1. Outcomes in all categories of patients
Ref: All Cause Mortality (13-16), Thrombosis (13-16), Survival without organ support 28 days (15), OSFD (14,15), Major Bleeding (13-16)
Explanations:
a. Not downgraded since risk of bias (RoB) assessment with RoB 2.0 tool scored 'some concerns' in only 1 domain of each of the studies, for this outcome. In the mpRCT studies [14;15], Domain 2 was marked down for 'some concerns' in view of significant deviations in intended interventions in trial, which probably did not affect outcomes. In HESACOVID trial [13], Domain 5 was marked down for 'some concerns' in view of not enough information being provided to completely rule out risk of bias in selection of the reported result.
b. Downgraded by 1 level for serious indirectness: the differences in mechanisms of action and drug delivery caused concerns in comparability between the 2 interventions (Rivaroxaban & Heparins) for these outcomes. Aspects like the anti-inflammatory effects of heparins and dosages used in the Rivaroxaban regimen were also discussed in this regard. These putative differences in pharmacological characteristics between Rivaroxaban and Heparin, beyond their direct antithrombotic effects, informed the decision to downgrade for Indirectness in the outcomes not related to thrombosis or bleeding.
c. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from appreciable benefit to harm.
d. Downgraded by 1 level for serious risk of bias; RoB assessment with RoB 2.0 tool scored 'some concerns' in 2 domains of the mpRCT studies [14;15]. In addition to Domain 2 as across all outcomes (see explanation a.), Domain 4 of RoB 2.0 tool was assessed to have some concerns because of the open-labelled nature of the trials which may have impacted aspects of assessment. This would not have a bearing on 'harder' outcomes like mortality, OSFD or major bleeding.
e. Data provided in mpRCT [15] for this outcome was an Adjusted Proportional Odds Ratio (using a bayesian approach) of 1.30 (1.06 - 1.62). We decided to compute the RR from the numbers provided in the supplementary data for this outcome, assuming a baseline risk of 75.4%.
f. Downgraded by 1 level for serious imprecision; 95% CI ranges from a clinically unimportant benefit to appreciable benefit.
g. Downgraded by 1 level for serious inconsistency: the I-squared is 84%, and there is minimal overlap of the CIs of individual ORs.
h. Downgraded by 1 level for serious indirectness: there was a very different baseline odds of higher organ-support free days: in one trial (mpRCT, Zarychanski et al. [14]) all participants were receiving organ support at baseline; in the other (mpRCT, Lawler et al. [15]) very few received organ support at baseline.
i. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from a clinically unimportant harm to appreciable harm.
2. Outcomes in the WHO moderate (on oxygen) and severe category of patients
Ref: All Cause Mortality (15,16), Thrombosis (15,16), Survival without organ support 28 days (15), OSFD (15), Major Bleeding (15,16)
Explanations
a. Not downgraded since RoB assessment with RoB 2.0 tool scored 'some concerns' in only 1 domain of each of the studies [15;16], for this outcome. Domain 2 was marked down for 'some concerns' in view of significant deviations in intended interventions in trial; these probably did not affect outcomes.
b. Downgraded by 1 level for serious inconsistency; the I-squared is 67%.
c. Downgraded by 1 level for serious indirectness: the differences in mechanisms of action and drug delivery caused concerns in comparability between the 2 interventions (Rivaroxaban & Heparins) for these outcomes. Aspects like the anti-inflammatory effects of heparins and dosages used in the Rivaroxaban regimen were also discussed in this regard. These putative differences in pharmacological characteristics between Rivaroxaban and Heparin, beyond their direct antithrombotic effects, informed the decision to downgrade for Indirectness in the outcomes not related to thrombosis or bleeding.
d. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from appreciable benefit to harm.
e. Downgraded by 1 level for serious risk of bias; RoB assessment with RoB 2.0 tool scored 'some concerns' in 2 domains for mpRCT (Lawler et al. [15]), and in one domain for the other trial [16], for measurement of this outcome. In the mpRCT (Lawler et al. [15]), In addition to Domain 2 (see explanation a.), Domain 4 of RoB 2.0 tool was assessed to have 'some concerns' because of the open-labelled nature of the trials which may have impacted aspects of assessment of this outcome [15;16]. This would not have a bearing on 'harder' outcomes like mortality, OSFD or major bleeding.
f. Not downgraded for indirectness in these outcomes (Thrombosis/Bleeding), as the direct anti-thrombotic effect of therapeutic anticoagulation form both Rivaroxaban and Heparins could be considered comparable.
g. Not downgraded for imprecision as, even at the upper 95% CI, the benefit was considered clinically significant by the expert working group.
h. Data provided in mpRCT (Lawler et al [15]), was as Adjusted Proportional Odds Ratios (using a Bayesian approach). We decided to compute the RR, assuming a baseline risk of 75.4%, which we have mentioned in effects column.
i. Downgraded by 1 level for serious imprecision; 95% CI ranges from a clinically unimportant benefit to appreciable benefit.
j. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from clinically low harmful effect to appreciable harm.
3. Outcomes in critical category of patients
Ref: All Cause Mortality (13,14), Thrombosis (13,14), OSFD (14), Major Bleeding (13,14)
Explanations
a. Not downgraded since RoB assessment with RoB 2.0 tool scored 'some concerns' in only 1 domain of each of the studies, for this outcome. In the mpRCT (Zarychanski et al. [14]), Domain 2 was marked down for 'some concerns' in view of significant deviations in the intended interventions in trial, which probably did not affect In HESACOVID trial [13], Domain 5 was marked down for 'some concerns' in view of not enough information being provided to completely rule out risk of bias in selection of the reported result.
b. Downgraded by 1 level for serious imprecision; 95% CI ranges from appreciable benefit to harm.
c. Downgraded by 1 level for serious risk of bias; RoB assessment with RoB 2.0 tool scored 'some concerns' in 2 domains for mpRCT (Zarychanski et al. [14]), and in one domain for other trials, for measurement of this outcome. In the mpRCT (Zarychanski et al. [14]), Domain 2 was marked down for 'some concerns' - see explanation a. Domain 4 was assessed to have 'some concerns' because of the open-labelled nature of the trials which may have impacted aspects of assessment of this outcome. This would not have a bearing on 'harder' outcomes like mortality, OSFD or major bleeding.
d. Not downgraded for imprecision as, even at the upper 95% CI, the benefit was considered clinically significant by the expert working group.
e. No data contributed towards this outcome from available trials in WHO Critical COVID-19 patients.
f. Downgraded by 1 level for serious imprecision; 95% CI is wide ranging, from appreciable benefit to appreciable harm.
Since December 2019, the worldwide pandemic of COVID-19, caused by the SARS-CoV-2 virus has adversely impacted humanity in diverse ways. Clinical studies of hospitalized patients with COVID-19 initially showed flu-like symptoms, most commonly cough, sore throat, fever, myalgia, and fatigue at onset of COVID-19 illness, which can then proceed to develop into a viral pneumonia with varying severity [3].
