This recommendation applies to acute COVID-19 in adults. Some of our recommendations vary according to the severity of COVID-19 illness. Definitions of the categories are based on the World Health Organization (WHO) criteria and can be viewed by clicking the plus (+) signs below.
RECOMMENDATION:
The guidelines group makes a strong recommendation against the use of neutralizing monoclonal Antibody Cocktail Casirivimab and Imdevimab (REGEN-COV™) in asymptomatic people with COVID-19. Few patients that are infected actually require hospitalization. The drug is extremely expensive and has little or no effect on progression to severe or critical disease though it may reduce progression to symptoms. Overall the potential benefits are tiny with enormous cost implications suggesting that routine implementation of this drug in asymptomatic COVID-19 infected individuals is not justified.
DATE OF RECOMMENDATION: 27/08/2021
No symptoms or clinical signs attributable to infection with SARS-CoV-2’
Targeted antiviral therapy in the pre-symptomatic and asymptomatic phase can possibly prevent development of symptoms and thus progression to moderate or severe COVID-19 infection. It is estimated that 20% of symptomatic COVID-19 infection will go on to progress to moderate or severe COVID-19 infection and 30-50% will continue to remain asymptomatic (1,2). This asymptomatic phase may be characterized by high viral load and shedding which can in turn can cause inadvertent transmission of infection thus perpetuating the pandemic (3).
This monoclonal antibody cocktail Casirivimab-Imdevimab (REGEN-COVTM) was studied in asymptomatic patients with COVID-19 postulating that there may be an antiviral effect if given sufficiently early in illness in individuals who are seronegative (i.e., have no evidence of prior infection or have not yet developed an endogenous immune response). The baseline serology test included presence of serum anti-SARS-CoV-2 antibodies (anti-spike [S1] IgA, anti-spike [S1] IgG, and anti-nucleocapsid IgG) (4). Pre-specified outcomes as per the PICO, drafted included hospitalization and progression to severe disease. The evidence of benefit is very uncertain with very high cost implications towards reducing admission into hospital. In a country like India, where this indicates that a huge number of patients will need to be given this drug to prevent progression to severe disease in a very small number of patients, or prevent transmission of infection which could be managed by appropriate low-cost preventive measures, it seems prudent to not advocate roll out of this drug in this group of patients. In addition, since this is an expensive drug and requires patients to access a health care facility just to be given this drug it could fuel transmission of COVID-19 which could otherwise have been prevented. At this point data are unavailable regarding a possible benefit in immunocompromised high-risk asymptomatic individuals with COVID-19. Hence at this time the group recommends against the use of REGEN-COVTM in asymptomatic COVID-19.
Date of latest search: 04th April 2023
Date of completion & presentation to the Expert Working Group: 14th July 2021
Date of planned review: 04th April 2024
Evidence Synthesis Team: Hanna Alexander, Naveena George, Richard Kirubakaran and Priscilla Rupali
5th April 2023-Comment on Updation: The evidence synthesis team did a search on 4th of April 2023 to update the evidence summary and recommendation. The team found one study that studied the intervention. However, the study did not include the outcomes of interest proposed by the panel and hence not included in the meta-analysis. The characteristics of the new study are included in the Table for ‘Summary of characteristics of included trials’. The authors have been contacted for disaggregated data but have not responded till now.
Explanations
a. Explanations Downgraded by one-level for risk of bias as study has some concerns
b. Downgraded by two levels for serious imprecision as the 95%CI is wide: 0.08 (0.00-1.40) and event rate is low.
c. Downgraded by two levels for serious imprecision as the 95%CI is wide:0.11 (0.01-2.06) and event rate is low.
d. Downgraded by two levels for serious imprecision as the 95%CI is wide: 6.04(0.74-49.58) and event rate is low.
