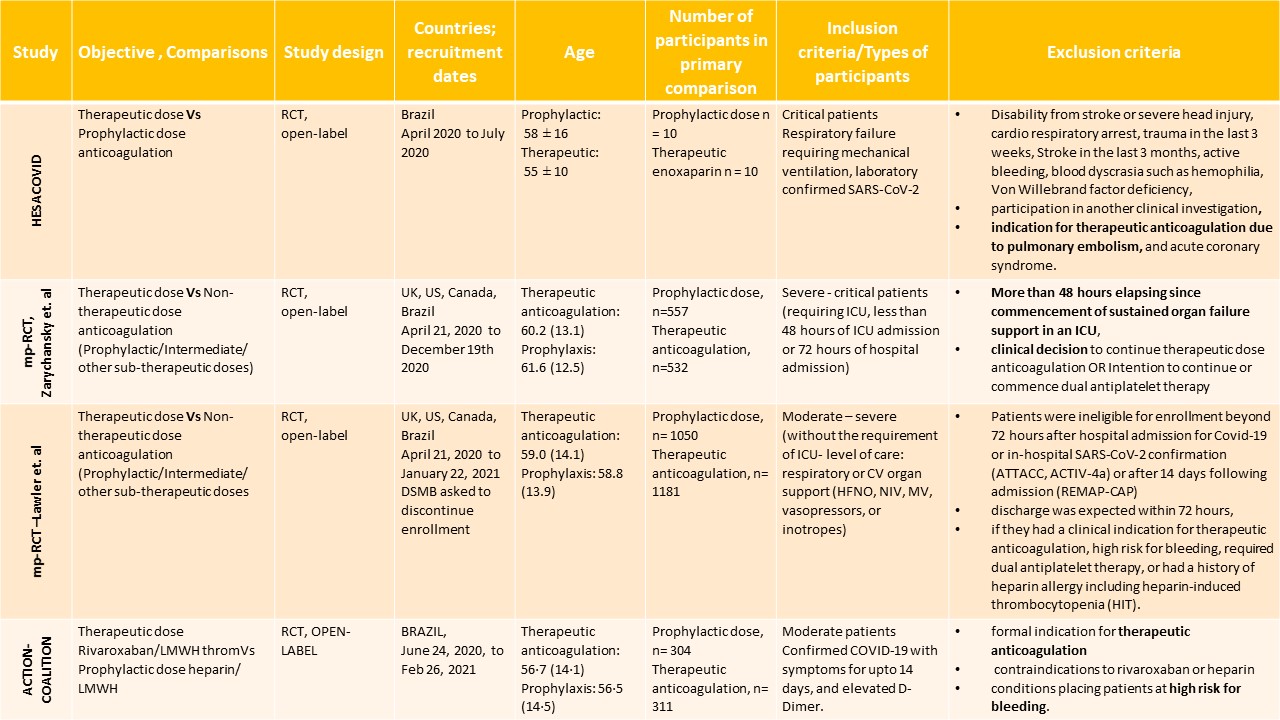
* ICU: intensive care unit; RCT: randomized controlled trial
Table 2. Summary of D-dimer values and co interventions administered in these 4 trials
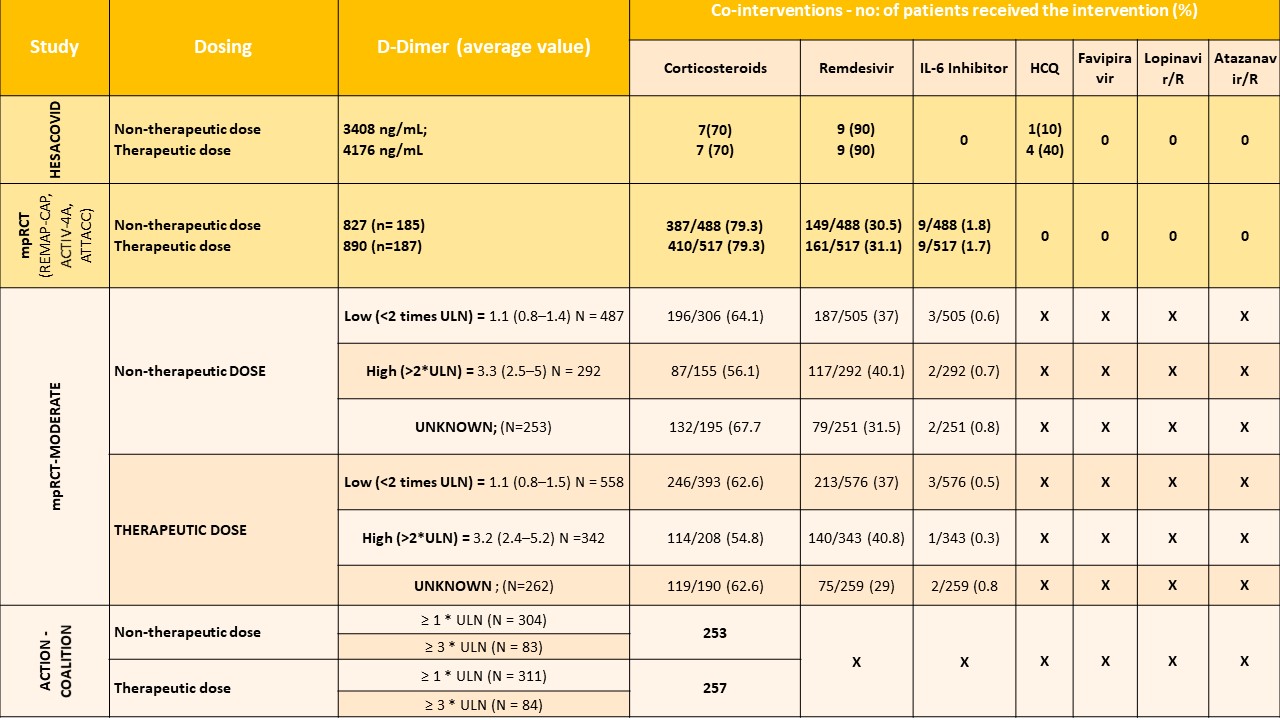
* R: ritonavir; ULN: upper limit of normal
THERAPEUTIC VS NON-THERAPEUTIC DOSE OF ANTI-COAGULATION ACROSS ALL SUBGROUPS OF SEVERITY
- All-cause mortality (21-30 days)
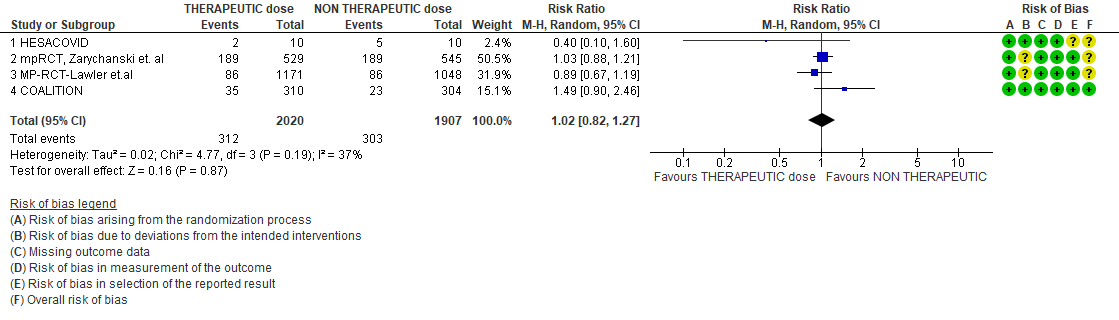
2. Thrombosis
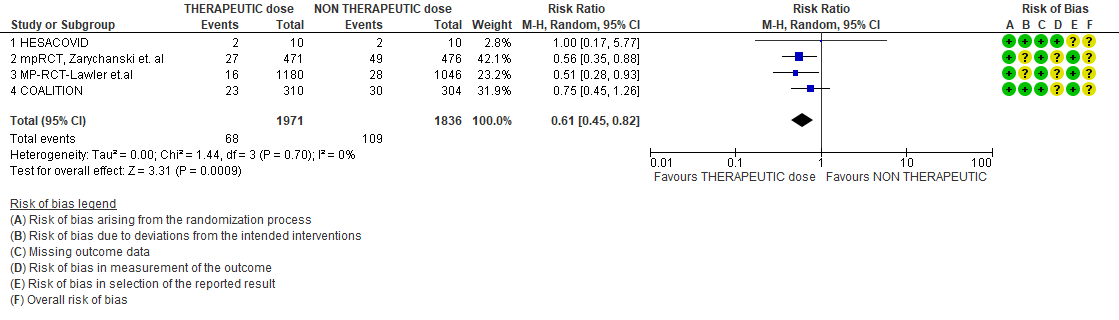