Abnormal coagulation profiles and thrombotic complications, both venous and arterial, are common among the hospitalized severe and critically ill patients [4], with pulmonary embolism the most common site [5].
In addition, multiple autopsy reports show unprecedented pulmonary microvascular thrombosis and endothelial damage [6] which could be related to the direct viral cytopathic effect on the endothelial cells due to shared receptors with the alveolar cells [7]. Other etiopathogenetic mechanisms include immune/cytokine mediated dysregulation of pro-coagulant & anti-fibrinolytic pathways.
Though hypercoagulability in COVID-19 is now well-recognized, uncertainty still exists as to how best to manage clotting risk in these patients. In addition, an increased risk of hypercoagulability leading to increased thrombotic events has been reported and recognized extensively in the media and among peers. There is a prevailing assumption that the delta variant in India may be contributing to an increased number of thrombotic events, however this needs to be systematically studied and documented.
Over the past year, several guidance documents have recommended the use of anticoagulation in hospitalized patients with COVID-19 [8]. Most of these guidelines recommend the use of unfractionated heparin (UFH) or low molecular weight heparin (LMWH), though the evidence is scarce with regard to which dose of anticoagulation i.e., prophylactic, intermediate, or therapeutic (“full”) dose should be employed in each severity group of COVID-19.
At present there is now fairly broad-based consensus from national and international guidelines that the standard of care is prophylactic dose anticoagulation to all in-patients with COVID-19 pneumonia. However, it remains unclear if specific severity sub-groups of patients will benefit from intermediate or therapeutic dose anticoagulation in the absence of a confirmed thrombotic event.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Pubmed), Epistemonikos (COVID Living Overview of Evidence [L*OVE] platform), and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language published from March 2020 up to 15th April 2021. We also reviewed reference lists of systematic reviews and included studies.
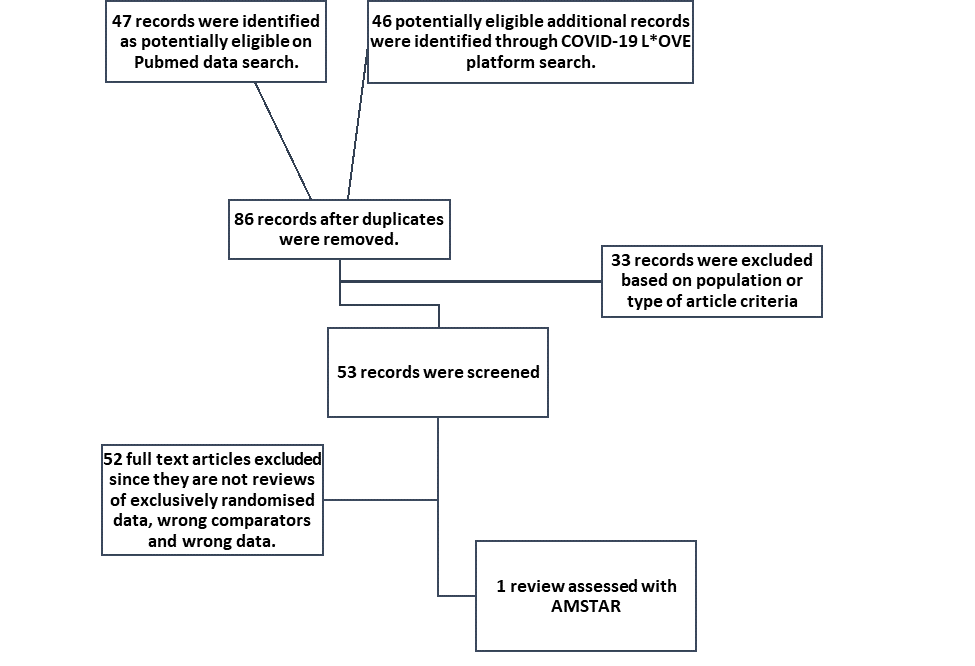
We searched the Pubmed database and found 47 systematic reviews, and from COVID Living Overview of Evidence(L*OVE) platform found 46 potentially eligible records. After removing duplicates, and excluding reviews that did not include exclusively randomized control trial (RCT) data or did not include intended interventions, we found only one systematic review looking specifically at the outcome of mortality [9]. When analysed with the AMSTAR 2 tool [10] it was found to be of low quality, and also did not include most outcomes of interest as defined by the working group’s PICO.
So, we decided to proceed with a rapid review of available RCTs that compare outcomes between anticoagulation doses in COVID-19 patients.
Two reviewers (SS & JSJ) independently assessed eligibility of search results. One reviewer extracted data from each included study, and both assessed risk of bias using the Cochrane Risk of bias (RoB) v2.0 tool [11].
We extracted data for the following outcomes, pre-defined by the Expert Working Group:
- Critical (primary for this review):
- Mortality (all-cause) – at 21-30 days, or in-hospital
- Thrombotic events
- Important (secondary):
- Time to clinical improvement
- Organ support free days (OSFD): - ventilator, inotropic requirements.
- Survival without organ support at day 28
- Duration of hospitalization
- Bleeding events
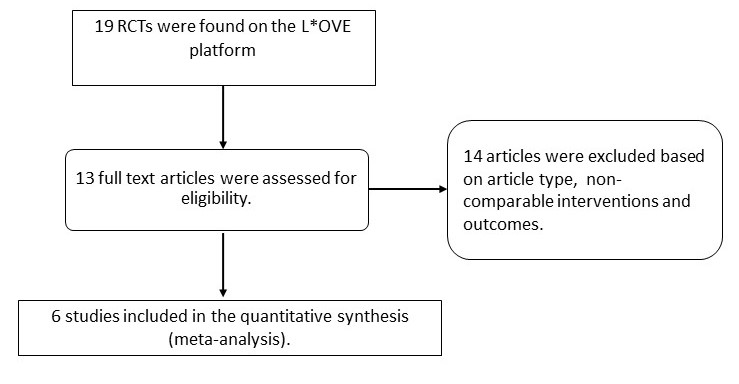
On searching Pubmed & COVID L*OVE platform and when restricting to RCTs, we found 19 records. After excluding extraneous records and trials that did not report any outcomes that could provide usable data for the review, we assessed 6 RCTs which compared differing doses of anticoagulation in COVID-19 patients.
We used RevMan v5.4 (12) to perform meta‐analysis using fixed-effect & random‐effects models for outcomes where pooling of effect estimates was appropriate. We used risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). Since the guidelines were going to be specific to each severity category we grouped studies as per their inclusion criteria into their severity categories and combined them as such to provide pooled estimates. We used the I2 statistic to measure residual heterogeneity. We used GRADE methodology to assess the certainty in the evidence, and documented this in a ‘Summary of findings’ table using GradeProGDT.
