According to World Health Organization, there have been 601,189,435 confirmed cases of COVID-19, including 6,475,346 deaths, reported to WHO, by September 2, 2022 (2) Systemic Inflammatory markers like Ferritin, IL-6, D-dimer are markedly elevated in severe Covid-19 patients. A meta-analysis of 6 studies showed that in complicated Covid-19 the marked elevation in the Interleukin-6 (IL-6) is significantly associated with ARDS and death (3) Tocilizumab, an IL-6 inhibiting monoclonal antibody, inhibits the inflammatory pathway via IL-6 and reduces the cytokine storm and other inflammatory responses in a severe to critical Covid-19 patient. Tocilizumab is used in the treatment of autoimmune diseases like rheumatoid arthritis and systemic sclerosis (4)
The updating of the evidence summary of the guideline for the Tocilizumab in Covid-19 focuses on evidence regarding the efficacy and safety of Tocilizumab in various severity of Covid-19 patients. The 2021 recommendations were recommending a conditional use of Tocilizumab in Severe to Critical Covid-19 patients.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language. We also reviewed reference lists of systematic reviews and included studies. We performed all searches up to 15th October 2021. We included randomized controlled trials (RCTs) testing Remdesivir in people with COVID‐19, and extracted data for the following pre-defined outcomes:
Critical (primary for this review):
- All-cause mortality
- Time to clinical recovery
- Non-invasive or mechanical ventilation
Important (secondary):
Secondary outcomes
- Admission to critical care
- Time to and duration of ventilation
- Length of critical care & hospital stay
- Organ supports other than ventilation
- Change in CRP at 48h (in %)
Adverse events
- All and Serious adverse events
- Nosocomial/opportunistic infections
- GI perforation/ Appendicitis
Two reviewers independently assessed eligibility of search results. One reviewer extracted data from each included study and assessed risk of bias using the Cochrane Risk of bias (RoB) v2.0 tool. Data and RoB assessments were checked by the second reviewer. The entire RoB assessment was scrutinized by the whole team for this review, to reach consensus.
We used RevMan 5.4 to perform meta‐analysis using a random‐effects model for outcomes where pooling of effect estimates was appropriate. We used risk ratios (RR) for dichotomous outcomes and hazard ratios for continuous outcomes, with 95% confidence intervals (CIs). We performed a subgroup analysis to explore the effect on mortality stratified by different oxygen and ventilation requirements at baseline.
We used the I2 statistic to measure residual heterogeneity. We used GRADE methodology to assess the certainty in the evidence and documented this in a ‘Summary of findings’ table using GradeProGDT.
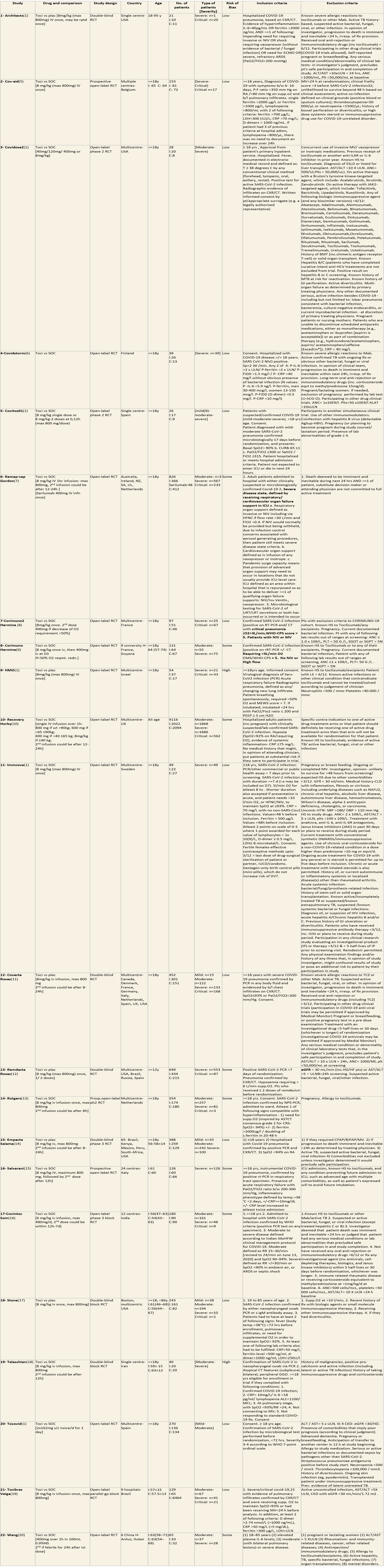
We included 22 trials involving 8436 adult participants, in which 4449 received Tocilizumab. Three studies were reported from US, 2 studies from Spain, and 5 studies were conducted in multiple nations. Rest of the 12 studies reported 1 each from Belgium, Brazil, China, Finland, France, India, Iran, Israel, Italy, Netherlands, Sweden and UK. Of the included trials only 4 trials included mild-moderate patients. Rest of the studies included severe to critically ill participants. Co-interventions varied among different trials, but 65% of the trial participants received glucocorticoids and 20% of the participants received Remdesivir as a co-intervention. The risk of Bias assessment was done among the trials and 11 studies were found to be of low risk of bias, out of 22, twelve studies (1,9–15) were found to have low risk of bias, 8 studies (9,16–22) have some concerns and 2 studies (1,23) were of High RoB.