3. Major Bleeding
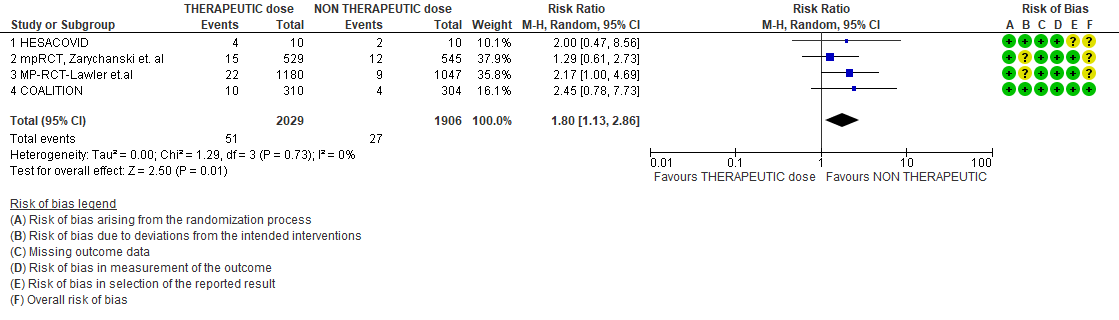
4. Organ support free days (OSFD)

THERAPEUTIC VS NON-THERAPEUTIC DOSE OF ANTI-COAGULATION ACROSS CRITICAL SEVERITY SUBGROUP
- All-cause mortality (21-30 days)
- Thrombosis
- Major Bleeding
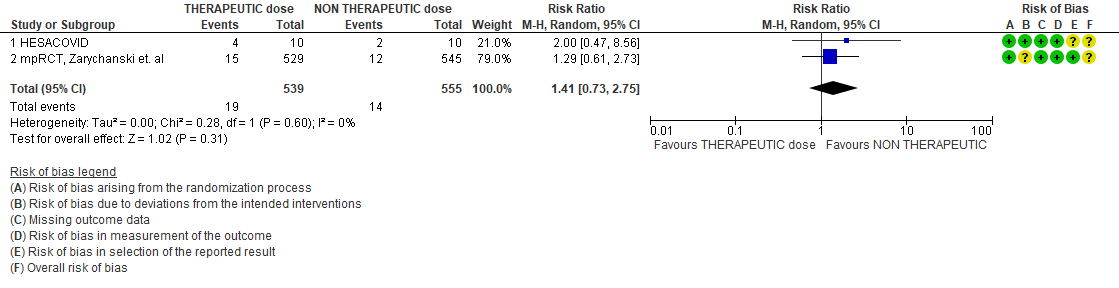
4. Organ support free days
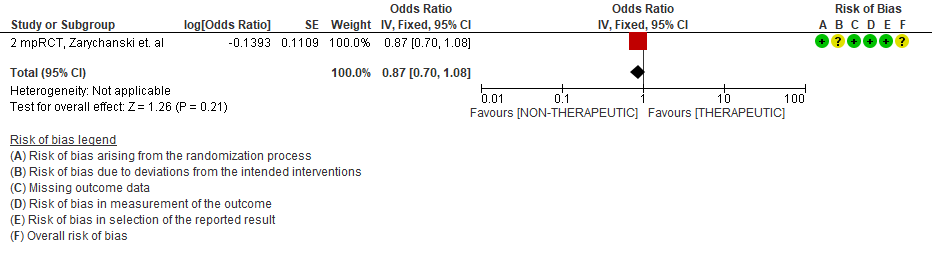
THERAPEUTIC VS NON-THERAPEUTIC DOSE OF ANTI-COAGULATION ACROSS MODERATE TO SEVERE SUBGROUP
- All cause mortality