There are myriad dosing strategies for anticoagulation based on indication, organ dysfunction, BMI and adverse drug reactions, based on available literature and package insert recommendations. For purposes of our guidelines in the expert working group meeting it was decided to broadly group prophylactic & intermediate doses as non-therapeutic anticoagulation (up to and including 1mg/kg of enoxaparin or equivalent subcutaneously once daily) AND higher doses would be therapeutic dose anticoagulation (usually 1mg/kg enoxaparin or equivalent subcutaneously twice daily).
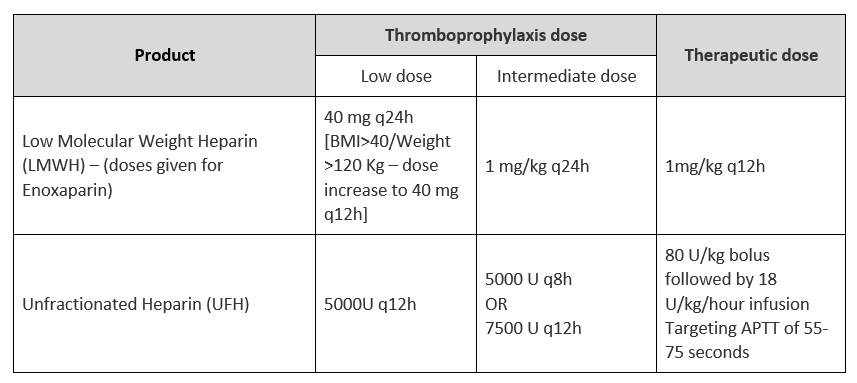
Though we are using the term anticoagulation, the intent of use of anticoagulation in all these trials was prophylaxis of thrombotic events, but two different doses are being compared in each of these trials: therapeutic vs. non-therapeutic, the latter of which may be prophylactic or intermediate dose.
Of the 6 RCTs found, four attempted to compare therapeutic dose anticoagulation with non-therapeutic doses:
- HESACOVID [13]: compared prophylactic vs. therapeutic dose of anticoagulation (20 participants).
- mpRCT (Critically ill; Zarychanski et al.) pre-print [14]: a collaboration of 3 RCT platforms, ACTIV-4a, REMAP-CAP and ATTACC, looking specifically at critically ill patients (total of 1074 participants) – comparing therapeutic vs non-therapeutic dose of anticoagulation.
- mpRCT (Non-critically ill; Lawler et al.) pre-print [15]: is a collaboration of above mentioned 3 RCT platforms, looking specifically at non-critically ill patients (total of 2291 participants) – comparing therapeutic vs non-therapeutic dose of anticoagulation.
- COALITION-ACTION [16]: Looked specifically at the WHO moderate to severe categories of participants.
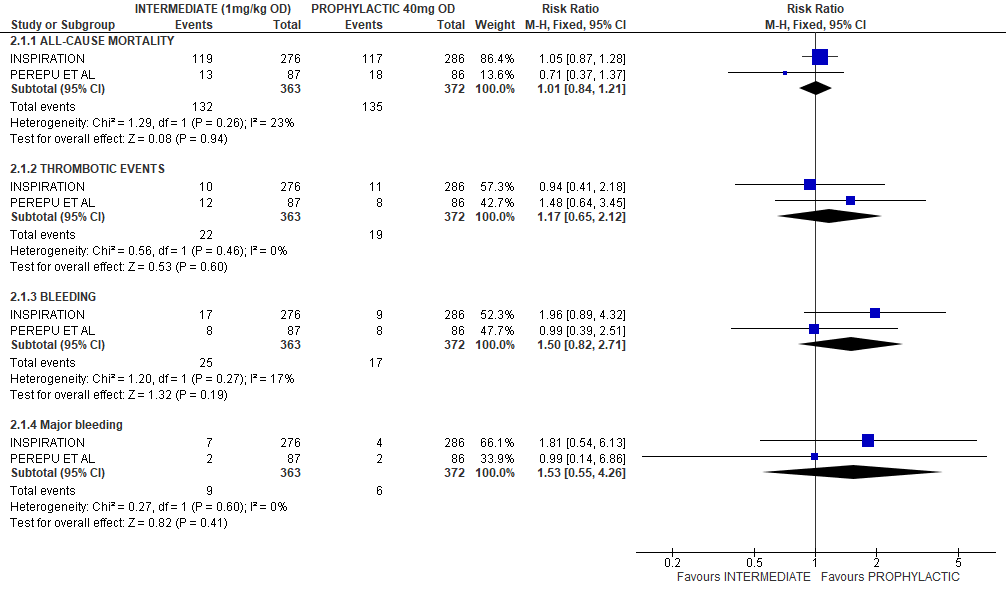
Two randomized trials studied the effect of intermediate vs. prophylactic dose anticoagulation:
- INSPIRATION [17]: compared prophylactic vs. intermediate dose of anticoagulation (562 participants).
- Usha Perepu et al, preprint [18]: compared prophylactic vs. intermediate dose of anticoagulation (176 participants).
Since we were providing category specific recommendations, HESACOVID [13] and mpRCT critical [14] were analysed together to enable evidence synthesis and a recommendation in the critical category of patients. In addition, mpRCT non-critically ill [15] and COALITION ACTION [16] were analysed together to provide a moderate-severe category specific recommendation as well. The results of INSPIRATION and Usha Perepu et al were kept separate from these other trials, in view of different comparator arms which were prophylactic and intermediate dose of anticoagulation. They are not presented in the results of this review.
The critical outcomes that were available and extracted for analysis from these studies included:
- All-cause mortality (21-30 days),
- Thrombosis,
- Organ support free days (i.e., number of days without need for ICU-level organ support, including invasive and non-invasive mechanical ventilation)
- Survival without organ support at 28 days
- Major bleeding events.
We included 4 trials with 3,927 patients of which 1 trial did not contribute much data (13). The two trials which did not include a therapeutic dose of anticoagulation as a comparator arm were excluded from this analysis (17,18). The patients were compared across different pre-specified COVID19 severity groups, for the different outcomes as mentioned above (See summary of characteristics tables below). The duration of administration of anticoagulation varied from a minimum of 96 hours to 14 days or till the patient got better.
We tailored the severity strata in the study according to WHO COVID-19 Clinical management: living guidance severity classification (Interim document originally published under the title "Clinical management of COVID-19: interim guidance, 27 May 2020"(19).
| | WHO severity criteria correlate |
|---|---|
| mpRCT (Critically ill) - Zarychanski et al. | WHO Critical |
| mpRCT (non-Critically ill) - Lawler et al. | WHO Severe & Moderate |
The group of patients studied in the mpRCT(Critically ill) study (14), correlated directly with WHO critical severity criteria. The group of patients studied in the mpRCT(non-Critically ill) study(15), correlated with WHO severe category also overlapping with a few in the WHO moderate category.