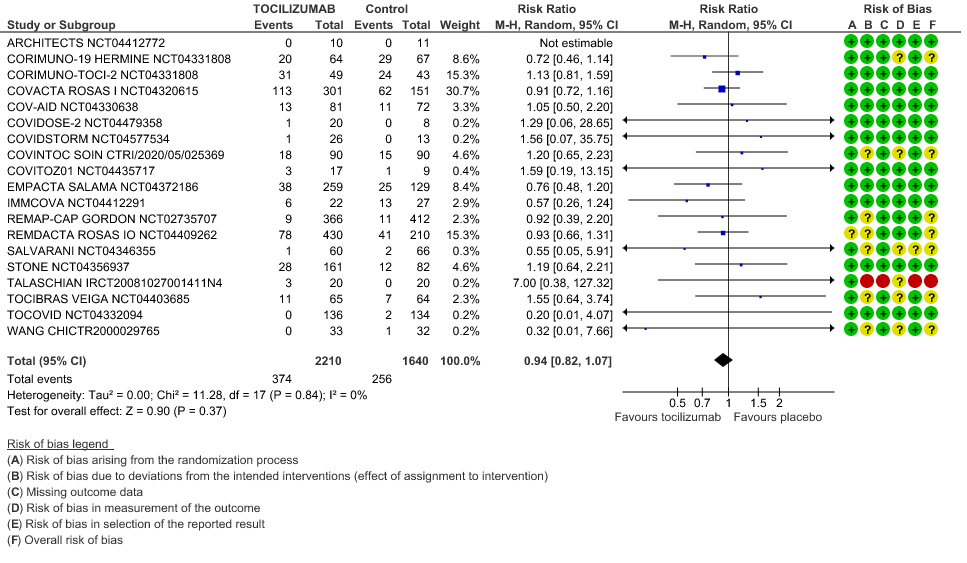
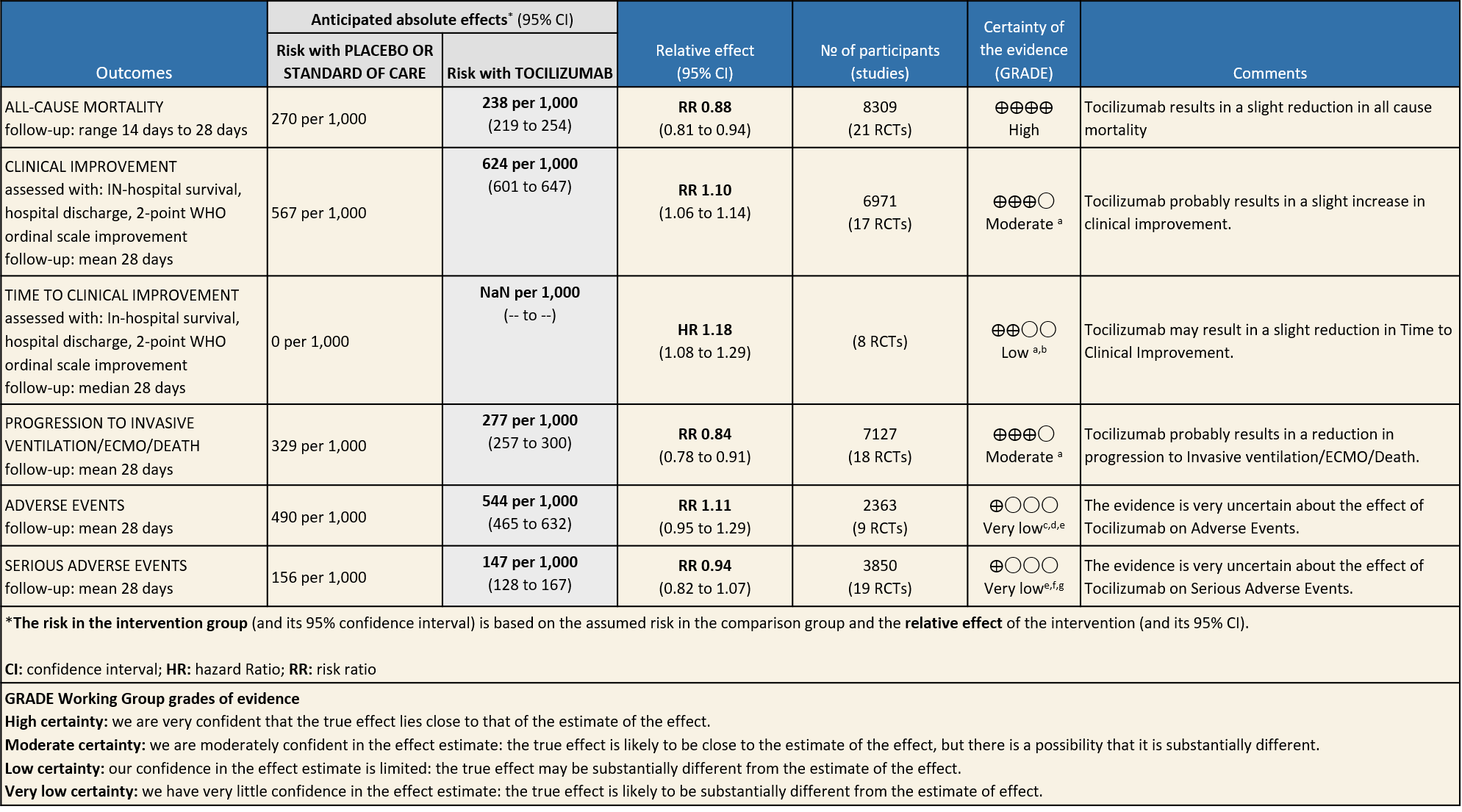
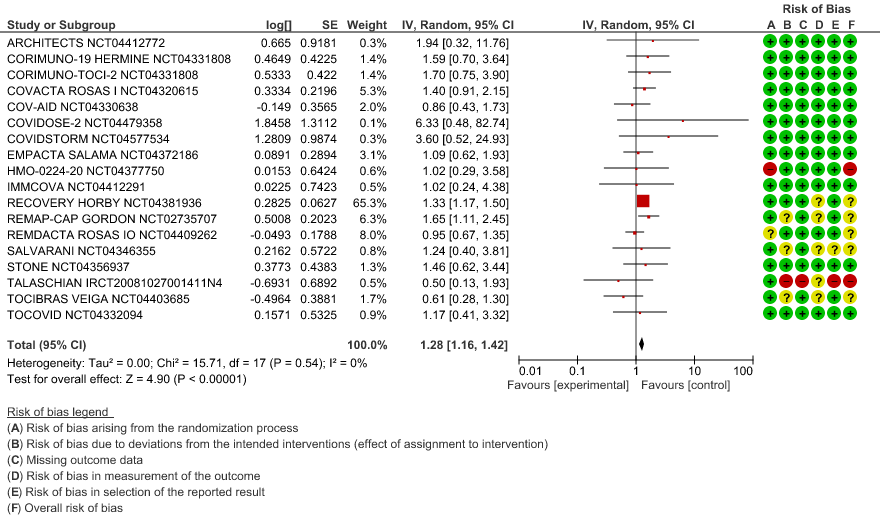
All-Cause Mortality: High certainty of evidence from 21 trials (1,9–26) including 8309 participants found that Tocilizumab reduces mortality in severe to critically ill COVID-19 patients [RR 0.88, 95% CI 0.81, 0.94].