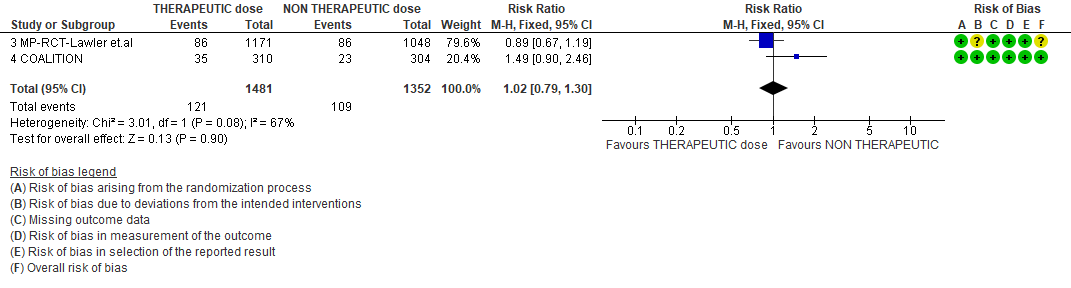
2. Thrombosis
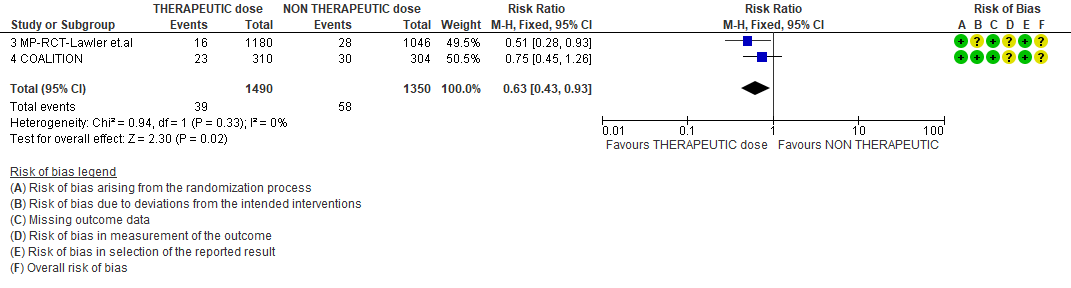
3. Bleeding
4. Organ support free days
 PROPHYLACTIC VS INTERMEDIATE DOSE OF ANTICOAGULATION
PROPHYLACTIC VS INTERMEDIATE DOSE OF ANTICOAGULATION
The Anticoagulation Expert Working Group met on 24th May 2021 to consider the use of therapeutic vs prophylactic dose anticoagulation in the management of COVID-19. A summary and then more detailed explanations of their judgements follow.