Another aspect to be factored into the analysis was that, in COALITION-ACTION trial (16), the overwhelming majority of patients in the therapeutic dose arm(90.3%) received Rivaroxaban. This may have led to indirectness. Aspects like the anti-inflammatory effects of heparins, absorption of oral drugs and dosages used in the Rivaroxaban regimen were also discussed in this regard. These putative differences in pharmacological characteristics between Rivaroxaban and Heparin, beyond their direct antithrombotic effects, informed the decision to downgrade for indirectness in the outcomes not related to thrombosis or bleeding. (See further detailed explanations in SoF tables)
Overall analysis: Using GRADE methodology certainty of evidence is shown in Summary of Findings tables.
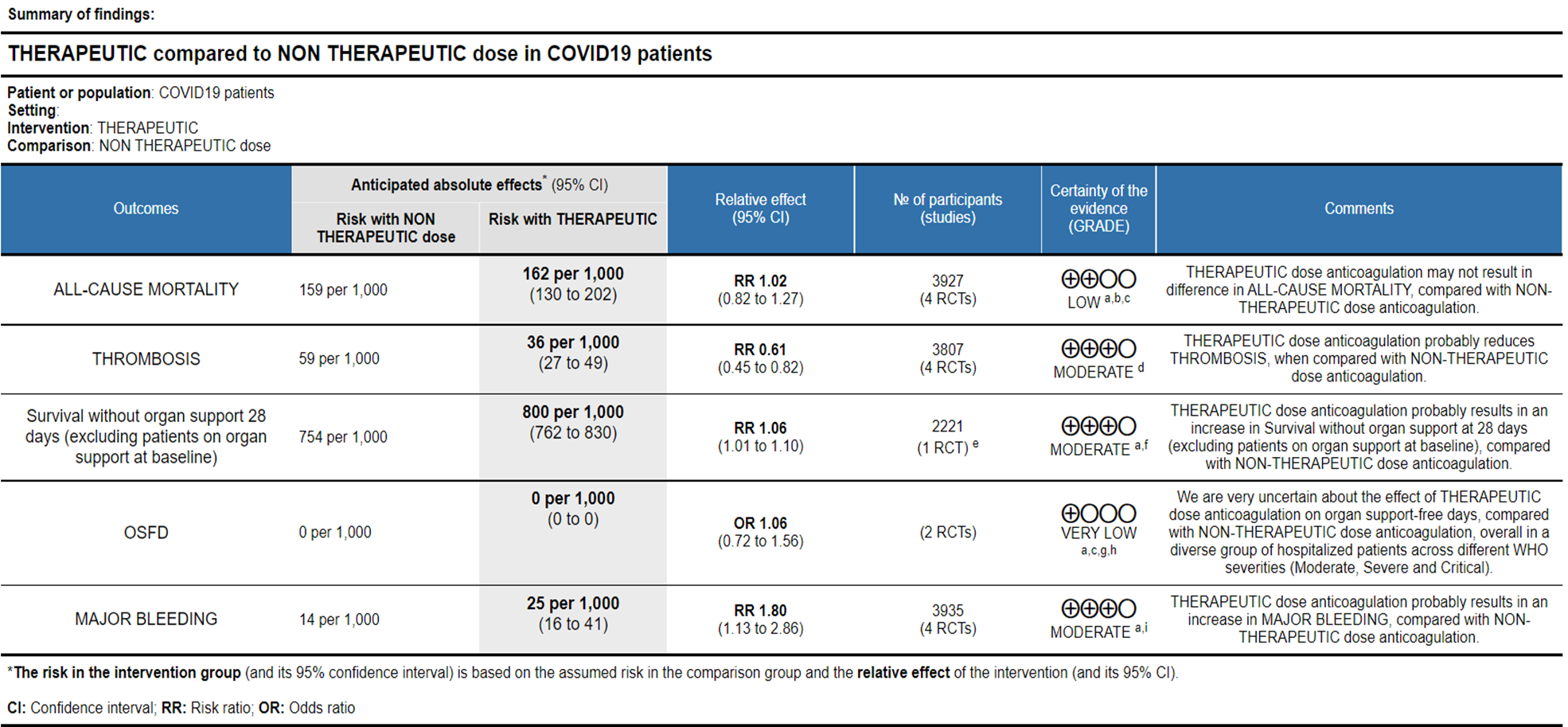
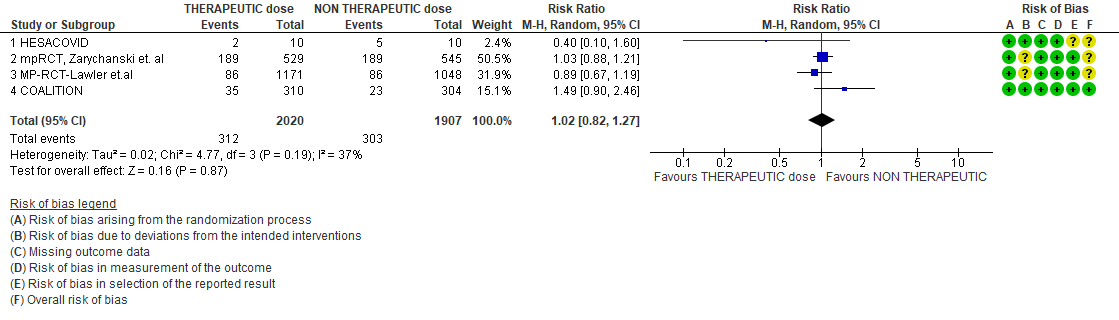
- All-cause Mortality- Low certainty of evidence in 4 trials with 3927 patients, revealed that therapeutic dose anticoagulation may result in little to no difference in all-cause mortality, compared with non-therapeutic dose anticoagulation (RR 1.02, 95 % CI=0.82 to 1.27).
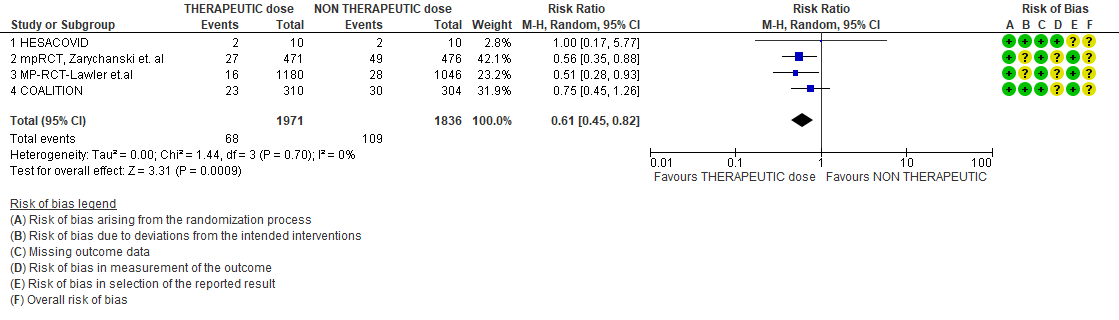
- Thrombosis- Moderate certainty of evidence in 4 trials with 3807 patients, revealed that therapeutic dose anticoagulation probably reduces thrombosis, when compared with non-therapeutic dose anticoagulation (RR= 61, 95 % CI = 0.45 to 0.82).