REGEN-COVT™ is a cocktail made up of two noncompeting, neutralizing human IgG1 antibodies that target the receptor binding domain of the SARS-CoV-2 spike protein, thereby preventing viral entry into human cells through the angiotensin-converting enzyme 2 (ACE2) receptor(4). The combination is manufactured by Regeneron pharmaceuticals. The drug was approved by the FDA for emergency use on 21 November 2020 in patients ≥12 yrs. of age, weighing at least 40 kilograms with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progressing to severe COVID-19. The authorized dosage is 1,200 mg of Casirivimab and 1,200 mg of Imdevimab administered together as a single intravenous (IV) infusion over 60min as soon as possible after positive viral test for SARS-CoV-2 and within 10 days of symptom onset. In this study however, this is used in a subcutaneous dose. The CDSCO in India, on 5 May 2021, granted an EUA to Roche (Genentech) and Regeneron for use of REGEN-C0VTM in the country. The announcement came in light of the second wave of the COVID-19 pandemic in India(5). This review aims to provide a summary of the available evidence from randomized clinical trials of REGEN-COVTM for treatment of COVID-19 which could guide clinicians and researchers regarding the appropriate use of this drug in the future.
We searched PubMed, Epistemonikos, and the COVID‐19‐specific resource www.covid‐nma.com, for studies of any publication status and in any language. We also reviewed reference lists of systematic reviews and included studies. We performed all searches up to 13th July 2021.
We searched the above databases and found 11 records. After removing duplicates and excluding those that did not match our PICO question, one randomized controlled trial was selected and included for the analysis.
We extracted data for the following outcomes, pre-defined by the Expert Working Group:
- Critical (primary for this review):
- Admission into hospital (all cause)
- Time to clinical recovery
- Progression to severe disease- requiring O2, non-invasive or invasive mechanical ventilation or ICU care
- Important (secondary):