Summary of judgements
EtD summary table
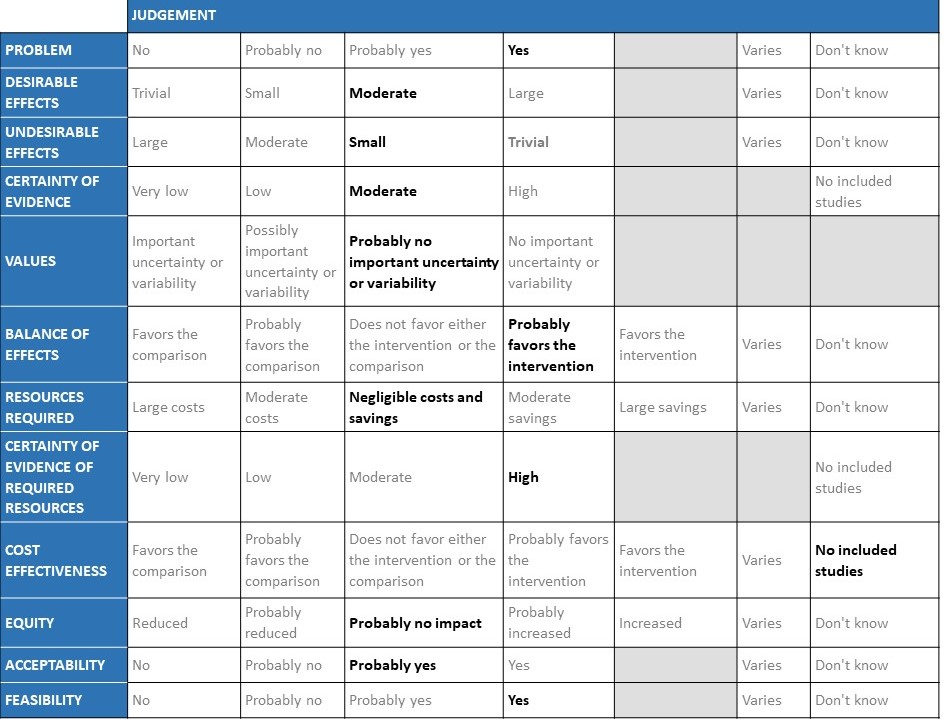
Problem
Hypercoagulability is a recognized phenomenon in COVID-19 and is believed to be multifactorial. SARS-CoV-2 virus both directly and indirectly causes endothelial injury, microvascular inflammation, endothelial exocytosis, endotheliitis contributing to acute respiratory distress syndrome. In post-mortem studies microvascular occlusion with platelet-fibrin thrombi have been reported. In addition, changes in circulating prothrombotic factors and stasis due to immobility encountered in the critically ill has led to a recognized prothrombotic state in COVID-19 infection, translating to increased arterial and venous thrombosis. This has led to the practice of prophylactic anticoagulation for all hospitalized COVID-19 patients. However there exists an area of equipoise whether patients with severe or critical COVID-19 infection will benefit with therapeutic (full) dose anticoagulation in the absence of a confirmed thrombotic event. This has especially become even more important as the country goes through a massive second wave probably caused by the delta variant B.1.617.2 starting in early February, which anecdotally seems to cause more thrombosis as per various reports in the media, however we need to await cohort studies for this to be accurately assessed.