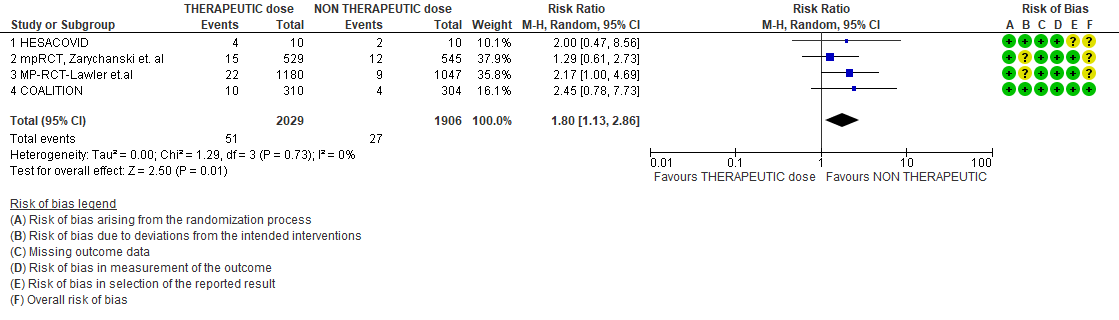
- Major bleeding- Moderate certainty of evidence in 3 trials with 3321 patients, revealed that therapeutic dose anticoagulation probably results in an increase in major bleeding, compared with non-therapeutic dose anticoagulation (RR 1.80, 95% CI = 1.13 to 2.86). There were 3 cases of fatal bleeds in mpRCT (non-critically ill) when given therapeutic dose anticoagulation vs 1 in the prophylactic dose anticoagulation.
- OSFD- Very low certainty of evidence in 2 trials with 3301 patients, revealed a very uncertain effect of therapeutic dose anticoagulation on organ support-free days in hospitalized patients, compared with non-therapeutic dose anticoagulation when patients from WHO moderate, severe & critical COVID19 strata were combined from available trials (RR=1.06,95%CI 0.72-1.56).
Since guidelines are to provide recommendations for each severity strata, disaggregated data was also assessed to provide analysis to help formulate recommendations specific to each severity strata.
Analysis according to severity strata
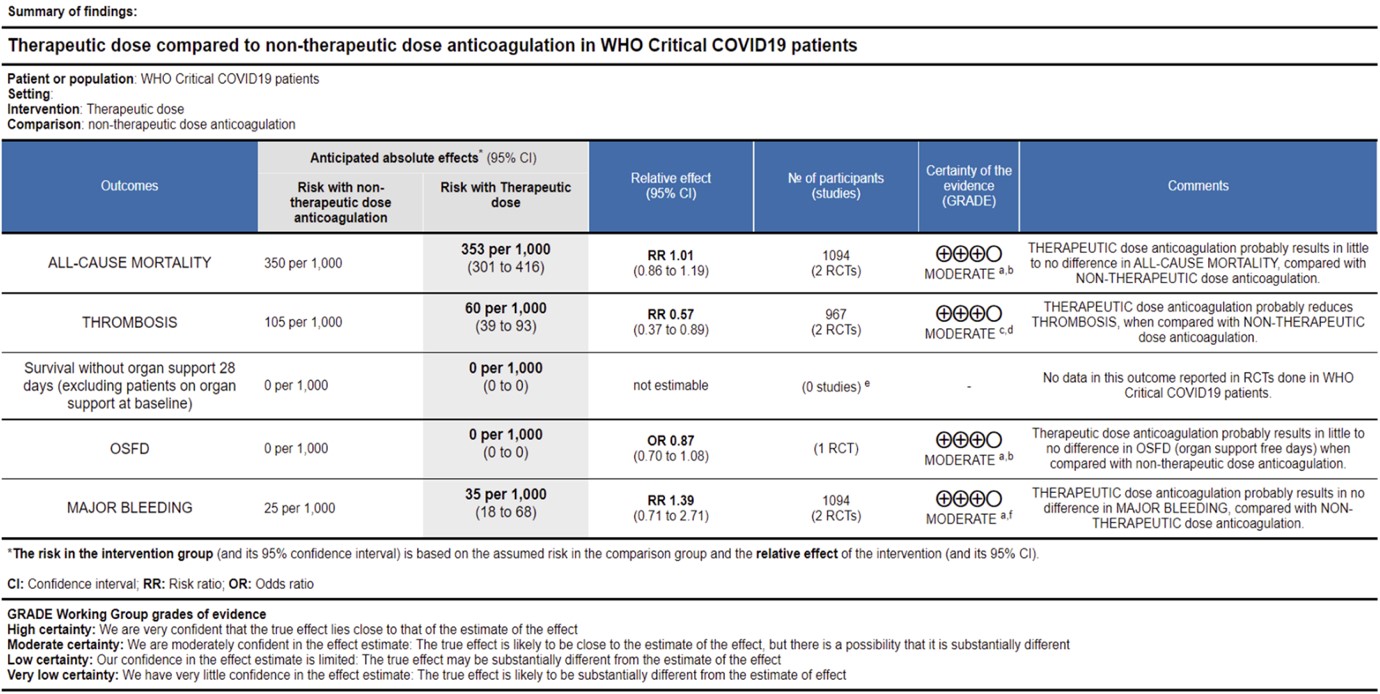
WHO Critical group: Very low certainty evidence from 2 trials (13,14) showed that using therapeutic dose anticoagulation did not significantly reduce mortality in critical COVID19 illness (RR-1.01, 95 % CI = 0.86 to 1.19). However, moderate certainty evidence did show decreased thrombosis in patients (RR-0.57, 95 % CI = 0.37 to 0.89) but with no increase in bleeding (RR-1.39, 95 % CI = 0.71 to 2.71). In addition, moderate certainty evidence from 1 trial (28), showed that therapeutic anticoagulation did not improve days free of organ support compared to prophylactic dose anticoagulation [Odds Ratio (OR) 0.87, 95% CI 0.70 to 1.08]. However, the median OSFD in the therapeutic dose anticoagulation group was 3 days vs 5 days in the prophylactic dose anticoagulation group. It was also noted that 41% received low dose prophylactic dose anticoagulation and 51% received an intermediate dose prophylactic dose anticoagulation.
Bayesian analysis from Zarychanski et al (14). showed a posterior probability of futility of 99.8% and posterior probability of inferiority of 89.4% for impact of therapeutic anticoagulation on OSFD. DSMB stopped recruitment since pre-specified futility boundary for therapeutic anticoagulation was achieved. However, this was for OSFD not for mortality or thrombotic events.