- Time to viral clearance (negative PCR)
- Death
- Progression to symptoms
- Adverse events:
- All
- Serious
- Infusion-related adverse events (if reported, record rate of infusion used)
Two reviewers independently assessed eligibility of search results using the online Rayyan tool. Data extraction was performed by one reviewer, and checked by another, using a piloted data extraction tool on Microsoft Word. Risk of bias (RoB) was assessed using the Cochrane RoB v2.0 tool, with review by a second reviewer and compared with covidnma database. If there was a difference in more than one domain it was assessed by a third independent author. We used risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs) using Review Manager (RevMan) v5.4. We used GRADE methodology to make summary of findings tables on GRADEPro GDT.
We found one RCT that matched the PICO question as pre-defined by the expert working group. This randomized trial included a total of 314 participants, all of whom were adults and adolescents ≥ 12 years of age and asymptomatic house hold contacts of index cases with SARS-COV2 virus infection. Study was performed at 112 sites in the United States, Romania, and Moldova.
The results are described below; characteristics of the trials are summarized in the Summary of characteristics of included trials table.
The following comparisons were investigated in the trial (we compared outcomes for arms randomized to Casirivimab and Imdevimab combination (REGN-COVTM) vs Placebo as pre specified in our PICO which was formulated by the expert working group.
Outcomes
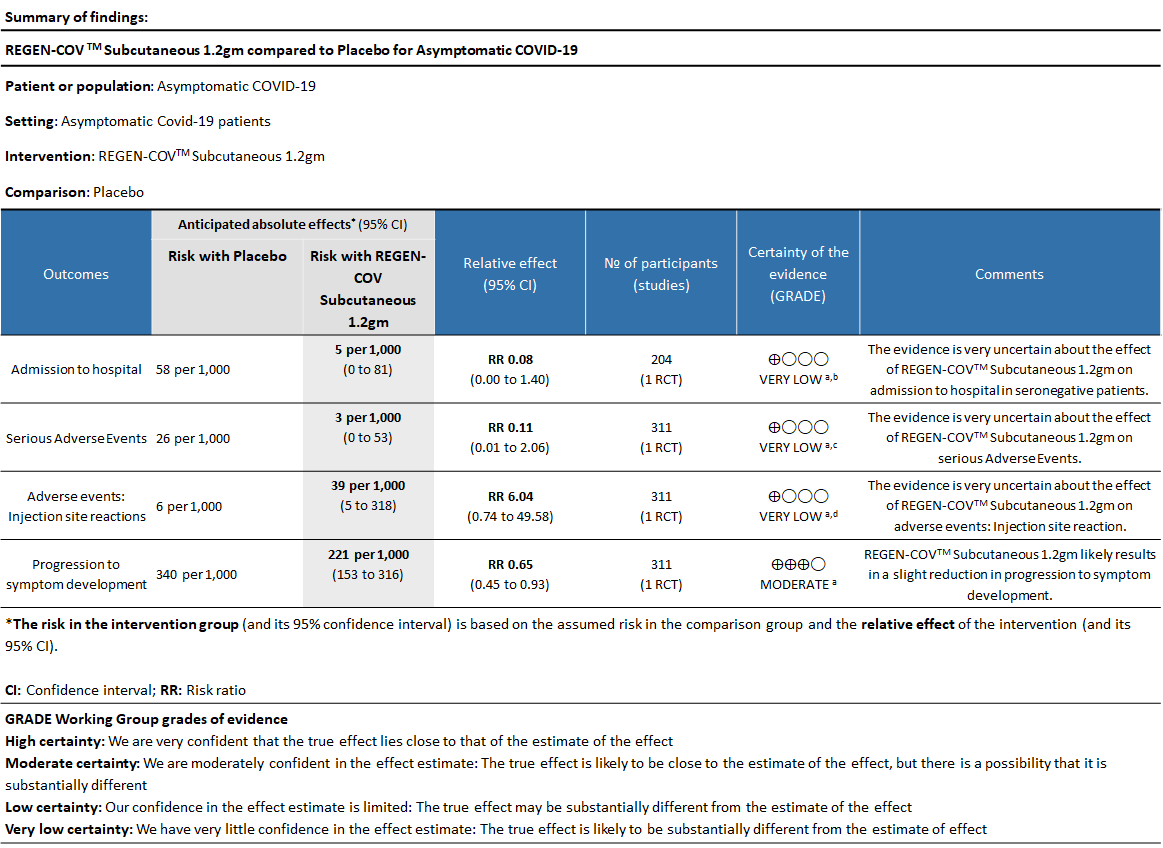
As presented in the ‘Summary of findings’ tables, the evidence is of very low certainty about the effect of Casirivimab and Imdevimab combination (REGEN-COVTM) 1.2 gm as a single dose subcutaneous injection on admission to hospital in seronegative patients and also on serious adverse events and injection site reactions.
a. Admission to Hospital in Seronegative patients:
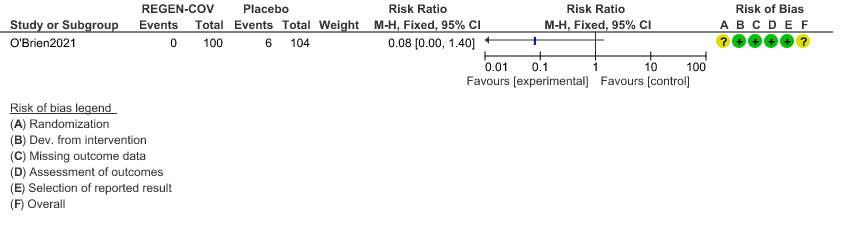