Desirable effects
WHO Critical: At present, evidence shows that using therapeutic dose anticoagulation probably does not significantly reduce mortality in critical COVID-19 but does appear to prevent thrombotic events by 42% (95% CI 11-63%) with a moderate certainty of evidence. Therapeutic dose anticoagulation also did not improve days free of organ support compared to prophylactic dose anticoagulation. The DSMB stopped recruitment in this category as it felt that therapeutic dose anticoagulation did not offer any advantage in OSFD (as a pre-specified Bayesian post probability of futility was achieved). The group discussed this at length and felt that preventing thrombosis was important even in the absence of mortality benefit. Panel also noted that most of the evidence was contributed to by the mPRCT critical trial [14] with very few patients in HESACOVID [13].
WHO Moderate (with hypoxia)/Severe: The group noted that using therapeutic dose anticoagulation probably reduced thrombosis and increased the probability of OSFD but did not significantly reduce mortality (very-low certainty evidence), though it probably increased bleeding in moderate/severe category of COVID-19. The group felt that right now thrombosis was an important event to prevent as it was difficult to recognize or confirm and probably would contribute to significant morbidity. However, the group felt that bleeding was easy to pick up and was rarely fatal. Hence the group that in an intensive care setting prevention of thrombosis was an important intervention. The various thrombotic events were reviewed and pulmonary embolism seemed to contribute to most of the events with other events recorded being myocardial infarction and strokes.
Undesirable effects
WHO Critical: The mPRCT (Critical)[14] had >500 participants in each arm and the incidence of bleeding in each group was similar. The absolute difference in bleeding between the two groups was 1%, suggesting no clinically important increase in the risk of bleeding. The DSMB of ACTIV-4 component of the mPRCT trial had recommended an early interim analysis to assess for effectiveness or harm, however the study was terminated as the futility threshold was reached suggesting that there was no benefit of therapeutic dose anticoagulation over non-therapeutic dose anticoagulation on the primary outcome of OSFD.
WHO Moderate (with hypoxia)/Severe: The mPRCT (Non-critical) [15] had >1000 patients in each arm and the incidence of bleeding in the therapeutic dose group was higher in this group. Despite the pooled risk ratio of 2.26, when considering absolute effects, this would translate to only 12 per 1000 (1.2 per 100) more bleeding events per patients treated, with a lower confidence interval bound of only 1 per 1000 difference between groups.
The panel also discussed the absence of data regarding anticoagulation in the mild and moderate groups without hypoxia and felt that decisions about anticoagulation in those groups could be based on evidence from non-randomized studies and clinical experience.
Certainty of evidence
Overall the quality of evidence in the WHO moderate (with hypoxia), severe and critical categories was felt to be moderate, though a minority of outcomes achieved only very low or very low certainty in the evidence.
Values
Outcomes noted were considered to be important in overall patient management especially in the setting of the pandemic. The group felt that the outcomes of mortality, thrombotic events, bleeding and organ support free days were certainly important to all stakeholders. They felt that there was no uncertainty or variability. However, they did note some unusual points in the way outcomes were assessed (for example, the Bayesian analyses were difficult to quantify and interpret). Other points noted include:
Critical group: The group discussed the uncertainty as to whether thrombotic events could have contributed to mortality. There is no clear breakup of mortality in the pre-print or supplement. Clinically significant pulmonary embolism seems to have contributed to most of the thrombotic events, but it was not specified as to whether they were all major arterial or just segmental embolism. It also seemed unlikely that a patient who is severely or critically ill would have been shifted out for a CT pulmonary angiogram (CTPA). Deep vein thrombosis was excluded in this group as a major thrombotic event.
Moderate: In the mpRCT non-critical [15] group, deep vein thrombosis was included as significant thrombosis which was excluded from mpRCT critical[14] group.
Balance of effects
Given that the country has recently faced a shortage of beds, oxygen and intensive care, if an intervention could reduce organ support and thrombosis it would be of benefit.
WHO Critical: There are very few trials overall to inform evidence and there seemed to be no mortality benefit or decrease in OSFD between therapeutic dose anticoagulation and non-therapeutic dose anticoagulation. There was a decrease in thrombotic events with no increase in bleeding noted. The DSMB stopped the larger trial [14] as futility end-point for OSFD was reached in interim assessment.
WHO Moderate/Severe: therapeutic dose anticoagulation appeared to increase OSFD, and prevent thrombotic events with a slightly increased risk for major bleeding. There was no mortality benefit between the 2 arms. OSFD should be given importance in the moderate category as it saves resources and prevents morbidity.