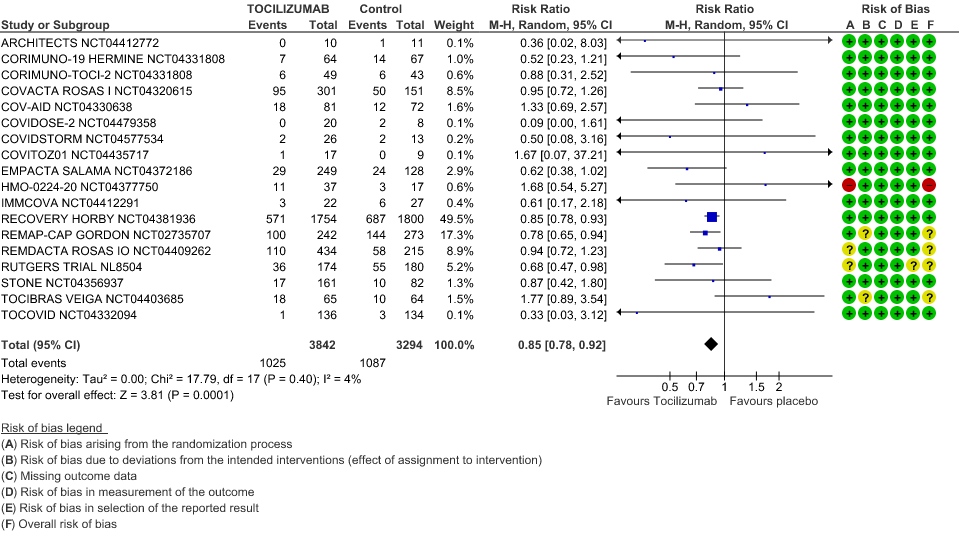
Progression to Invasive Ventilation/ECMO/Death: Moderate certainty of evidence from 18 RCTs (1,9–15,17–19,21,25) including 7127 participants found that Tocilizumab probably results in a reduction in the progression to Ventilation/ECMO/Death [RR 0.85, 95% CI 0.78,0.92].
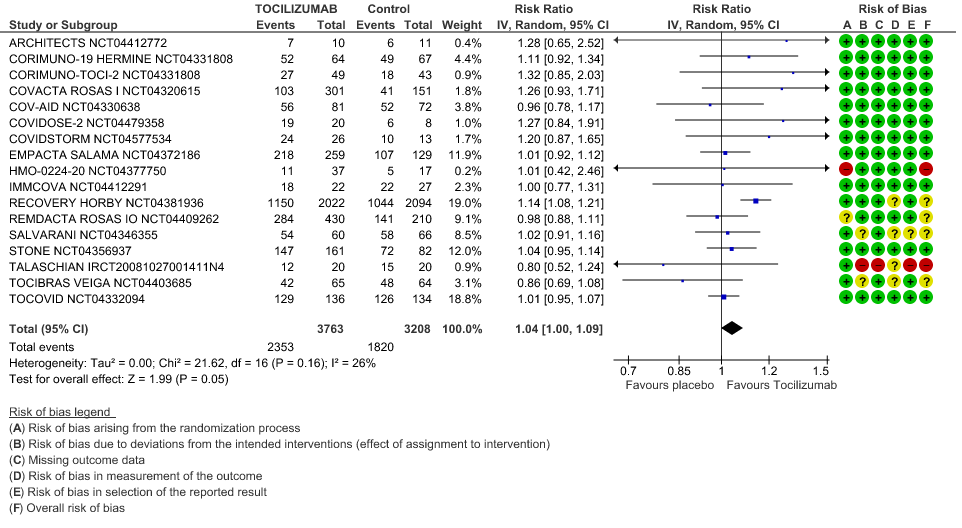
Clinical improvement: Moderate certainty of evidence from 17 RCTs (1,9–15,18,20,21,23,25) including 6971 participants, showed that Tocilizumab probably results in a slight increase in clinical improvement in severe to critically ill Covid 19 patients [RR 1.04, 95% CI 1.00, 1.09]
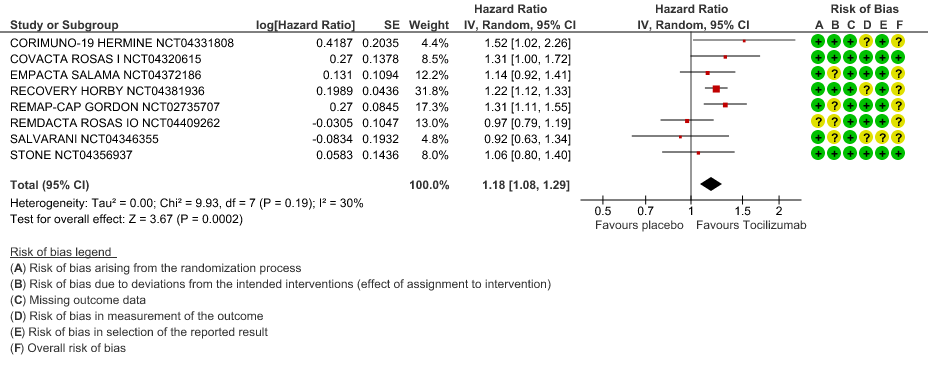
Time to Clinical improvement: Low certainty of evidence from 8 RCTs (9,10,13–15,17,18,20) showed, that treating with Tocilizumab may result in slight reduction for the time to clinical improvement [HR 1.18, 95% CI 1.08,1.29]