Very low certainty of evidence in 206 patients from 1 trial (3) found that the evidence is very uncertain about the effect of REGEN-COVTM subcutaneous 1.2gm injection on reduction of hospital admission in seronegative patients. (RR 1.08; 95 % CI 0.00-1.4)
b. Serious Adverse events:
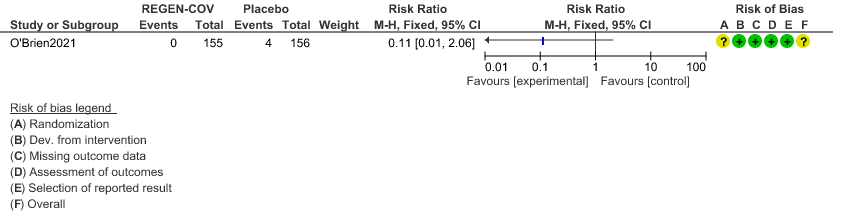
Very low certainty evidence in 311 patients from 1 trial (3) revealed that the evidence is very uncertain about the effect of REGEN-COVTM Subcutaneous 1.2gm on serious Adverse Events (RR 0.11;95% CI 0.01-2.06)
c. Injection site reactions:
Very low certainty evidence in 311 patients from 1 trial revealed a very uncertain effect of REGEN-COVTM Subcutaneous 1.2gm on Injection site reactions (RR 6.04 ;95% CI 0.74 - 49.58)
d. Progression to symptoms
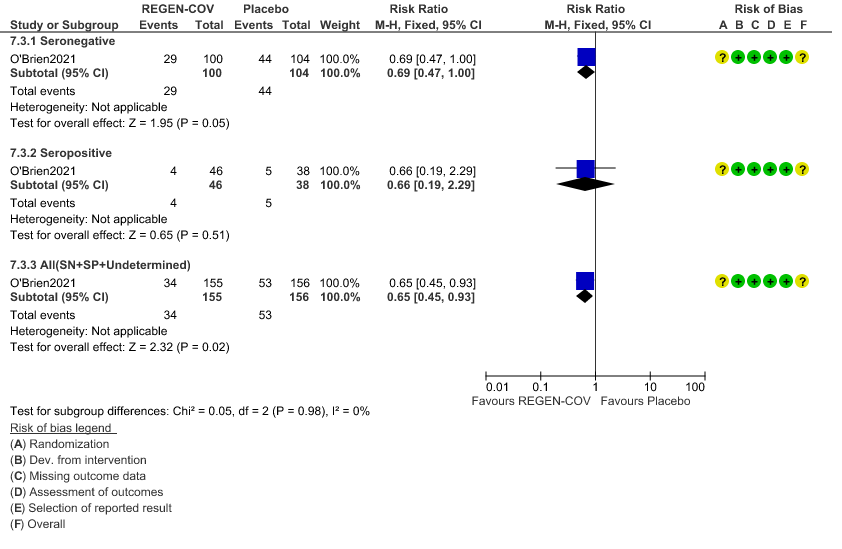
Moderate certainty evidence from 1 trial in 206 patients found that REGEN-COVTM probably reduces progression to symptoms RR 0.65; 95% CI 0.45-0.93).
Seronegative: Progression to symptom development was slightly reduced by REGEN-COV™ with a RR=0.69 (95% CI 0.47-1.00).
Seropositive: Progression to symptom development was not reduced by REGEN-COV™ with a RR=0.66 (95% CI 0.19-2.29).
Subgroup analysis
Disaggregated data could not be obtained for co-morbidities, different age groups, pregnancy, lactation, renal failure, liver failure were excluded from this trial. Also, no data were available separately for immunocompromised individuals or patients with 1 or more risk factors which had a high risk of progression to severe disease or death.
a. Admission to Hospital in Seronegative patients:
b. Serious Adverse events:
c. Injection site reactions:
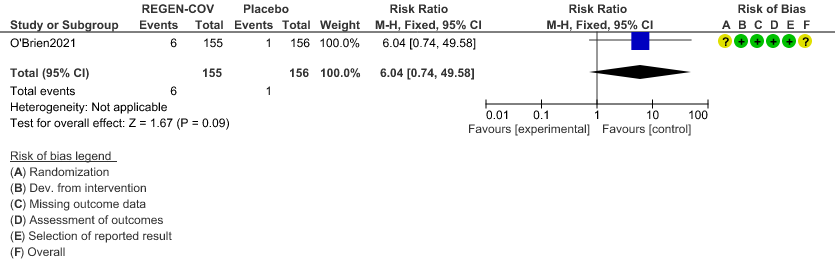
d. Progression to symptom development
Summary of judgements
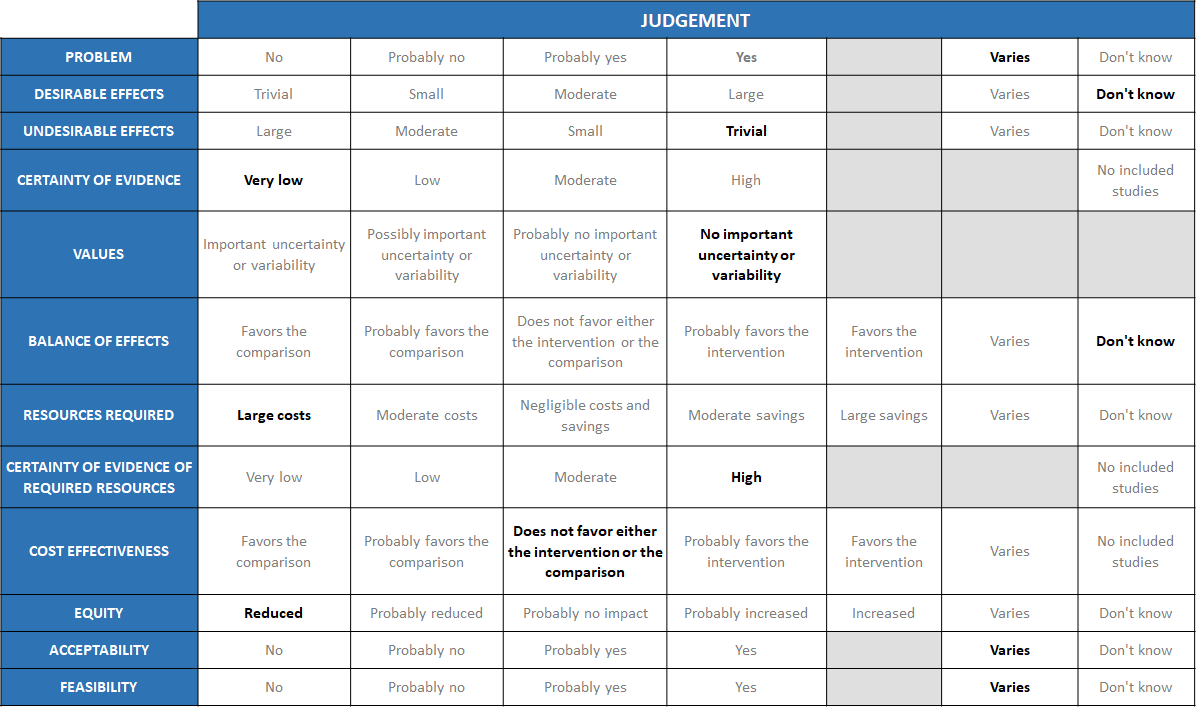
The Antibody Expert Working Group met on 14th July 2021 to consider Casirivimab and Imdevimab combination 1.2g as a treatment for patients with asymptomatic COVID-19. Conflict of interest declarations were reviewed by the Steering Committee; none were found to be relevant to Casirivimab and Imdevimab.
A summary and then more detailed explanations of the Expert Working Group's judgements follow.
Problem