Resource requirements
There are likely to be negligible savings or costs pertaining to implementation of therapeutic dose anticoagulation. Drugs used for this are relatively cheap, widely available and unlikely to incur a significant cost nor are they likely to result in significant savings. Health care workers in the country are fairly experienced with the use of the same and are aware of the various monitoring implications. Cost of implementation is low and needs to be weighed against hospitalization and intensive care costs. Though monitoring of anticoagulation efficacy with Anti-Xa testing will be possible only in an advanced hemostasis laboratory, if widely employed to ensure therapeutic efficacy it may also protect against unnecessary bleeding. However, this will increase costs and probably unnecessary in most instances. There is also possible ineffectiveness of therapy in view of high rates of heparin resistance documented in published data but overall costs of implementing therapeutic dose anticoagulation are likely low if we are able to save on costs of hospitalisation and intensive care beds.
Certainty of evidence of required resources
Resources required for implementation of therapeutic dose anticoagulation are minimal and the certainty of evidence for this is high.
Cost effectiveness
At the moment there are no data relating to the cost effectiveness of this intervention and studies need to be done to be sure of the same. The group recognized the minimal costs and resources required to deliver as it is a subcutaneous injection which can be delivered by most health care workers easily. It is also likely that even if patients in the moderate category are given therapeutic dose anticoagulation, hospitalization will be dictated by disease severity rather than administration of anticoagulation, given that most health care settings and health professionals are comfortable with delivery of this intervention in the outpatient settings. There are also many novel oral anticoagulants available which have been proven to have similar efficacy as heparin and could potentially be employed as substitutes, however data with these are scarce in the COVID-19 setting.
Equity
As the cost and resource requirements of anticoagulation delivery are reasonable, implementation can be equitable.
Acceptability
This intervention would be considered acceptable to patients and health care workers as it is in common use for a variety of indications other than COVID-19.
Feasibility
Therapeutic dose anticoagulation is a feasible intervention which can be easily implemented in all health care settings by any health care professional.
There is anecdotal evidence that thrombotic risk is increased with the newer delta variant [20], so thrombosis is increasingly being noticed in the second wave of COVID-19 in India. Whether this is more apparent due to the increased numbers of patients in a short space of time, or whether there is a true correlation of the delta variant with an increased risk of thrombosis remains to be determined. In mild or moderate non-hospitalized patients, there seems to be no routine role for anticoagulation.
Moderate (hypoxic) and severe: The group felt that for these severity groups the evidence suggests a positive benefit vs. risk balance in favour of therapeutic anticoagulation. Other considerations, such as feasibility and low cost, were also favourable. Important clinical outcomes like prevention of thrombotic events and reduced need for ICU-level organ support, including invasive and non-invasive mechanical ventilation (OSFD) were noted with minimal harm or bleeding risk. Overall the group given the evidence felt therapeutic dose anticoagulation should definitely be considered in this group of patients.
Critical: However, the group was less certain in the critical category of illness. Evidence revealed there was prevention of thrombotic events but no improvement with regard to mortality or organ support. In addition, no harm was demonstrated. Anecdotally most clinical members of the expert working group reported that they use either intermediate or therapeutic doses routinely in their intensive care areas and felt that this decision should be individualized according to the clinical picture of the patient or with monitoring of anti-Xa levels.
Overall, anticoagulation is feasible to implement widely and easily. There is evidence for Injectable low molecular weight heparin and Unfractionated Heparin through the data presented. However, Direct Oral Anti Coagulants (DOACs) like Rivaroxaban/Apixaban are being used in COVID-19 patients widely in clinical practice. However, in our analysis they were studied only in one of the smaller trials and hence data regarding its efficacy in COVID-19 is scarce. Given the increased risk in bleeding noted and difficulty in immediate reversal of anticoagulant effect of DOACs, decision to prescribe these should weigh these benefits and harms especially when administering at therapeutic doses.
Enoxaparin achieves anticoagulant effect by activating antithrombin. Routine laboratory monitoring for efficacy is not usually necessary. However, in special situations such as obesity, renal insufficiency, and pregnancy, laboratory monitoring may be required. The peak anti-factor Xa (anti-Xa) level is the recommended test for monitoring enoxaparin efficacy. Blood samples should be withdrawn about 3–5 hours after dose administration. In patients on therapeutic anticoagulation with unfractionated heparin, APTT monitoring can be done and maintained 1.5 to 2 times the control. In other anticoagulation regimens using low molecular weight heparin or Fondaparinux, regular anti-Xa monitoring is not recommended other than in obese, pregnancy or in renal insufficiency patients. In addition, in obese patients that intermediate dose of anticoagulation may need to be considered over prophylactic dose anticoagulation to achieve the required prophylactic levels. Renal insufficiency may prompt also prompt dose modification. In pregnancy data is still evolving and decisions regarding the required dose may need to take into consideration indications other than COVID-19. In the setting that heparin resistance is suspected, even in the setting of unfractionated heparin, anti-Xa levels should be monitored. Target peak anti-Xa for the treatment doses of twice-daily enoxaparin is 0.6–1.0 IU/mL. The target peak anti-Xa level for prophylactic doses of enoxaparin is 0.2–0.5 IU/mL [22].