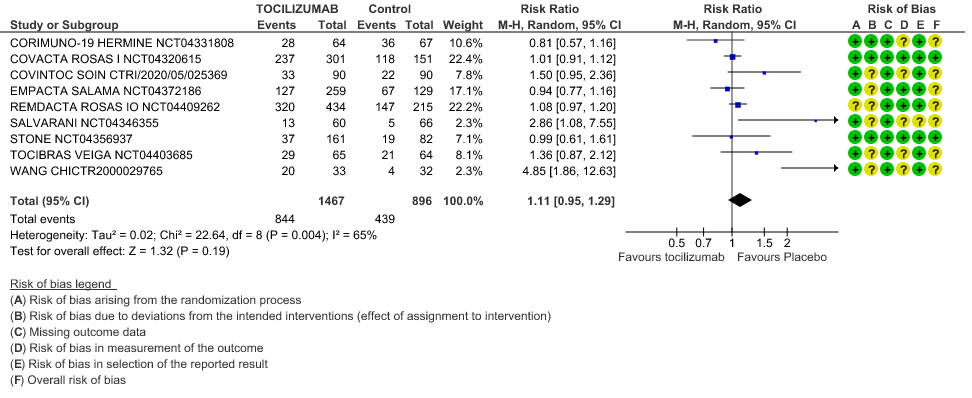
Adverse events: Very low certainty of evidence from 9 RCTs (10,13,15,16,18,20–22,25) with 2363 participants are very uncertain about the effects of Tocilizumab in serious adverse effects [RR 1.11 95% CI 0.95, 1.29].
Serious Adverse Events: Very low certainty of evidences from 19 RCTs (1,9–13,15–18,20–23,25) with 3850 participants is very uncertain about the effects of Tocilizumab in Serious Adverse events [RR 0.94, 95% CI 0.82, 1.07].
1. All-Cause Mortality
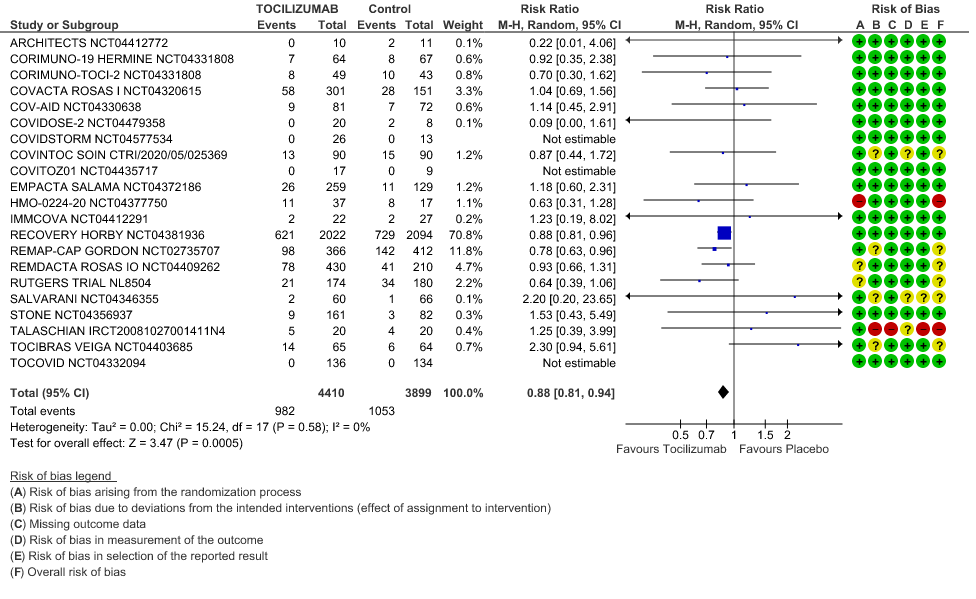
2. Progression to Invasive Ventilation/ECMO/Death
3. Clinical Improvement
When event rate [common event] >20% OR may overestimate. Hence, we calculated RR with 95% CI – 1.14 [1.08, 1.18]
4. Time to Clinical Improvement
5. Adverse Events
6. Serious Adverse Events