The COVID-19 pandemic in India, which has resulted in more than 30 million illnesses and 0.39 million deaths, has wreaked havoc on the country's health system. The country is experiencing a significant health crisis due to a lack of intensive care unit beds, oxygen, and skilled people. This has resulted in many irrational and experimental treatment interventions, such as the use of Casirivimab and Imdevimab combination in all severity of patients across the country in patients hospitalized in Covid-19. Often this is done with no clear indication or evidence of which subgroup of the population or disease severity the drug is effective for. In this study the population is asymptomatic COVID positive individuals with a history of contact with a symptomatic COVID positive individuals. The panel acknowledged the ambiguity that the study did not specify the difference between healthy and high-risk patients. So, the panel judged that the problem of utility of treatment with REGEN-COVTM 1200 mg varies across patient population. While it may be considered beneficial and cost effective in certain high risk groups, it would not be cost effective or considered a priority in healthy asymptomatic individuals, who anyway have a low risk of mortality and morbidity.
Desirable effects
The group discussed the anticipated desirable effects of the drug in covid-19. In PCR-positive/seronegative asymptomatic participants, REGEN-COVTM 1200mg SC treatment had very low certainty evidence and had an uncertain effect on admission into hospital RR=0.08(0.00-1.4).
In PCR-positive/seronegative asymptomatic participants, REGEN-COVTM 1200mg administered subcutaneously (SC) treatment did reduce the risk of developing a symptomatic infection compared with placebo (31.5% relative risk reduction; 29/100 [29.0%] vs. 44/104 [42.3%]; odds ratio 0.54 [95% CI, 0.30 to 0.97]; P=0.0380) – this was specified a priori by the guideline group as progression to severe disease which in this case is progression to symptomatic phase. However, preventing the progression to symptomatic phase did not mean preventing progression to severe or critical disease.