The following points are to be considered prior to initiating anticoagulation in patients:
- Contra-indications to anticoagulation:
- Absolute: Platelets <20,000/mm3,
- Relative: platelets <50,000/mm3; Brain metastases; Recent major trauma; Major abdominal surgery within the past 2 days; Gastrointestinal or genitourinary bleeding within the past 14 days; Endocarditis; Severe hypertension (systolic BP >200 or diastolic BP >120 mmHg). - Specific contraindications and dosing considerations:
- Enoxaparin (taken from literature for Lovenox®): Known hypersensitivity to enoxaparin, heparin or other LMWHs; History of immune mediated heparin-induced thrombocytopenia (HIT) within the past 100 days or in the presence of circulating antibodies; Active major bleeding and conditions with a high risk of uncontrolled haemorrhage including recent haemorrhagic stroke. (Refer to product literature for further details, including dosing for renal failure.)
For alternative drugs, consult product literature.
The subgroups of children <18 years of age, pregnant women, asymptomatic, mild and out-patients were excluded from most studies. Some studies also excluded those on dialysis for chronic kidney disease, chronic liver and lung diseases as well as those on antiplatelet therapy.
The utility of D-dimer was also evaluated as part of a subgroup analysis. In the mpRCT (non-critical [15]) study it was noted that a high D-dimer is associated with a high risk of mortality and organ support and thus the adjusted absolute treatment benefits were more apparent, as the groups were stratified according to a high D-dimer, low D-dimer and unknown D-dimer values. However, in this trial, it seemed that the benefit of therapeutic dose anticoagulation was irrespective of the D-dimer values. The COALITION ACTION trial [16] included only patients with an elevated D-dimer with the values ranging from 1-3 times the upper limit of normal in 75% of participants to >3 times the upper limit of normal in 25% of participants in each arm. In the mpRCT (critical[14]) and HESACOVID [13] trials, no stratification was done according to the D-dimer values. The mean D-dimer values were 827 ng/ml in 48% of therapeutic dose anticoagulation vs 890 ng/mL in 46% of non-therapeutic dose anticoagulation (both values nearly twice the upper limit of normal) in mpRCT (critical [12]). In HESACOVID [13] the mean value of D-dimer was >4 times normal in the intervention and control groups.
There is no defined role for monitoring of D-dimer in non-hospitalised COVID-19 patients. A high D-dimer is known to be associated with an increased risk of venous thromboembolism in patients without COVID-19, and in COVID-19 it has been correlated with a poorer prognosis and increased mortality (21). However, whether a high D-dimer value can be extrapolated to an increased risk of arterial or venous thrombosis in COVID-19, as in the non-COVID-19 literature and thus higher mortality due to this is yet to be determined. Hence in hospitalized patients with COVID-19 with no clinical or radiological evidence of thrombosis, there is insufficient data to recommend regular monitoring of D-dimer or to base management strategies on the same.
At this point, it seems that the balance of effects considering the incidence of thrombosis vs risk of bleeding favours therapeutic dose anticoagulation over prophylactic dose anticoagulation in the moderate with hypoxia to severe categories of patients. This conditional recommendation regarding use of anticoagulation may be revisited as evidence emerges. In addition to evidence of benefit, with its widespread use in India, there may be additional cohort evidence emerging regarding incidence of thrombosis and bleeding with therapeutic dose anticoagulation which the group will monitor. In addition, the COVID Guidelines India anticoagulation expert working group is embarking on a survey to assess if the risk of thrombosis has increased in the second wave as compared to the first which may provide supporting evidence towards institution of therapeutic anticoagulation. However, we need to be cautious towards interpretation of any data relating to outcomes with different dosing strategies, as this would be non-randomized data.