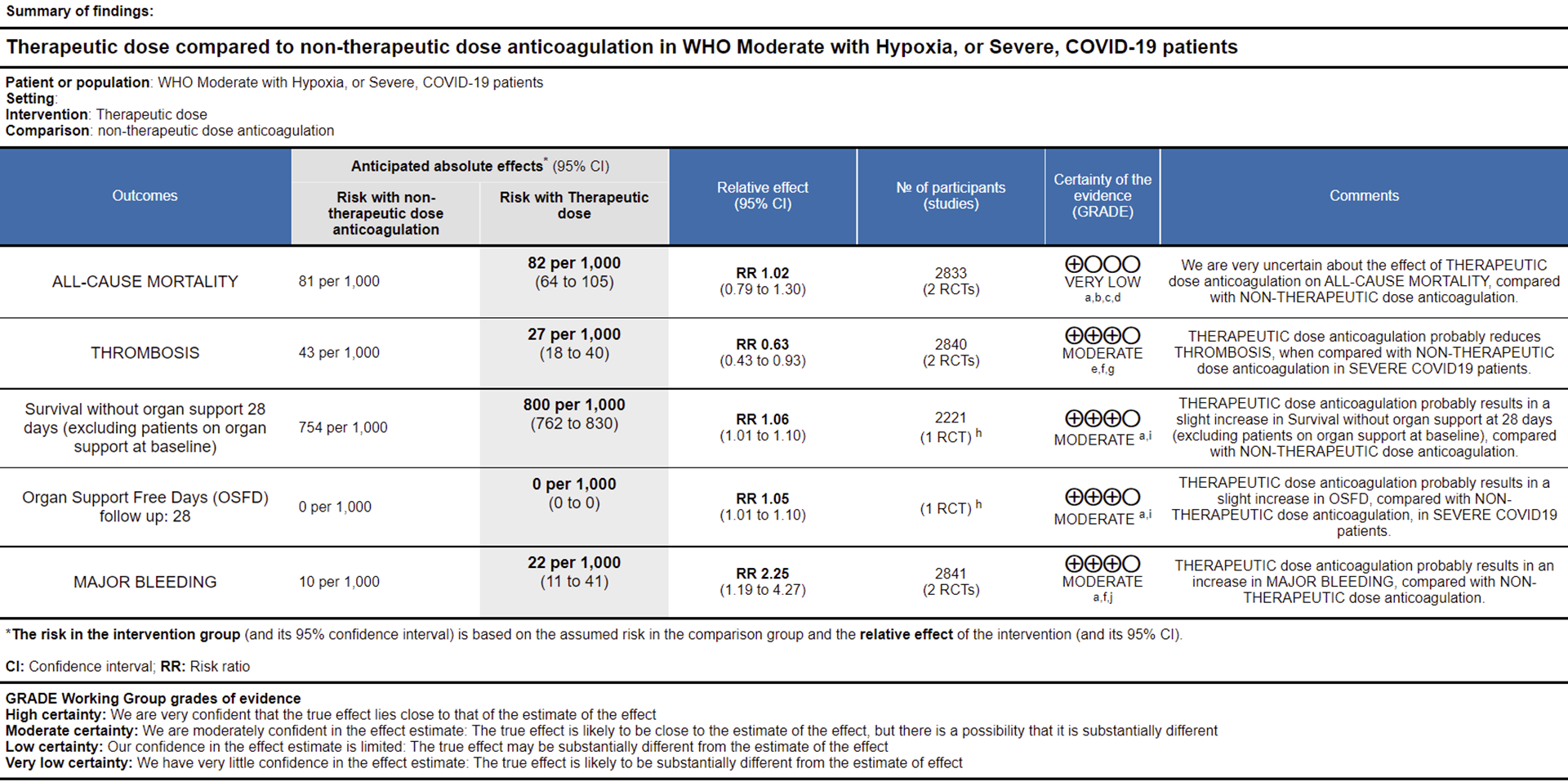
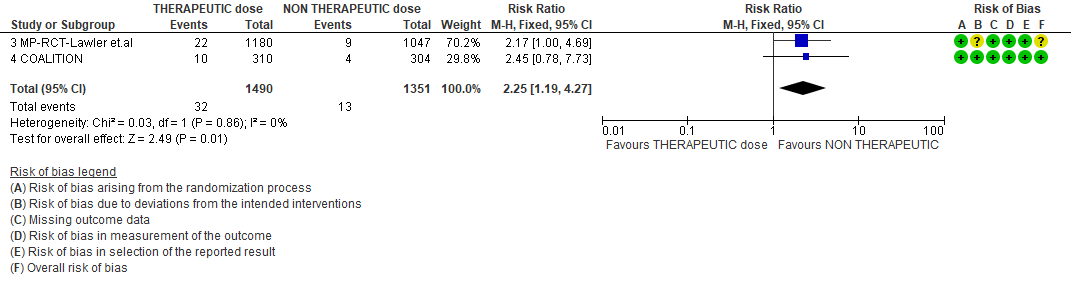
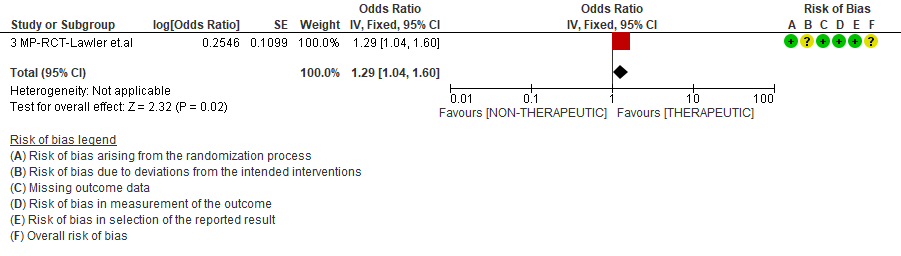
WHO Moderate/Severe group: Very low certainty evidence from 2 trials (15,16) suggested that therapeutic anticoagulation provided no mortality benefit RR 1.02 (95% CI 0.79 to 1.30). However there was moderate certainty evidence that therapeutic dose anticoagulation did appear to prevent thrombosis by 37% (RR 0.63, 95 % CI 0.43 to 0.93) with a clinically appreciable risk of major bleeding of greater than 19%; RR 2.25, 95 % CI 1.19 to 4.27).In addition there was moderate certainty evidence from 1 trial (29) showing that therapeutic dose anticoagulation increased the probability of OSFD with OR 1.05, 95 % CI 1.01 to 1.10 and similarly survival without organ support RR 1.06 (95% CI 1.01 to 1.10). However fatal bleeds in mpRCT non- critically ill [15] revealed 3 bleeds in therapeutic dose anticoagulation vs 1 in the prophylactic dose anticoagulation group.
Bayesian analysis from mpRCT (non-critical) (15) showed a posterior probability of superiority of 99% for therapeutic anticoagulation improving OSFD. In this trial, deep venous thrombosis (DVT) was included as significant thrombosis which seems to have been excluded from mpRCT critical group (14).