There was a more rapid decline in viral load in REGEN-COVTM 1200mg SC treated participants as compared to those treated with placebo, with an adjusted mean difference in viral load of -1.5 log10 copies/mL in favor of the antibody combination at Day 8. However, this was not not specified a priority by the guideline group and hence cannot be considered as in most viral infection only a decrease in 2 log viral loads is considered significant.
The group made the decision of “don’t know” due to the uncertainty of the evidence as to the benefit in this population
Undesirable effects
A lower proportion of participants in the REGEN-COVTM group experienced treatment emergent adverse events (33.5%) compared with placebo (48.1%), including both Covid-19-related (25.8% vs. 39.7%, respectively) and non-Covid-19-related (11.0% vs. 16.0%, respectively) events. However, RR=0.11 (0.01-2.06) widely varying confidence intervals suggested that there was no appreciable difference between the two groups.
Injection site reactions (grade 1–2) occurred in 6 participants (4%) in the REGEN-COVTM group and 1 participant (1%) in the placebo group. Overall the group felt that the undesirable effects were trivial.
Certainty of evidence
Using GRADE methodology, the evidence synthesis team rated the certainty of evidence as very low for progression to hospital admission in seronegative patients, serious adverse events and injection site reactions with moderate certainty of evidence for progression to symptoms which would not really contribute to a mortality or morbidity reduction. The group felt that the overall certainty of evidence reviewed was very low.
Values
The group reviewed the results and concluded that there is no significant uncertainty or variability with regard to the outcomes. They also felt that the progression to symptoms was an important outcome.
Balance of effects
The group felt that the balance of effects did not favor the intervention. The cost implications were too high in a group of patients normally considered low risk for progression to severe disease or death. The group felt that the desirable effects did not show a difference in this population of asymptomatic healthy COVID-19 positive individuals, but it is possible that this drug may make a difference in a high-risk population with 1 or more co-morbidities which have been shown to progress to severe disease, however that population has not been studied in this trial. Hence the group felt that there was still significant equipoise remaining and hence voted “don’t know”.
Resources required
The group believed that the resources needed to implement this intervention are significant and large, as the cost includes both the drug cost and the resources needed to securely give the drug in a healthcare facility without risk to family and health care workers alike.
Certainty of evidence of required resources
No studies reporting this have been evaluated by the group, but because the physicians in the group were aware of the expense, infrastructure and personnel required to deliver this intervention, they agreed that there was high certainty of evidence with regard to the resources required to implement this intervention.
Cost effectiveness
This drug is expensive and the study was not powered to answer our PICO outcomes of hospitalization/death. The group felt that there was uncertainty with regard to benefit with this drug vs placebo in this group of asymptomatic COVID-19 patients. This category of patients would be considered relatively low risk for progression to severe disease or death and hence the administration of the drug in this group would not considered justified.
Equity
This drug is extremely expensive and certainly cannot be afforded or accessed by those with a low socioeconomic background. In addition, it may not be stocked by smaller pharmacies due to cost implications and hence may be inaccessible in the time of need and hence the group voted for reduced equity.
Acceptability
The acceptability of this intervention is likely to vary depending on the population or the health care setting. Asymptomatic otherwise healthy individuals as well as the regulatory authorities would not probably be accepting of an intervention that is expensive and will only reduce symptoms or viral load but would not prevent hospital admission. On the other hand, patients with multiple serious co-morbidities may be accepting of this expensive intervention on the premise that if it reduced symptoms and viral load it would likely be beneficial and hospital authorities may be accepting if it brought in extra revenue. Clinicians also may consider use in this category of patients only if they expected a high risk of progression to severe disease or death.
Feasibility
The group concluded that feasibility to implement the intervention varies because it depends on the type of setting and population it is advised for.
The recommendations being made are primarily from a single randomized trial done at multiple trial sites in asymptomatic COVID-19 patients who are seronegative based on a validated antibody test. REGEN-COVTM at a dose of 1200mg was given subcutaneously in asymptomatic patients with COVID-19 infection in this trial. As the data is scarce with this route of administration it should be used only as an alternative to intravenous infusion in this category of patients.