It has been shown that therapeutic dose anticoagulation probably improves clinical outcomes in patients with moderate (with hypoxia) and severe COVID-19, but this remains uncertain in critically ill COVID-19 patients. This seems counterintuitive, though it could be explained by the premise that critically ill patients are too far into the thrombo-inflammatory phase of illness where it may be futile. However, more studies are required to explore this critical group further; to identify which subpopulations might benefit most, based on risk factors and comorbidities; and to determine the utility of biomarkers like D-dimer. Studies also need to be done in mild and moderate COVID-19 illness without hypoxia, including in outpatients, to ascertain whether any form of anticoagulation can prevent progression of illness and/or hospital admission. Dose and duration of post-discharge anticoagulation in the absence of a suspected or confirmed thrombotic event is another area where considerable equipoise exists. All of these are urgent research priorities considering the country’s recent manpower, oxygen, and intensive care unit bed shortages.
- NIH(National Institute for Health) COVID-19 treatment guidelines. Anti-thrombotic therapy in patients with COVID-19. https://www.covid19treatmentguidelines.nih.gov/therapies/antithrombotic-therapy/ last updated February 11,2021 accessed last 23 June 21
- NICE(National institute for Health Care Excellence). COVID-19 rapid guideline: Reducing the risk of venous thromboembolism in over 16s with COVID-19 NICE guideline [NG191]; Published: 23 March 2021; Last updated: 03 June 2021; accessed on 23st June 21 via URL https://www.nice.org.uk/guidance/NG191 or https://app.magicapp.org/#/guideline/L4Qb5n/rec/LwomXL
- Wang, D. et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 323, 1061–1069 (2020).
- Tang, N., Li, D., Wang, X. & Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 18, 844–847 (2020).
- Klok, F. A. et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. (2020) doi:10.1016/j.thromres.2020.04.013.
- Ackermann, M. et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 0, null (2020).
- Hoffmann, M. et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181, 271-280.e8 (2020).
- Cuker, A. et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 5, 872–888 (2021).
- Ongoing Living Update of Potential COVID-19 Therapeutics Options Summary of Evidence. Rapid Review. Pan American Health Organization. Available online :- https://iris.paho.org/handle/10665.2/52294 Accessed on 6th May 2021 .
- Shea, B. J. et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358, j4008 (2017).
- Cochrane Handbook for Systematic Reviews of Interventions. ‘Assessing risk of bias in a randomized trial’. accessed at URL https://training.cochrane.org/handbook/current on 25 May 2021.
- Review Manager (RevMan) [Computer program]. Version 5.4, The Cochrane Collaboration, 2020 Available at revman.cochrane.org
- Lemos, A. C. B. et al. Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial (HESACOVID). Thromb. Res. 196, 359–366 (2020).
- Remap-Cap, T., ACTIV-4a, Investigators, A. & Zarychanski, R. Therapeutic Anticoagulation in Critically Ill Patients with Covid-19 – Preliminary Report. medRxiv 2021.03.10.21252749 (2021) doi:10.1101/2021.03.10.21252749.
- The ATTACC, A.-4a et al. Therapeutic Anticoagulation in Non-Critically Ill Patients with Covid-19. medRxiv 2021.05.13.21256846 (2021) doi:10.1101/2021.05.13.21256846.
- Lopes DR, de Barrose Silva PGM, Furtado RH et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID19 and elevated D-Dimer concentration (ACTION): an open label,multicentre, randomised controlled trial. https://doi.org/10.1016/ S0140-6736(21)01203-4.
- INSPIRATION Investigators et al. Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA 325, 1620–1630 (2021).
- Perepu, U. et al. Standard Prophylactic Versus Intermediate Dose Enoxaparin in Adults with Severe COVID-19: A Multi-Center, Open-Label, Randomised Controlled Trial. https://papers.ssrn.com/abstract=3840099 (2021) doi:10.2139/ssrn.3840099.
- WHO Clinical management of COVID-19: interim guidance, 25 January 2021 https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1.
- Doctors spot new trend in Covid 2nd wave: Strokes in recovering or discharged patients [Internet]. [cited 2021 Jun 22]. Available from: https://theprint.in/health/doctors-spot-new-trend-in-covid-2nd-wave-strokes-in-recovering-or-discharged-patients/655613/
- Zhang L, Yan X, Fan Q et al. D‐dimer levels on admission to predict in‐hospital mortality in patients with Covid‐19. J Thromb Haemost.2020 Apr 19 : 10.1111/jth.14859. doi: 10.1111/jth.14859 [Epub ahead of print]
- Tahaineh L, Edaily SM, Gharaibeh SF. Anti-factor Xa levels in obese patients receiving enoxaparin for treatment and prophylaxis indications. Clin Pharmacol. 2018;10:63-70
https://doi.org/10.2147/CPAA.S161599
Covid Management Guidelines India Group - Anticoagulation Working Group. Prophylactic Vs Therapeutic dose anticoagulation. Covid Guidelines India; Published online on June 9, 2021; URL: https://indiacovidguidelines.org/anti-coagulation/ (accessed ).