Analysis of studies using non-therapeutic doses
In a brief analysis of RCTs INSPIRATION & Perepu et al.[17,18] which compared prophylactic vs intermediate dose anticoagulation (see table above), no statistically significant differences were noted in the outcomes of all-cause mortality [RR1.01,95% CI 0.84-1.21];thrombotic events [RR 1.17;95% CI 0.65-2.12];bleeding [RR 1.5;95%CI 0.82-2.71]; major bleeding [RR1.53;95%CI 0.55-4.26] assessed. (See relevant forest plot)
Table 1: The four trials analysed for various outcomes
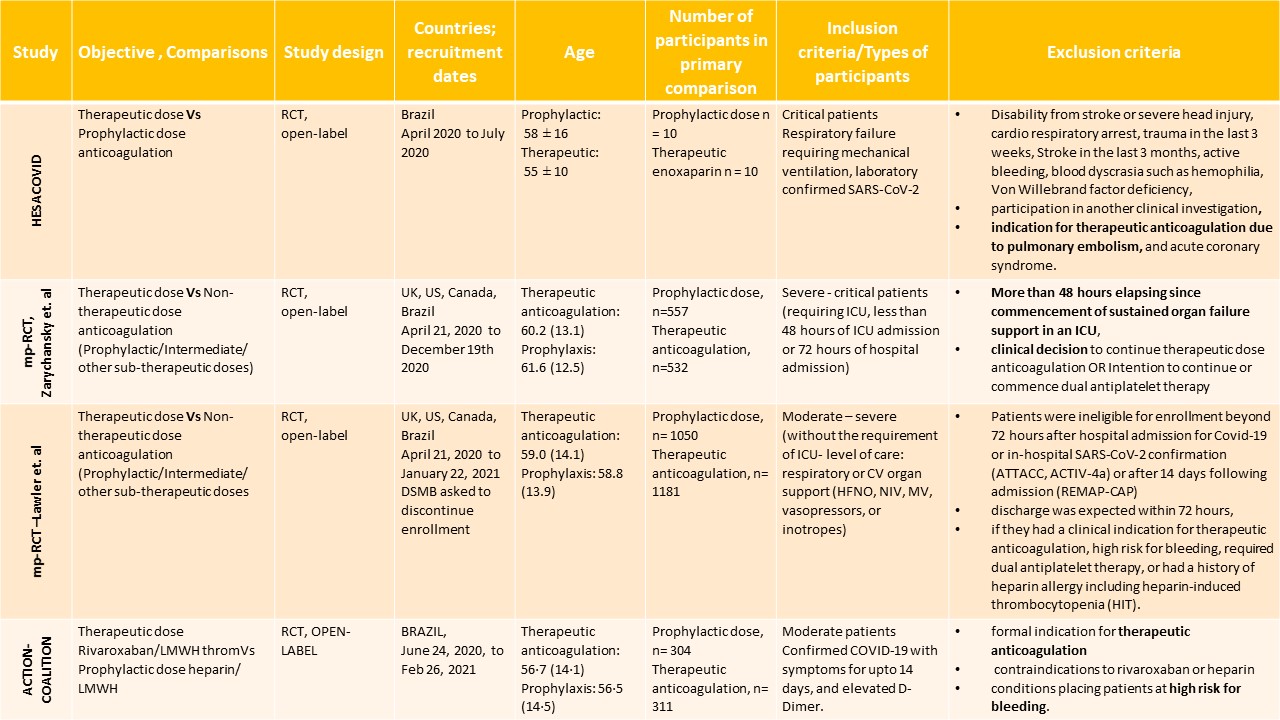
* ICU: intensive care unit; RCT: randomized controlled trial
Table 2. Summary of D-dimer values and co interventions administered in these 4 trials
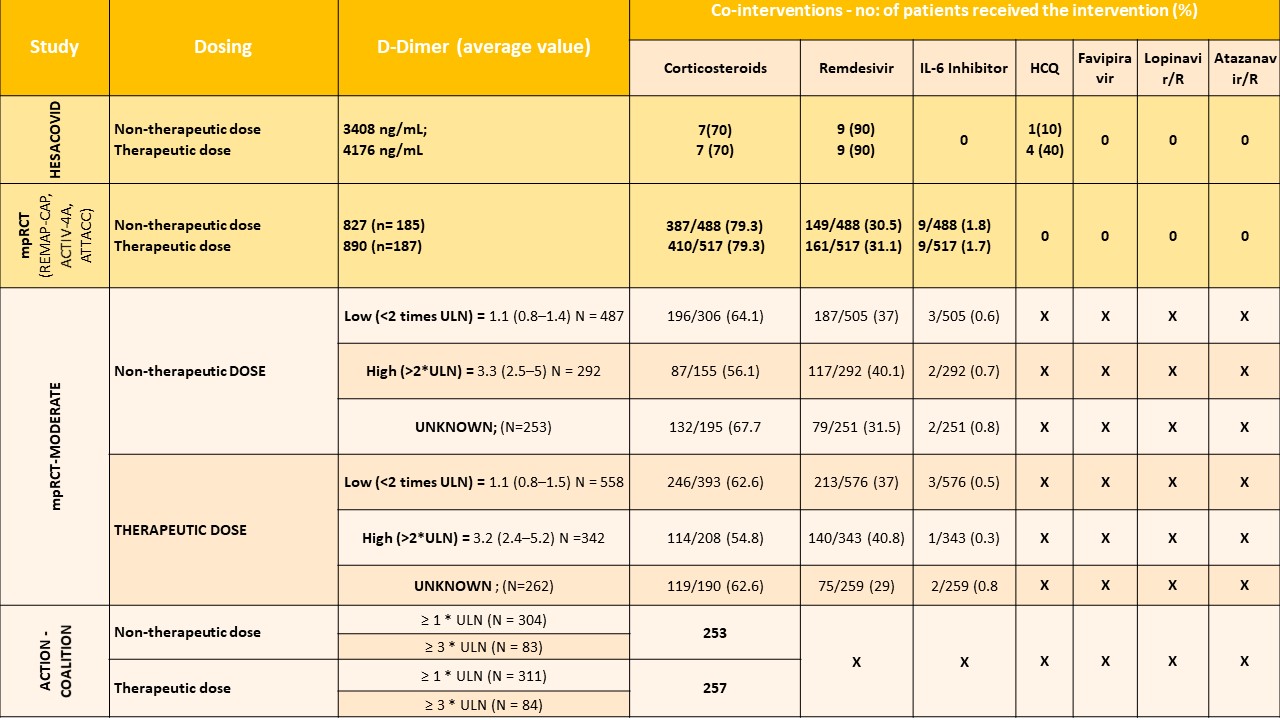
* R: ritonavir; ULN: upper limit of normal
THERAPEUTIC VS NON-THERAPEUTIC DOSE OF ANTI-COAGULATION ACROSS ALL SUBGROUPS OF SEVERITY