The decision to administer REGEN-COVTM in this category of patients should be made judiciously and cautiously as it is an expensive intervention with a low certainty of evidence that it decreases hospital admission in patients with early COVID-19 illness. A rapid antibody test result needs to be available with a quick turn-around of a couple of hours for this intervention to be implemented. This is out of the reach of many secondary or even tertiary care settings in the public or private sector. In addition, cost of the drug, resources and infrastructure required to deliver this drug on an outpatient basis are considerable and need to be taken in to account when delivering this drug. Being a protein moiety, though the reported drug related reactions were very low, it would be prudent to be prepared to handle anaphylaxis and other allergic reactions at the health care setting that it is being administered at. Since the costs of the drug are huge, therefore its widespread use in the Indian setting will not be practical or feasible.
It is possible that in the future it may be of benefit in selected high-risk sub-groups with significant immunocompromise, where costs are less of a consideration but at this point in an asymptomatic group with a positive Covid test, we would discourage its use in the Indian setting as the natural history of progression to severe disease or death is very small and does not justify its use.
Disaggregated subgroup data was not available for pregnant women, those with co-morbidities and immunosuppressed patients and hence at this point it is not possible to comment whether there are specific benefits or harms in this subgroup with the use of this antibody cocktail. Though it would have interesting to note variables like prior vaccination, infecting strain and prior infection and studied their effect on efficacy this study did not do so.
The current evidence does not support the use of the antibody cocktail in asymptomatic PCR+/ seronegative household contacts of COVID-19 since it has an uncertain effect on reducing hospital admission. There was a possible signal towards reduction in progression to symptoms, however the trial was not designed to assess progression to severe disease or death and hence preventing just preventing symptoms is not clinically relevant. Therefore, the group will continue to monitor literature for a trial adequately powered to assess this important outcome of hospitalization or severe COVID-19 disease. It will also be important to identify whether a particular subgroup of patients including the immunocompromised or those with multiple comorbidities may benefit with this intervention.
There is a subgroup of patients i.e., those with co-morbities or immunocompromised state who would have a high risk of progression to severe disease or death and in this group of patients, there may be a role for REGEN-COVTM. However, the role of REGEN-COVTM in asymptomatic COVID positive individuals remains uncertain and further trials are required to elaborate on the benefits of the same.
1. Identify the subgroup of patients who is most likely to note a benefit if asymptomatic.
2. Cost effective analysis to determine if use of REGEN-COVTM in this group of patients is justified.
- Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. 2020;323(13):1239–1242. doi:10.1001/jama.2020.2648.
- Oran DP, Topol EJ. Prevalence of Asymptomatic SARS-CoV-2 Infection : A Narrative Review. Ann Intern Med. 2020 Sep 1;173(5):362-367. doi: 10.7326/M20-3012. Epub 2020 Jun 3. PMID: 32491919; PMCID: PMC7281624.
- Zou L, Ruan F, Huang M, et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med 2020; 382:1177-9.
- O'Brien MP, Forleo-Neto E, Musser BJ, et al. Subcutaneous REGEN-COV Antibody Combination for Covid-19 Prevention. New England Journal of Medicine Published August 4, 2021 R 10.1056/NEJMoa2109682 accessed on August 8, 2021 from https://www.nejm.org/doi/full/10.1056/NEJMoa2109682.
- Commissioner O of the. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 [Internet]. FDA. FDA; 2020 [cited 2021 Jun 29]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19
- Roche receives Emergency Use Authorisation in India for its investigational Antibody Cocktail (Casirivimab and Imdevimab) used in the treatment of Covid-19 | Cipla [Internet] accessed June 29,2021 from: https://www.cipla.com/press-releases-statements/roche-receives-EUA-India-investigational-antibody-cocktail-Casirivimab-Imdevimab-covid.
Covid Management Guidelines India Group – Anti-body Working Group. Casirivimab – Imdevimab. Covid Guidelines India; Published online on August 27, 2021; URL : https://indiacovidguidelines.org/casirivimab-imdevimab-asymptomatic/ (accessed )