- All-cause mortality (21-30 days)
2. Thrombosis
3. Major Bleeding
4. Organ support free days (OSFD)

THERAPEUTIC VS NON-THERAPEUTIC DOSE OF ANTI-COAGULATION ACROSS CRITICAL SEVERITY SUBGROUP
- All-cause mortality (21-30 days)
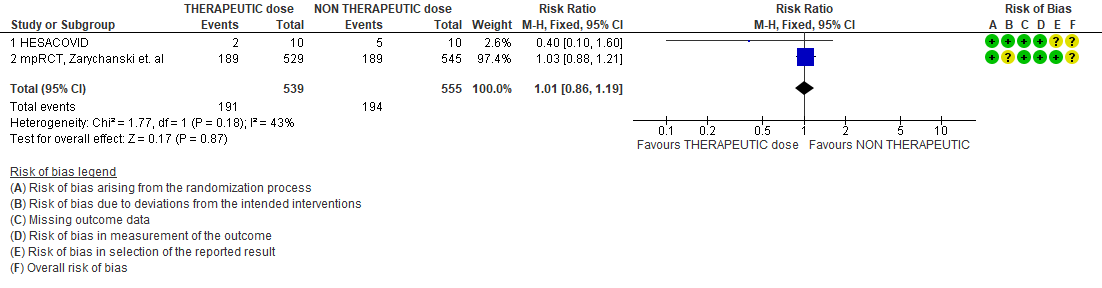
- Thrombosis
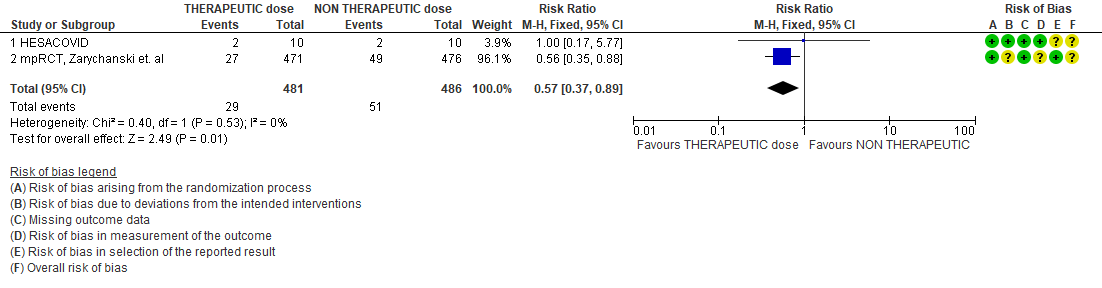
- Major Bleeding
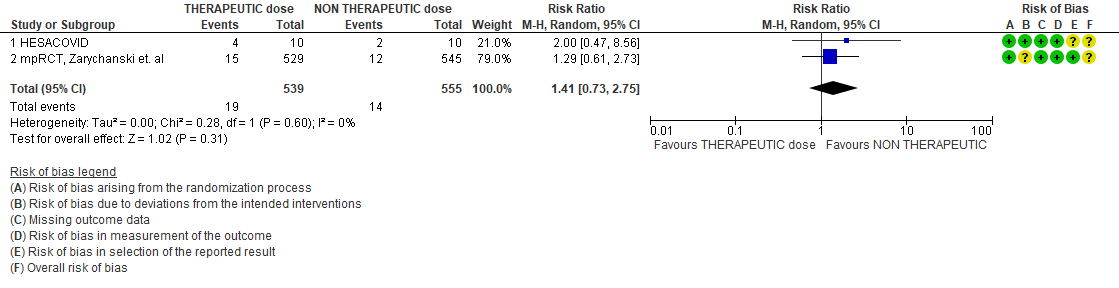
4. Organ support free days
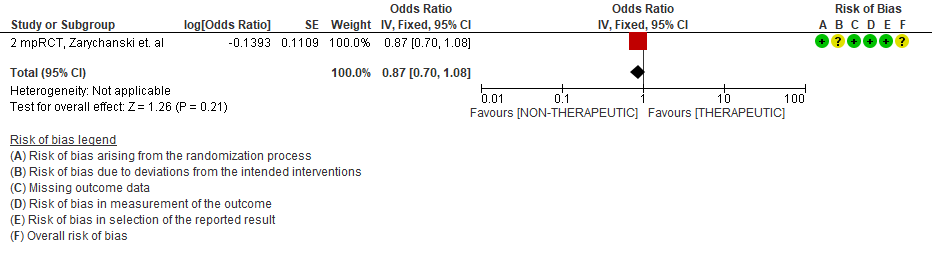
THERAPEUTIC VS NON-THERAPEUTIC DOSE OF ANTI-COAGULATION ACROSS MODERATE TO SEVERE SUBGROUP
- All cause mortality
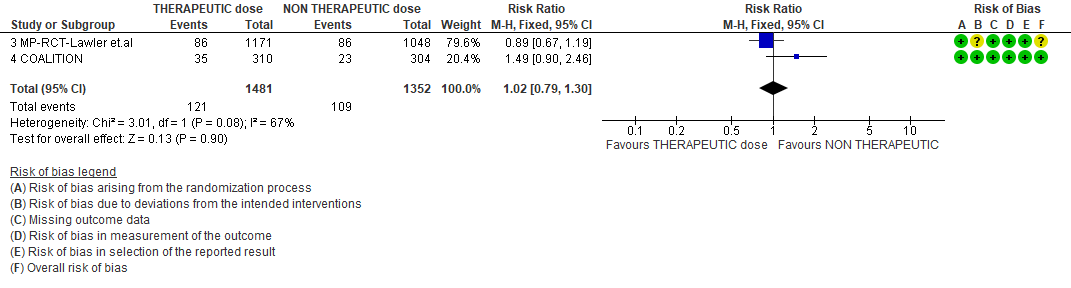
2. Thrombosis
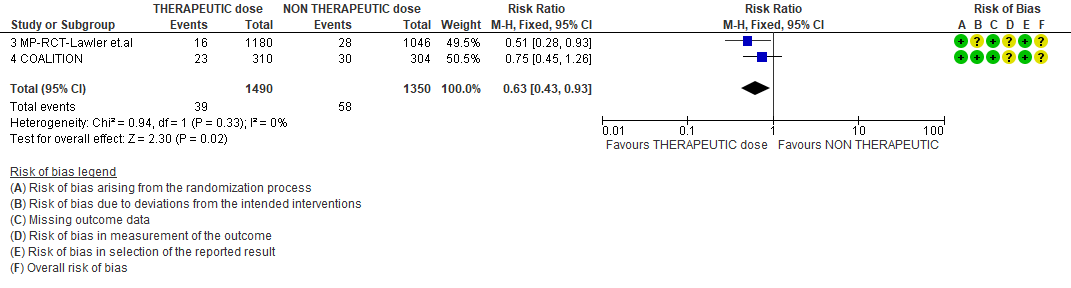
3. Bleeding
4. Organ support free days
 PROPHYLACTIC VS INTERMEDIATE DOSE OF ANTICOAGULATION
PROPHYLACTIC VS INTERMEDIATE DOSE OF ANTICOAGULATION